Abstract
Optimal iron nutrition in utero is essential for development of the fetus and helps establish birth iron stores adequate to sustain growth in early infancy. In species with hemochorial placentas, such as humans and rodents, iron in the maternal circulation is transferred to the fetus by directly contacting placental syncytiotrophoblasts. Early kinetic studies provided valuable data on the initial uptake of maternal transferrin, an iron-binding protein, by the placenta. However, the remaining steps of iron trafficking across syncytiotrophoblasts and through the fetal endothelium into the fetal blood remain poorly characterized. Over the last 20 years, identification of transmembrane iron transporters and the iron regulatory hormone hepcidin has greatly expanded the knowledge of cellular iron transport and its regulation by systemic iron status. In addition, emerging human and animal data demonstrating comprised fetal iron stores in severe maternal iron deficiency challenge the classic dogma of exclusive fetal control over the transfer process and indicate that maternal and local signals may play a role in regulating this process. This review compiles current data on the kinetic, molecular, and regulatory aspects of placental iron transport and considers new questions and knowledge gaps raised by these advances.
Keywords: iron, fetal development, metabolism, placenta, regulation.
INTRODUCTION
During pregnancy, the placenta actively transports iron from the mother to the fetus. Iron is an essential component of many enzymes and hemoproteins vital for normal function of all cells, and iron demand increases during rapid growth and development. Iron deficiency in human infants is associated with a number of short- and long-term neurodevelopmental deficits that persist even after iron repletion. Animal data have attributed these effects to neural processes most vulnerable to iron deficiency in early life, including neurotransmitter production, neuronal energy metabolism, and myelination.1 Recent work in rodents has further implicated epigenetic changes and abnormal gene expression in the brain in the pathophysiology of long-term neurological dysfunction associated with early iron deficiency.1,2 In addition to impaired brain function, emerging human and animal data have demonstrated other developmental consequences of prenatal iron deficiency, including elevated blood pressure, altered nephron morphology, and an increased risk of iron deficiency during infancy.3–6 The identification of gestational windows of organ development most profoundly impacted by iron deficiency further underlines the importance of adequate iron supply throughout fetal life.7,8 Finally, the relatively frequent occurrence of iron deficiency in breastfed infants before 6 months of age, both in industrialized (6%–15%)9–11 and in developing (12%–37%)10 countries, suggests that a considerable number of newborns may not have adequate iron stores at birth.
The importance of iron nutrition in utero has prompted sustained interest in research on placental iron transport since the early 20th century. While early studies provided valuable data on the kinetics and chemical nature of placental iron transfer, it was not until the introduction of modern biochemical and molecular genetic tools that a detailed characterization of the transport process became possible. Even so, fundamental questions remain regarding the roles of key cellular iron transporters and the systemic iron regulatory hormone hepcidin in iron transport across the placenta. This review summarizes decades of human and animal data on the mechanism and regulation of placental iron transport and identifies important gaps to address in future research.
NONHEME IRON TRANSPORT
Iron uptake
In humans, approximately 80% of fetal iron accrues in the last trimester of pregnancy,12 with the peak transfer rate estimated to be as high as 7 mg/d.13 Expressed as a proportion of the basal plasma iron turnover in nonpregnant adults, this represents 23% to 35% of plasma iron flux, second only to iron directed to the bone marrow.
In species with hemochorial placentas such as humans and most primates and rodents, maternal blood is in direct contact with fetal tissue, namely the trophoblasts of the placental villi, to promote efficient exchange of gas, nutrients, and waste. Transferrin-iron is the major, if not only, maternal iron source for placental transfer, and its uptake is mediated by transferrin receptor 1 (TFR1) (Figure 1). Transferrin receptor 1 is a homodimeric transmembrane protein with high affinity for diferric transferrin and is a part of the principal mechanism of cellular iron acquisition in vertebrates.14 Expressed in virtually all cells except mature erythrocytes, TFR1 is found at uniquely high levels in nucleated erythroid precursors and placental syncytiotrophoblasts, two cell types that transport iron for distinctly different purposes: hemoglobin synthesis and fetal iron acquisition, respectively.15 In fact, the placenta was one of the first tissues from which TFR1 was isolated and characterized16,17 and has been the major source of TFR1 standards used in serum TFR1 immunoassays.18–20
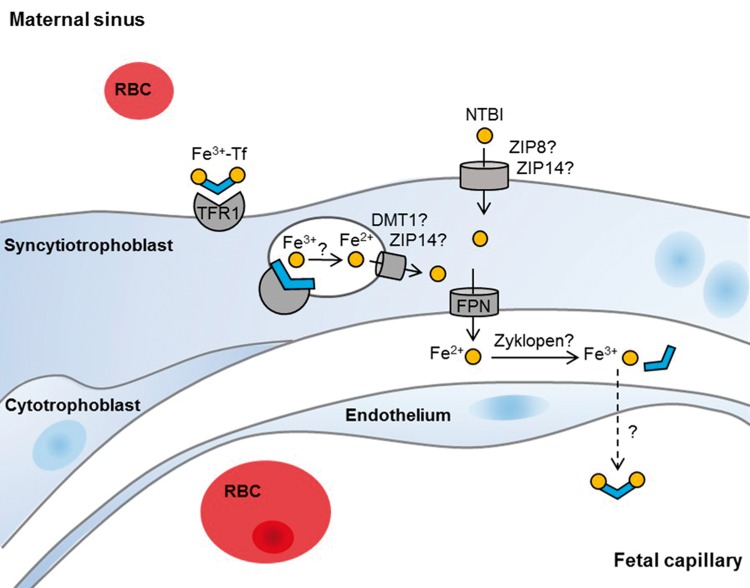
Figure 1.
