The ability to generate transgenic plants without regard to cultivar or genotype can be considered a holy grail of cereal crop transformation. Despite years of effort, it has been remarkably difficult to develop efficient methods for transformation of cereals. The preferred methods generally involve Agrobacterium tumefaciens-mediated transformation of cultured tissue or immature embryos, followed by callus culture to regenerate plants (reviewed in Shrawat and Lörz, 2006). Unfortunately, the capacity of Agrobacterium to infect monocots is limited to a narrow range of genotypes and the utility of the technique is further limited by the recalcitrance of many genotypes to callus formation and regeneration. A Breakthrough Report from Lowe et al. (2016) describes an exciting new approach to boost monocot transformation rates in a broad range of genotypes.
Lowe et al. took advantage of morphogenic genes to create a biological context conducive to transformation efficiency. Their transformation constructs included maize (Zea mays) Baby boom (Bbm) and Wuschel2 (Wus2), homologs of which in Brassica napus and Arabidopsis thaliana promote transition from vegetative to embryonic growth (Boutilier et al., 2002; Zuo et al., 2002). The authors used these constructs for Agrobacterium-mediated transformation of immature embryos from four maize inbred lines. These lines showed markedly higher transformation rates, going from 0 to 2% in the controls to 25 to 51% in the presence of Wus and Bbm.
Lowe et al. designed their constructs with flanking LoxP recombination sites and CRE recombinase driven by a drought-inducible promoter. Accordingly, desiccation treatment of the transgenic tissue allowed excision of the Wus, Bbm, and CRE expression cassette. The final transformation efficiency, accounting for all three stages of the process (transformation, excision, regeneration of healthy plants), for the four tested lines ranged from 3 to 11% (calculated based on the number of initial embryos used and the final number of healthy transgenic plants obtained). This level of recovery of transgenic plants is high enough to be useful for large-scale production both for commercial purposes and in primary research.
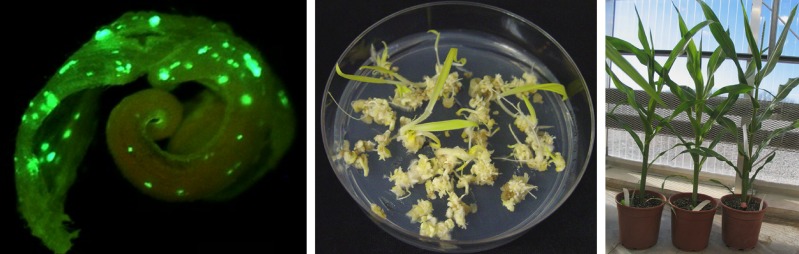
Among a panel of 50 maize inbred lines, 33 successfully produced transgenic calli upon transformation with Wus and Bbm. Thus, although not yet fully genotype independent, this approach greatly increases the range of cultivars available for transformation. Importantly, it can be used on a variety of initial tissues. Maintaining a consistent supply of immature embryos can be costly and time-consuming, but Lowe et al. obtained healthy transgenic plants by directly transforming mature seed tissues or seedling leaf segments (see figure), skipping the usual explant culture step before transformation and further facilitating large-scale transformation efforts.
Morphogenic gene-based transformation of a maize leaf segment. Left: green fluorescent calli on leaf segments from maize seedlings transformed with expression cassettes including Wus2 and Bbm. Middle, right: after excision of Wus2 and Bbm, calli grow and readily regenerate into leaf-derived T0 plants (right). (Figure courtesy of Ning Wang.)
This morphogenic gene-based approach also worked in other monocot crops, increasing transformation frequency from 1.9% to 18% in sorghum and from 0–3% to 15–43% in rice, whereas sugarcane callus showed an almost fantastical increase from 2% to 274–885% (i.e., multiple transgenic events recovered per initial starting tissue). In extending the range of species, genotypes, and tissues that can be used for efficient transformation, Lowe et al. have given us a beautiful example of what can be accomplished by combining basic research, technical expertise, and knowledge of practical problems facing mainstream applications.
Supplementary Material
Footnotes
Articles can be viewed without a subscription.
References
- Boutilier K., Offringa R., Sharma V.K., Kieft H., Ouellet T., Zhang L., Hattori J., Liu C.-M., van Lammeren A.A.M., Miki B.L.A., Custers J.B.M., van Lookeren Campagne M.M. (2002). Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14: 1737–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe K., et al. (2016). Morphogenic regulators Babyboom and Wuschel improve monocot transformation. Plant Cell 18: 1998–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrawat A.K., Lörz H. (2006). Agrobacterium-mediated transformation of cereals: a promising approach crossing barriers. Plant Biotechnol. J. 4: 575–603. [DOI] [PubMed] [Google Scholar]
- Zuo J., Niu Q.-W., Frugis G., Chua N.-H. (2002). The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J. 30: 349–359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.