Mechanisms of iron transport across human placenta. Cross-section of a placental villus bathed in maternal blood and surrounded by the multinucleated syncytiotrophoblast. The basal side of the syncytiotrophoblast is in contact with a discontinuous layer of mononucleated cytotrophoblasts or the basement membrane. In terminal villi, fetal capillaries are in close vicinity to the syncytiotrophoblast and are separated only by fetal endothelium. Maternal diferric transferrin (Tf) binds to transferrin receptor 1 (TFR1) on the apical side of the syncytiotrophoblast and is internalized by receptor-mediated endocytosis. Within the endosomes, iron dissociates from Tf and is reduced and released into the cytoplasm via an unknown mechanism that may involve ferrous iron transporters divalent metal transporter 1 (DMT1) and/or Zrt and Irt-like protein 14 (ZIP14). Iron is exported from the syncytiotrophoblast by the iron exporter ferroportin (FPN) and is oxidized to a ferric state by ferroxidases with uncertain identities. Ferric iron likely binds to fetal Tf after exiting the syncytiotrophoblast and is subsequently transported across the fetal endothelium through unknown mechanisms. The placenta may also take up non-Tf-bound iron from the maternal circulation by ZIP8 or ZIP14, expressed on apical side of the syncytiotrophoblast. Abbreviations: DMT1, divalent metal transporter 1; Fe2+, ferrous iron; Fe3+, ferric iron; FPN, ferroportin; NTBI, non-transferrin-bound iron; RBC, red blood cell; Tf, transferrin; TFR1, transferrin receptor 1; ZIP, Zrt- and Irt-like protein
The strong placental expression of TFR1 during peak fetal demand,21,22 the efficient transfer of maternally injected ferric iron to the fetus,23 and the profoundly anemic phenotype of Tfr1-null mouse embryos24 suggest a potential role of TFR1 in placental iron transport. Despite the likely importance of TFR1, the mechanism of iron transport in the placenta remains poorly characterized, partly because of the lack of in vitro systems that mimic the polar iron physiology of the placental syncytiotrophoblast and because placental iron transport has not been studied with modern molecular genetic techniques, including tissue-specific gene targeting in vivo.
Certain aspects of the transferrin cycle have, however, been studied in isolated trophoblasts and the BeWo human choriocarcinoma cell line; they have yielded results similar to those found in other tissues that express high levels of TFR1, such as erythroblasts. Immunohistochemical studies have localized TFR1 and transferrin to the apical membrane of syncytiotrophoblasts.25,26 Concordantly, immunoelectron microscopy demonstrated the presence of transferrin26–28 and TFR129 on the membrane of cellular invaginations and intracellular vesicles, likely representing clathrin-coated endosomes that contain transferrin,30,31 TFR1,32 and TFR1-transferrin complexes.30,32 Kinetic studies in BeWo cells33 and placental microvillous membrane preparations17,34,35 showed that diferric transferrin uptake is a specific and saturable process, with binding affinities similar to values reported in K562 erythroleukemia cells36 and reticulocytes.37,38 The transferrin cycle time in BeWo cells33 is also comparable to that in HepG239 and K562 cells.40 Finally, the weak base chloroquine, which disrupts endosomal acidification, inhibits placental accumulation and fetal transfer of transferrin-iron in a dose-dependent manner.41 Studies in rat placenta suggest that the inhibitory effect of high pH is due to reduced iron release from internalized transferrin,42 also consistent with the requirement of acidification in transferrin-iron utilization in the classic transferrin cycle. Collectively, these data suggest that syncytiotrophoblasts utilize a mechanism of transferrin-iron endocytosis similar to that observed in other cells.
Questions remain regarding the metabolic fate of transferrin-iron following uptake. It is unclear how transferrin-iron in endosomes enters the cytoplasm and reaches the basal membrane for export. In erythroid endosomes, ferric iron (Fe3+) is reduced to ferrous iron (Fe2+) by the ferrireductase STEAP3 (six-transmembrane epithelial antigen of the prostate 3) and is subsequently transported across the endosomal membrane by divalent metal transporter 1 (DMT1, solute carrier family 11 member 2 [SLC11A2]). Whether STEAP3 and DMT1 play similar roles in the placenta is unclear, but strongly suspected. STEAP3 is highly expressed in mouse and human placenta. The STEAP3 homolog, STEAP4 (six-transmembrane epithelial antigen of the prostate 4), which likewise exhibits ferrireductase activity,43 is also highly expressed in the human placenta.43,44 Iron reduction in the placenta might also involve a cytochrome B561 isoform expressed on the late endosomal/lysosomal membranes.45
Similar to the uncertainty regarding the requirement for and the identity of a placental endosomal reductase, the protein responsible for endosomal iron export has been presumed to be DMT1. DMT1 is critical for luminal iron uptake in enterocytes and for endosomal iron release in erythroid cells.46 In human syncytiotrophoblasts, DMT1 has variably been reported to have a punctate cytoplasmic distribution and to be localized predominantly to the apical47,48 or basal membranes.25,49 (Table 1) This dispersed pattern of DMT1 distribution may suggest multiple sites of action. The necessity of DMT1 in placental iron transport requires experimental confirmation, especially in light of the normal body iron concentration in neonatal Dmt1-null mice.50 Another potential endosomal iron transporter is ZIP14. ZIP14 is a member of the SLC39A (solute carrier family 39 A) zinc transporter family that also transports Fe2+.51 ZIP14 is highly expressed in mouse placenta52 and has been shown to mediate plasma membrane uptake of non-transferrin-bound iron53 as well as transferrin-iron assimilation from endosomes.54 Targeted Zip14 mutants have no abnormal birth phenotype except lower birth weight, which might be attributed to abnormal bone morphogenesis and endocrine abnormalities.55 Another member of the SLC39A family, ZIP8, which transports Fe2+, is also highly expressed in placenta.56 Targeted deletion of Zip8 in mice leads to complete preweaning mortality.57 Finally, mucolipin 1, a ubiquitous membrane protein, was recently found to function as an Fe2+-permeable channel in late endosomes and lysosomes.58 The role of ZIP14, ZIP8, and mucolipin 1 in placental iron transport is unknown, and it is possible that these three, together with DMT1, play redundant roles in endosomal iron export.
Table 1.
Function and localization of iron transporter proteins in human and mouse placentaea
| Proposed function | Protein | Localization in human placenta | Localization in mouse placenta |
|---|---|---|---|
| Apical iron uptake | |||
| Transferrin | No localization data | ||
| TFR1 | Strong, predominant staining on apical membrane of labyrinth trophoblasts; also stains fetal endothelium at E1020 | ||
| HFE | No localization data | ||
| SCARA5 | No localization data | ||
| ZIP8 | No localization data | ||
| Intracellular iron trafficking | |||
| ZIP14 | Syncytiotrophoblast: strong cytoplasmic and membranous staining50 | No localization data | |
| DMT1 | No localization data | ||
| Ferritin | No localization data | ||
| STEAP3 | Syncytiotrophoblast: weak and diffuse cytoplasmic staining; intense and granular staining along basal membrane50 | No localization data | |
| STEAP4 | No localization data | No localization data | |
| Basolateral iron export | |||
| FPN1 | Strong, continuous staining in labyrinth trophoblasts at E16.554 | ||
| Ceruloplasmin | No localization data | ||
| Hephaestin | Syncytiotrophoblast: membranous and cytoplasmic staining 50 | No localization data | |
| Zyklopen | No localization data | Labyrinth trophoblasts and spongiotrophoblasts at E15.557 | |
| Heme iron metabolism | |||
| HRG1 | No localization data | No localization data | |
| LRP1 | Strong staining in ectoplacental cone at E7.559 | ||
| PCFT | Weak staining in labyrinth trophoblasts, spongiotrophoblasts, trophoblast giant cells, and fetal endothelium at E12.5–18.561 | ||
| FLVCR1 |
|
|
|
| FLVCR2 | No localization data | ||
| HO1 | Labyrinth trophoblasts2: positive staining at E1367; negative staining at E14.568; strong staining in spongiotrophoblasts at E13 and E14.567,68 | ||
| HO2 | Moderate staining in labyrinth trophoblasts, spongiotrophoblasts, and trophoblast giant cells at E13; weak staining in fetal endothelium67 | ||
aAll human data are from studies in term healthy placentae; mouse studies represent expression after the formation of mature placenta at E10.5, if data are available. Subcellular locations of some proteins are not described because the immunohistochemistry images are at low resolution.
bDenotes inconsistent findings.
Abbreviations: DMT1, divalent metal transporter 1; E, embryonic day; FLVCR, feline leukemia virus subgroup C cellular receptor; FPN, ferroportin; HO, heme oxygenase; HRG, heme-responsive gene; LRP, low-density lipoprotein receptor-related protein; PCFT, protein-coupled folate transporter; SCARA5, scavenger receptor class A member 5; STEAP, six-transmembrane epithelial antigen of the prostate; TF, transferrin; TFR1, transferrin receptor 1; ZIP, Zrt- and Irt-like protein.
Radioisotope studies in perfused human placentas,41 cultured trophoblasts,35 and BeWo cells33 showed that the majority of iron derived from transferrin-iron is incorporated into ferritin, with a small fraction found in a low-molecular-weight cellular fraction35 and the reminder recovered in the extracellular media. Ferritins in the placenta contain mainly the heavy chain subunit.59 There is also evidence for a placental-specific ferritin heavy chain homolog with immunosuppressive activity.60 Immunohistochemical studies of ferritin expression in syncytiotrophoblasts are conflicting, with the majority of reports showing a lack of expression,25,61–63 while others demonstrate some staining.64,65 (Table 1) This discrepancy may be due to the use of antibodies that react differentially with each isoferritin. In contrast to the syncytiotrophoblast, fetal villous stromal cells consistently show pronounced ferritin staining.25,61,62,64,65 This raises the possibility that villous stroma may serve as a buffer between the syncytiotrophoblast and fetal circulation to ensure adequate, but not excessive, iron supply. Whether synthesis and degradation of ferritin in the stromal cells respond to fetal iron demand is unknown. Thus, although it can be inferred that TFR1, DMT1, STEAP3, and possibly other proteins are essential for iron uptake in the placenta, experiments designed to directly test these hypotheses have not been performed.
Basal transport of iron
Iron export from the syncytiotrophoblast to the fetal stroma is likely mediated by the iron exporter ferroportin 1 (FPN1), also known as SLC40A1 (solute carrier family 40 member A1). FPN1 is abundantly expressed along the basal membrane of human syncytiotrophoblasts25,66 and mouse labyrinthine trophoblasts.67 Studies in transgenic animals with aberrant Fpn1 expression provide further evidence for a role of Fpn1 in maternal–fetal iron transport. Mouse embryos with a hypomorphic mutation in Fpn1 are severely iron deficient at E12.5 and exhibit defects in neural tube closure and forebrain patterning.68 Iron-chelating experiments suggest that the developmental abnormalities in the Fpn1 hypomorphs are due to impaired iron delivery from the visceral endoderm to the embryo.68 In addition, a deletion of the iron-responsive element in the Fpn1 5′ untranslated region results in dysregulation of Fpn1 in multiple organs and markedly reduced FPN1 protein expression in the placenta, causing severe anemia and tissue iron deficiency at birth.67
In contrast to the global Fpn1 knockout mice that die early in gestation, animals with selective retention of Fpn1 in primitive endoderm (the precursor of the visceral yolk sac) and trophoectoderm (the precursor of the placenta) survived to term and were indistinguishable from their wild-type littermates at birth,69 suggesting that Fpn1 expression in the maternal–fetal interface is essential for normal embryonic development. However, it is not clear whether Fpn1 expression in the visceral endoderm, the placenta, or both conferred embryonic viability in the conditional mutants. While all Fpn1 transgenic mouse studies suggest a role for Fpn1 in placental iron transfer, not one directly address its role in mediating iron efflux from the syncytiotrophoblast.
Following export by FPN1, iron must be oxidized to the ferric state before binding to transferrin. Three multicopper ferroxidases have been identified, all of which can be found in the placenta: ceruloplasmin, hephaestin, and zyklopen). Ceruloplasmin is a soluble copper-dependent ferroxidase that facilitates iron efflux from macrophages and hepatocytes.70 Immunohistochemical staining in human placenta demonstrated ceruloplasmin in syncytiotrophoblasts71,72 and fetal capillaries71; however, whether the former is due to local production is uncertain, since ceruloplasmin gene (Cp) mRNA is largely restricted to the liver, retina, and endothelial cells, although mRNA expression has been detected in cultured human syncytiotrophoblasts.71 Cp-null animals exhibit a normal phenotype at birth, suggesting that Cp is not essential for placental iron transport.70 On the basis of the phenotype of sex-linked anemia (sla) mice that carry a mutation, the membrane-bound Cp homolog Heph appears to be important for iron egress from enterocytes.73 Heph has not been localized to human placenta, but expression of Heph mRNA has been demonstrated in rat placenta74 and human BeWo choriocarcinomas.75 Hemoglobin levels in sla pups is usually decreased compared with that in wild-type pups, though there is some overlap in the phenotypes.76 Placental transfer of maternally injected radioiron in the second half of pregnancy was not different between sla and wild-type mice, although sla pups accumulated less radioiron given in the maternal diet throughout pregnancy.76 It is worth pointing out that the sla allele of Heph retains partial ferroxidase activity,77 so the minimally perturbed placental iron transfer in sla pups may underrepresent the degree to which Heph is important for placental iron transport. A third ferroxidase in the placenta has been described by Danzeisen et al.,78 who detected an intracellular, membrane-bound protein in BeWo cells that exhibited ceruloplasmin-like oxidase activity and reacted with a ceruloplasmin antibody.72 Recently, this group identified zyklopen as a placenta-specific ferroxidase79 that has approximately 50% protein identity with ceruloplasmin and hephaestin. Zyklopen contains a transmembrane domain and an extracellular ferroxidase domain with appropriate topology to interact with FPN1.79 Absent in liver and intestine, zyklopen is abundantly expressed in the placenta and has been localized to the labyrinth, spongiotrophoblasts, and yolk sac of mouse placenta.79 Thus, there is evidence that all 3 mammalian multicopper ferroxidases are expressed in the placenta, but little is known definitively regarding their functions in placental iron transport. Research is needed to determine the subcellular locations of the ferroxidases in placental tissue and to elucidate the function and possible interaction of the ferroxidases with one another and/or with FPN1 in facilitating iron export from the syncytiotrophoblast.
The specific events following iron exit from syncytiotrophoblasts, like those following iron exit from enterocytes, the intestinal counterparts of syncytiotrophoblasts, are obscure. In terminal placental villi, the syncytiotrophoblast comes in close contact with fetal capillaries to facilitate gas, nutrient, and waste exchange.80 It is possible that a fraction of iron released from syncytiotrophoblasts is not bound by transferrin and is utilized by fetal tissues through non-transferrin-mediated pathways, but the majority likely binds to transferrin in the extracellular space25 before traversing the fetal endothelium into the fetal circulation. How iron is trafficked across the endothelial layer is unknown. The weak cytoplasmic staining of TFR181 and the nondetectable expression of FPN125 in placental fetal endothelium suggest a mechanism of transport that is different from the syncytiotrophoblast. Large molecules such as immunoglobulin G traffic across fetal endothelium by vesicular transport,82 but it is unclear if this type of mechanism applies to iron or iron chelates. Thus, it seems likely that some unique mechanisms and regulatory processes are involved in iron transport across the fetal vascular endothelium. It should be noted that, in contrast to the nonfenestrated endothelium in human placenta, the villous endothelium in rodents has numerous fenestrae that confer greater permeability to small solutes such as glucose.83,84 It is unclear whether larger molecules such as transferrin can move across fetal endothelium through fenestrations, but this may represent an additional route in addition to vesicular or receptor-mediated pathways. Future research to examine the possible role of fetal endothelium in placental iron transport should be of interest.
Other cells in the placental stroma that may participate in iron transfer are the villous macrophages known as Hofbauer cells. The function of Hofbauer cells is not well defined but may include the support of trophoblast differentiation, stromal development, angiogenesis, and erythroid cell maturation.85,86 Located in close vicinity to fetal capillaries, Hofbauer cells express most of the major heme and nonheme iron transporters and storage proteins,25,49 suggesting a role in iron transport and/or regulation.86,87 It is tempting to speculate that Hofbauer cells may function as the temporary iron storage site in villous stroma, storing iron when maternal supply exceeds fetal demands and releasing iron when supply is insufficient.
NON-TRANSFERRIN-BOUND IRON TRANSPORT
In addition to maternal transferrin-iron, other circulating forms of iron such as non-transferrin-bound iron and heme may also be taken up by the placenta. There is no definitive data on whether transferrin-iron is the exclusive source of iron for fetal growth. Although global deletion of Tfr1 in mice leads to embryonic lethality by E12.5, some Tfr1−/− embryos have appreciable numbers of hemoglobinized red blood cells as late as E10.5, and cultured yolk sac hematopoietic progenitors from these embryos stained positively for hemoglobin,24 suggesting that the transferrin cycle may not be essential for erythropoiesis during early development. Furthermore, it is unclear whether anemia in Tfr1-null embryos is due to insufficient placental iron transport or to defects in erythroid iron uptake, or both. Targeted deletion of Tfr1 in the placenta is needed to resolve this controversy.
Although its physiological relevance is unclear, there is some evidence of ferritin transport in the placenta. Radioisotope studies in rabbits and guinea pigs demonstrated transfer of maternally injected ferritin to the fetus.88,89 Electron microscopy of placental villi from ferritin-injected animals showed ferritin-containing endocytic vesicles88 and ferritin accumulation in the basement membranes,89 suggestive of ferritin endocytosis. This is consistent with human data showing specific ferritin binding by placental villous membranes90,91 and appreciable amounts of ferritin-containing vesicles in the placenta.31 The molecular mechanism of ferritin uptake by the placenta is unknown and may involve TFR192 and/or the renal ferritin receptor SCARA5 (scavenger receptor class A member 5).93 Furthermore, the relevance of this process to maternal delivery of iron to the fetus is uncertain, as plasma ferritin is a processed and glycosylated form of L-ferritin that does not bind iron.
In addition to utilizing nonheme iron, the placenta may be able to utilize heme iron sources, as suggested by its high expression of heme iron transporters and catabolic enzymes (Table 1). Several of these proteins, including lipoprotein receptor-related protein 1, proton-coupled folate transporter, heme oxygenase 1, and heme oxygenase 2, have been localized to the syncytiotrophoblast and/or stroma (Table 1), but it is unknown whether there is co-localization at the subcellular level. Data are lacking on placental localization of the more recently identified heme transporters such as feline leukemia virus subgroup C receptor 1 and heme-responsive gene 1. Research is needed to characterize the function of these heme-transport proteins in the placenta and to elucidate the placental heme metabolic pathway, especially in light of the recent recognition of placenta as a site for hematopoietic stem cell production and erythroid differentiation.86,94,95
REGULATION OF IRON TRANSPORT ACROSS THE PLACENTA
Placental iron transfer can be viewed as a balancing act between the mother and the fetus for a limited iron supply and reflects the capacity of the regulatory mechanism to maintain normal fetal iron content. The significance of maternal and fetal factors in regulating placental–fetal iron transfer has been the focus of many scientific inquiries since the early 20th century, and yet fundamental questions regarding the mechanisms behind this regulation remain to this day.
The observation that infants of anemic women are generally born with normal hemoglobin status forms the basis of the widely accepted notion that the fetus is a perfect parasite for maternal iron, able to acquire adequate iron irrespective of the mother’s iron status.96 The strong linear correlation between iron content and body weight in human fetuses provides further evidence that fetal need drives placental iron transport.12 Fetal signals, such as iron status97,98 and gestational age,99 have been shown to impact the expression of placental iron transporters.
At this time, it is unclear how the placenta senses fetal demand, but this is likely mediated by hormonal action. Hepcidin, the iron regulatory “hormone” expressed by the liver, negatively regulates cellular iron transport via an FPN1-dependent mechanism and has been detected in mouse embryos at midgestation.21 It may fulfill the role of relaying information on fetal iron status to the placenta. Whether fetal hepcidin inhibits placental iron transport has not been shown conclusively, but the anemic, iron-deficient phenotype of transgenic mouse embryos overexpressing hepcidin100 suggests this is likely the case. Interestingly, the reduced iron level in hepcidin transgenic embryos was associated with lower placental Tfr1, with no change in Fpn1 mRNA.101 Likewise, studies in rats showed that fetal liver hepcidin correlates negatively with placental Tfr1 mRNA but not with Fpn1 protein.102 Thus, available evidence suggests that TFR1-mediated iron uptake may be the primary target of hepcidin action in the placenta, although it cannot be ruled out that hepcidin also affects iron efflux by regulating subcellular localization of FPN1.102 Interestingly, recent human studies failed to find significant relationships between cord hepcidin levels and either placental TFR1 expression103 or placental transfer of maternal dietary iron,104 suggesting differences between humans and rodents in hepcidin regulation of placental iron transfer. More research is needed to clarify the role of hepcidin in regulating iron homeostasis during the prenatal period and to identify other fetal factors regulating placental iron transport, such as those related to growth and development.
While the fetus may provide the driving force for iron transfer across the placenta, there is ample evidence that maternal iron status also impacts the transport process. The relative immunity of fetal hemoglobin to mild maternal anemia highlights the ability of the placenta to respond to variations in maternal supply. Stable isotope data in human pregnancies has shown that more iron in the maternal diet is transferred to the fetus when the maternal stores are low.104,105 This is likely accomplished by upregulation of intestinal and placental iron transporters. Elevated placental TFR1 expression has been consistently observed in human and animal models of gestational iron deficiency97,103,106,107 and is perhaps the best-known compensatory change in maternal deficiency. The mechanisms underlying this regulation is not well characterized and may involve placental iron regulatory protein 1 and intracellular iron.98 Data are limited regarding how other placental iron transporters respond to maternal iron deficiency. Cell culture and rat studies show that iron deficiency increases placental DMT1 expression but has little effect on FPN1 expression.75,106,107 A study in 40 healthy pregnant women found no difference in placental FPN1 expression between anemic and iron-replete women.108 These data echo the observations between fetal iron status and placental iron transporter expression and again suggest that placental TFR1 is the major target of regulation by systemic iron status.
In addition to affecting the expression of placental iron transporters, maternal iron status appears to play a role in determining the maximum iron available for placental transfer. This is not surprising, considering the competition between the fetal–placental unit and the maternal bone marrow for the same limited supply of iron from maternal diet and liver stores. As discussed above, the placenta exhibits great capacity to mobilize iron for fetal use over a wide range of maternal statuses. However, there is evidence that the regulatory system can no longer sustain transfer with increasing severity of maternal deficiency. Several studies showed significantly lower cord hemoglobin levels in the infants of severely anemic women compared with infants born to iron-sufficient mothers.109–112 Likewise, diet-induced maternal anemia in rhesus monkeys caused a significant reduction in hemoglobin, mean corpuscular volume, and bone marrow colony-forming units in the newborns,113 suggesting a limited capacity of the placenta to support fetal erythroid needs in the face of severe maternal deficiency.
The relationship between maternal and fetal iron stores mirrors that of maternal and fetal hemoglobin levels, with an interdependence becoming evident when maternal iron reserves are depleted. Maternal iron stores are generally improved by iron supplementation, with no resultant changes observed in cord ferritin,114,115 and maternal ferritin exhibits a weak or no relationship with neonatal ferritin levels in small observational studies. In contrast, animal studies consistently show compromised fetal liver iron status in maternal iron deficiency,102,116,117 suggesting an interdependence between maternal and fetal stores. A recent study in China, which included a large sample of mother–child pairs (n = 3702), detected a strong correlation between maternal and neonatal ferritin in women whose plasma ferritin levels fell below a threshold of depletion, with every unit of decrease in maternal serum ferritin corresponding to a 2.4-unit drop in cord ferritin.118 This finding is consistent with rodent data indicating a “broken stick” relationship between maternal and fetal iron parameters, suggesting a critical point below which the two become dependent.102
While most mechanistic studies are modeled on healthy pregnancy, it is worth noting that conditions with underlying placental abnormalities, such as intrauterine growth restriction and gestational diabetes, may disrupt the normal regulatory mechanism of the placenta and negatively affect fetal iron transfer. For example, despite the presence of fetal hypoxia and low fetal iron stores,119 expression of placental TFR1 in intrauterine growth restriction is significantly lower compared with that in normal term pregnancies,81 indicating impaired ability of TFR1 to sense and/or respond to its classic stimuli. Gestational diabetes is another common pregnancy complication associated with decreased infant iron stores at birth.119 Unlike expression of placental TFR1 in intrauterine growth restriction, expression of placental TFR1 in gestational diabetes shows expected relationships with fetal iron stores and placental iron regulatory protein 1.98 The defect appears to be a reduced affinity of placental TFR1 to bind transferrin.120 Studies are needed to determine the factors underlying the regulatory and functional aberrations of placental TFR1 in intrauterine growth restriction and gestational diabetes and to identify other components of the placental iron transport mechanism that may contribute to low fetal iron levels associated with these pregnancy complications.
Taken together, these data indicate that, under most circumstances, maternal iron status modulates the expression of placental iron transporters in favor of fetal demands. However, there appears to be a breakpoint in maternal iron status below which the mother can no longer maintain supply, resulting in a disruption in the normal hierarchy between the fetal–maternal partitioning.
Despite years of public health efforts, maternal iron deficiency remains prevalent, accounting for half of the anemia burden (38.2%) in pregnant women worldwide121 and affecting 29.5% of pregnant women in the third trimester in the United States.122 Besides reflecting the markedly increased requirement for iron during pregnancy, the high prevalence of maternal iron deficiency also indicates that most women do not have sufficient iron reserves (≈300 mg) at the start of pregnancy123 and that maternal dietary iron intakes consistently fall below recommended levels, even in developed countries.124 As discussed above, adequate maternal iron status is needed for sustained placental iron transfer to support fetal demands. Thus, it is important to improve iron nutrition in women of reproductive age to build adequate iron stores prior to pregnancy and to target women with suboptimal iron status early in pregnancy for dietary interventions. It is worth pointing out that a large portion of the interindividual variation in placental iron transfer and transporter expression cannot be explained by maternal and fetal factors alone. The continued placental accumulation of iron in fetectomized rat dams125 and the absence of a relationship between placental transfer of an intravenous iron isotope and either maternal or neonatal iron status in pregnant women105 suggest that other factors, such as local regulation by the placenta, play important roles in the transfer process.
CONCLUSION
Optimal transfer of iron across the placenta is essential for fetal development in utero and for the establishment of adequate birth iron stores to sustain growth in early infancy. This review summarizes decades of research on placental iron transport and regulation, identifies major knowledge gaps, and proposes directions for future research. With the exception of the initial step of iron uptake, the molecular details of iron trafficking in the placenta remain poorly characterized. Many cellular iron transporters are expressed at very high levels in the placenta, but very few have been definitively localized to the syncytiotrophoblast, and their functions are largely inferred from studies in other tissues and global knockout mice. Thus, there is a compelling need to generate placental-specific deletions of these proteins to conclusively study their physiological significance in placental iron transport. Furthermore, there is virtually no information on the movement of iron after it exits the syncytiotrophoblast and before it enters the fetal circulation. Research is needed to better characterize iron passage through villous stroma and to determine whether this process is subject to regulation. In addition, there is still very little known about the systemic and local mechanisms that regulate placental iron transport. How the placenta senses and integrates signals from maternal and fetal compartments and whether hepcidin is the mediator of this regulation are both important questions to address. Finally, it may be of interest to assess the potential contribution of non-transferrin-bound iron to placental iron transport in light of recent data showing appreciable levels of plasma non-transferrin-bound iron in pregnant women receiving intravenous iron supplementation.126
Acknowledgments
Funding. This research is supported by grant no. 5T32HL110852 from the National Heart, Lung, and Blood Institute, Bethesda, Maryland, USA.
Author contributions. C.C. and M.D.F. conceived the review topic and wrote the manuscript. All authors approved the final draft. C.C. and M.D.F. had responsibility for the final content.
Declaration of interest. The authors have no relevant interests to declare.
References
- 1.Georgieff MK. Long-term brain and behavioral consequences of early iron deficiency. Nutr Rev. 2011;69(suppl 1):S43–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tran PV, Kennedy BC, Lien Y-C, et al. Fetal iron deficiency induces chromatin remodeling at the Bdnf locus in adult rat hippocampus. Am J Physiol Regul Integr Comp Physiol. 2015;308:R276–R282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gambling L, Dunford S, Wallace DI, et al. Iron deficiency during pregnancy affects postnatal blood pressure in the rat. J Physiol. 2003;552:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lisle SJM, Lewis RM, Petry CJ, et al. Effect of maternal iron restriction during pregnancy on renal morphology in the adult rat offspring. Br J Nutr. 2003;90:33–39. [DOI] [PubMed] [Google Scholar]
- 5.Lewis RM, Petry CJ, Ozanne SE, et al. Effects of maternal iron restriction in the rat on blood pressure, glucose tolerance, and serum lipids in the 3-month-old offspring. Metabolism. 2001;50:562–567. [DOI] [PubMed] [Google Scholar]
- 6.Hay G, Refsum H, Whitelaw A, et al. Predictors of serum ferritin and serum soluble transferrin receptor in newborns and their associations with iron status during the first 2 y of life. Am J Clin Nutr. 2007;86:64–73. [DOI] [PubMed] [Google Scholar]
- 7.Andersen HS, Gambling L, Holtrop G, et al. Maternal iron deficiency identifies critical windows for growth and cardiovascular development in the rat postimplantation embryo. J Nutr. 2006;136:1171–1177. [DOI] [PubMed] [Google Scholar]
- 8.Mihaila C, Schramm J, Strathmann FG, et al. Identifying a window of vulnerability during fetal development in a maternal iron restriction model. PLoS One. 2011;6:e17483 doi:10.1371/journal.pone.0017483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makrides M, Leeson R, Gibson RA, et al. A randomized controlled clinical trial of increased dietary iron in breast-fed infants. J Pediatr. 1998;133:559–562. [DOI] [PubMed] [Google Scholar]
- 10.Yang Z, Lönnerdal B, Adu-Afarwuah S, et al. Prevalence and predictors of iron deficiency in fully breastfed infants at 6 mo of age: comparison of data from 6 studies. Am J Clin Nutr. 2009;89:1433–1440. [DOI] [PubMed] [Google Scholar]
- 11.Dube K, Schwartz J, Mueller MJ, et al. Iron intake and iron status in breastfed infants during the first year of life. Clin Nutr. 2010;29:773–778. [DOI] [PubMed] [Google Scholar]
- 12.Widdowson EM, Spray CM. Chemical development in utero. Arch Dis Child. 1951;26:205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finch CA, Huebers HA, Miller LR, et al. Fetal iron balance in the rat. Am J Clin Nutr. 1983;37:910–917. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence CM. Crystal structure of the ectodomain of human transferrin receptor. Science. 1999;286:779–782. [DOI] [PubMed] [Google Scholar]
- 15.Ponka P, Lok CN. The transferrin receptor: role in health and disease. Int J Biochem Cell Biol. 1999;31:1111–1137. [DOI] [PubMed] [Google Scholar]
- 16.Seligman PA, Schleicher RB, Allen RH. Isolation and characterization of the transferrin receptor from human placenta. J Biol Chem. 1979;254:9943–9946. [PubMed] [Google Scholar]
- 17.Wada HG, Hass PE, Sussman HH. Transferrin receptor in human placental brush border membranes. Studies on the binding of transferrin to placental membrane vesicles and the identification of a placental brush border glycoprotein with high affinity for transferrin. J Biol Chem. 1979;254:12629–12635. [PubMed] [Google Scholar]
- 18.Huebers HA, Beguin Y, Pootrakul P, et al. Intact transferrin receptors in human plasma and their relation to erythropoiesis. Blood. 1990;75:102–107. [PubMed] [Google Scholar]
- 19.Kohgo Y, Nishisato T, Kondo H, et al. Circulating transferrin receptor in human serum. Br J Haematol. 1986;64:277–281. [DOI] [PubMed] [Google Scholar]
- 20.Speeckaert MM, Speeckaert R, Delanghe JR. Biological and clinical aspects of soluble transferrin receptor. Crit Rev Clin Lab Sci. 2010;47:213–228. [DOI] [PubMed] [Google Scholar]
- 21.Yoon D, Pastore YD, Divoky V, et al. Hypoxia-inducible factor-1 deficiency results in dysregulated erythropoiesis signaling and iron homeostasis in mouse development. J Biol Chem. 2006;281:25703–25711. [DOI] [PubMed] [Google Scholar]
- 22.Drake BL, Head JR. Transferrin receptor expression in early postimplantation mouse trophoblast and associated tissues. Placenta. 1990;11:535–547. [DOI] [PubMed] [Google Scholar]
- 23.Seal US, Sinha AA, Doe RP. Placental iron transfer: relationship to placental anatomy and phylogeny of the mammals. Am J Anat. 1972;134:263–269. [DOI] [PubMed] [Google Scholar]
- 24.Levy JE, Jin O, Fujiwara Y, et al. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat Genet. 1999;21:396–399. [DOI] [PubMed] [Google Scholar]
- 25.Bastin J, Drakesmith H, Rees M, et al. Localisation of proteins of iron metabolism in the human placenta and liver. Br J Haematol. 2006;134:532–543. [DOI] [PubMed] [Google Scholar]
- 26.Parmley RT, Barton JC, Conrad ME. Ultrastructural localization of transferrin, transferrin receptor, and iron-binding sites on human placental and duodenal microvilli. Br J Haematol. 1985;60:81–89. [DOI] [PubMed] [Google Scholar]
- 27.King BF. Localization of transferrin on the surface of the human placenta by electron microscopic immunocytochemistry. Anat Rec (Hoboken). 1976;186:151–159. [DOI] [PubMed] [Google Scholar]
- 28.Baker E, van Bockxmeer FM, Morgan EH. Distribution of transferrin and transferrin receptors in the rabbit placenta. Q J Exp Physiol. 1983;68:359–372. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs R, Ellinger I. Endocytic and transcytotic processes in villous syncytiotrophoblast: role in nutrient transport to the human fetus. Traffic. 2004;5:725–738. [DOI] [PubMed] [Google Scholar]
- 30.Booth AG, Wilson MJ. Human placental coated vesicles contain receptor-bound transferrin. Biochem J. 1981;196:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearse BM. Coated vesicles from human placenta carry ferritin, transferrin, and immunoglobulin G. Proc Natl Acad Sci U S A. 1982;79:451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turkewitz AP. Concentration of transferrin receptor in human placental coated vesicles. J Cell Biol. 1989;108:2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Ende A, du Maine A, Simmons CF, et al. Iron metabolism in BeWo chorion carcinoma cells. Transferrin-mediated uptake and release of iron. J Biol Chem. 1987;262:8910–8916. [PubMed] [Google Scholar]
- 34.Brown PJJ, Molloy CMM, Johnson PMM. Transferrin receptor affinity and iron transport in the human placenta. Placenta. 1982;3:21–27. [DOI] [PubMed] [Google Scholar]
- 35.Douglas GC, King BF. Uptake and processing of 125I-labelled transferrin and 59Fe-labelled transferrin by isolated human trophoblast cells. Placenta. 1990;11:41–57. [DOI] [PubMed] [Google Scholar]
- 36.D’Alessandro AM, D’Andrea G, Di Ciccio L, et al. 3’-Azido-3'-deoxythymidine reduces the rate of transferrin receptor endocytosis in K562 cells. Biochim Biophys Acta. 1999;1450:232–241. [DOI] [PubMed] [Google Scholar]
- 37.Morgan EH. Effect of pH and iron content of transferrin on its binding to reticulocyte receptors. Biochim Biophys Acta. 1983;762:498–502. [DOI] [PubMed] [Google Scholar]
- 38.Blight GD, Morgan EH. Ferritin and iron uptake by reticulocytes. Br J Haematol. 1983;55:59–71. [DOI] [PubMed] [Google Scholar]
- 39.Ciechanover A, Schwartz AL, Dautry-Varsat A, et al. Kinetics of internalization and recycling of transferrin and the transferrin receptor in a human hepatoma cell line. Effect of lysosomotropic agents. J Biol Chem. 1983;258:9681–9689. [PubMed] [Google Scholar]
- 40.Klausner RD, Van Renswoude J, Ashwell G, et al. Receptor-mediated endocytosis of transferrin in K562 cells. J Biol Chem. 1983;258:4715–4724. [PubMed] [Google Scholar]
- 41.Contractor SF, Eaton BM. Role of transferrin in iron transport between maternal and fetal circulations of a perfused lobule of human placenta. Cell Biochem Funct. 1986;4:69–74. [DOI] [PubMed] [Google Scholar]
- 42.McArdle HJ, Douglas AJ, Morgan EH. Uptake of transferrin and iron by cultured rat placental cells. J Cell Physiol. 1985;122:405–409. [DOI] [PubMed] [Google Scholar]
- 43.Ohgami RS, Campagna DR, McDonald A, et al. The Steap proteins are metalloreductases. Blood. 2006;108:1388–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korkmaz CG, Korkmaz KS, Kurys P, et al. Molecular cloning and characterization of STAMP2, an androgen-regulated six transmembrane protein that is overexpressed in prostate cancer. Oncogene. 2005;24:4934–4945. [DOI] [PubMed] [Google Scholar]
- 45.Zhang DL, Su D, Bérczi A, et al. An ascorbate-reducible cytochrome b561 is localized in macrophage lysosomes. Biochim Biophys Acta. 2006;1760:1903–1913. [DOI] [PubMed] [Google Scholar]
- 46.Fleming MD, Romano MA, Su MA, et al. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci U S A. 1998;95:1148–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li YQ, Bai B, Cao XX, et al. Divalent metal transporter 1 expression and regulation in human placenta. Biol Trace Elem Res. 2012;146:6–12. [DOI] [PubMed] [Google Scholar]
- 48.Chong WS, Kwan PC, Chan LY, et al. Expression of divalent metal transporter 1 (DMT1) isoforms in first trimester human placenta and embryonic tissues. Hum Reprod. 2005;20:3532–3538. [DOI] [PubMed] [Google Scholar]
- 49.Georgieff MK, Wobken JK, Welle J, et al. Identification and localization of divalent metal transporter-1 (DMT-1) in term human placenta. Placenta. 2000;21:799–804. [DOI] [PubMed] [Google Scholar]
- 50.Gunshin H, Fujiwara Y, Custodio AO, et al. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest. 2005;115:1258–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinilla-Tenas JJ, Sparkman BK, Shawki A, et al. Zip14 is a complex broad-scope metal-ion transporter whose functional properties support roles in the cellular uptake of zinc and nontransferrin-bound iron. Am J Physiol Cell Physiol. 2011;301:C862–C871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Girijashanker K, He L, Soleimani M, et al. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol Pharmacol. 2008;73:1413–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liuzzi JP, Aydemir F, Nam H, et al. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci U S A. 2006;103:13612–13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao N, Gao J, Enns CA, Knutson MD. ZRT/IRT-like protein 14 (ZIP14) promotes the cellular assimilation of iron from transferrin. J Biol Chem. 2010;285:32141–32150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hojyo S, Fukada T, Shimoda S, et al. The zinc transporter SLC39A14/ZIP14 controls G-protein coupled receptor-mediated signaling required for systemic growth. PLoS One. 2011;6:e18059 doi: 0.1371/journal.pone.0018059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang C-Y, Jenkitkasemwong S, Duarte S, et al. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J Biol Chem. 2012;287:34032–34043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown SD, Moore MW. The International Mouse Phenotyping Consortium: past and future perspectives on mouse phenotyping. Mamm Genome. 2012;23:632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dong X-P, Cheng X, Mills E, et al. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455:992–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collawn JF, Donato H, Fish WW. Evidence that H-enriched human placental ferritin is structurally similar to L-enriched ferritins of other tissues. Biochim Biophys Acta. 1986;871:235–242. [DOI] [PubMed] [Google Scholar]
- 60.Moroz C, Traub L, Maymon R, et al. PLIF, a novel human ferritin subunit from placenta with immunosuppressive activity. J Biol Chem. 2002;277:12901–12905. [DOI] [PubMed] [Google Scholar]
- 61.Maymon R, Jauniaux E, Greenwold N, et al. Localization of p43 placental isoferritin in human maternal-fetal tissue interface. Am J Obstet Gynecol. 2000;182:670–674. [DOI] [PubMed] [Google Scholar]
- 62.Oliva K, Barker G, Riley C, et al. The effect of pre-existing maternal obesity on the placental proteome: two-dimensional difference gel electrophoresis coupled with mass spectrometry. J Mol Endocrinol. 2012;48:139–149. [DOI] [PubMed] [Google Scholar]
- 63.Galbraith GM, Galbraith RM, Faulk WP. Immunological studies of transferrin and transferrin receptors of human placental trophoblast. Placenta. 1980;1:33–46. [DOI] [PubMed] [Google Scholar]
- 64.Brown PJ, Johnson PM, Ogbimi AO, et al. Characterization and localization of human placental ferritin. Biochem J. 1979;182:763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nasir Y, Nergiz Y, Aktaş A, et al. Immunohistochemical evaluation of iron accumulation in term placenta of preeclamptic patients. Afr J Biotechnol. 2013;10:11273–11279. [Google Scholar]
- 66.Donovan A, Brownlie A, Zhou Y, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–781. [DOI] [PubMed] [Google Scholar]
- 67.Mok H, Mendoza M, Prchal JT, et al. Dysregulation of ferroportin 1 interferes with spleen organogenesis in polycythaemia mice. Development. 2004;131:4871–4881. [DOI] [PubMed] [Google Scholar]
- 68.Mao J, McKean DM, Warrier S, et al. The iron exporter ferroportin 1 is essential for development of the mouse embryo, forebrain patterning and neural tube closure. Development. 2010;137:3079–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Donovan A, Lima CA, Pinkus JL, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. [DOI] [PubMed] [Google Scholar]
- 70.Harris ZL, Durley AP, Man TK, et al. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci U S A. 1999;96:10812–10817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guller S, Buhimschi CS, Ma YY, et al. Placental expression of ceruloplasmin in pregnancies complicated by severe preeclampsia. Lab Invest. 2008;88:1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Danzeisen R, Ponnambalam S, Lea RG, et al. The effect of ceruloplasmin on iron release from placenta (BeWo) cells; evidence for an endogenous Cu oxidase. Placenta. 2000;21:805–812. [DOI] [PubMed] [Google Scholar]
- 73.Vulpe CD, Kuo YM, Murphy TL, et al. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet. 1999;21:195–199. [DOI] [PubMed] [Google Scholar]
- 74.Frazer DM, Vulpe CD, McKie AT, et al. Cloning and gastrointestinal expression of rat hephaestin: relationship to other iron transport proteins. Am J Physiol Gastrointest Liver Physiol. 2001;281:G931–G939. [DOI] [PubMed] [Google Scholar]
- 75.Li Y-Q, Bai B, Cao X-X, et al. Ferroportin 1 and hephaestin expression in BeWo cell line with different iron treatment. Cell Biochem Funct. 2012;30:249–255. [DOI] [PubMed] [Google Scholar]
- 76.Kingston PJ, Bannerman CEM, Bannerman RM. Iron deficiency anaemia in newborn sla mice: a genetic defect of placental iron transport. Br J Haematol. 1978;40:265–276. [DOI] [PubMed] [Google Scholar]
- 77.Chen H, Attieh ZK, Su T, et al. Hephaestin is a ferroxidase that maintains partial activity in sex-linked anemia mice. Blood. 2004;103:3933–3939. [DOI] [PubMed] [Google Scholar]
- 78.Danzeisen R, Fosset C, Chariana Z, et al. Placental ceruloplasmin homolog is regulated by iron and copper and is implicated in iron metabolism. Am J Physiol Cell Physiol. 2002;282:C472–C478. [DOI] [PubMed] [Google Scholar]
- 79.Chen H, Attieh ZK, Syed BA, et al. Identification of zyklopen, a new member of the vertebrate multicopper ferroxidase family, and characterization in rodents and human cells. J Nutr. 2010;140:1728–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feneley MR, Burton GJ. Villous composition and membrane thickness in the human placenta at term: a stereological study using unbiased estimators and optimal fixation techniques. Placenta. 1991;12:131–142. [DOI] [PubMed] [Google Scholar]
- 81.Mandò C, Tabano S, Colapietro P, et al. Transferrin receptor gene and protein expression and localization in human IUGR and normal term placentas. Placenta. 2011;32:44–50. [DOI] [PubMed] [Google Scholar]
- 82.Leach L, Eaton BM, Firth JA, et al. Immunogold localisation of endogenous immunoglobulin-G in ultrathin frozen sections of the human placenta. Cell Tissue Res. 1989;257:603–607. [DOI] [PubMed] [Google Scholar]
- 83.Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 2002;23:3–19. [DOI] [PubMed] [Google Scholar]
- 84.Takata K, Hirano H. Mechanism of glucose transport across the human and rat placental barrier: a review. Microsc Res Tech. 1997;38:145–152. [DOI] [PubMed] [Google Scholar]
- 85.Tang Z, Abrahams VM, Mor G, et al. Placental Hofbauer cells and complications of pregnancy. Ann N Y Acad Sci. 2011;1221:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van Handel B, Prashad SL, Hassanzadeh-Kiabi N, et al. The first trimester human placenta is a site for terminal maturation of primitive erythroid cells. Blood. 2010;116:3321–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Drachenberg CB, Papadimitriou JC. Placental iron deposits: significance in normal and abnormal pregnancies. Hum Pathol. 1994;25:379–385. [DOI] [PubMed] [Google Scholar]
- 88.Lamparelli RD, Friedman BM, MacPhail AP, et al. The fate of intravenously injected tissue ferritin in pregnant guinea-pigs. Br J Haematol. 1989;72:100–105. [DOI] [PubMed] [Google Scholar]
- 89.Thornburg KL, Faber JJ. The steady state concentration gradients of an electron-dense marker (ferritin in the three-layered hemochorial placenta of the rabbit. J Clin Invest. 1976;58:912–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takami M, Mizumoto K, Kasuya I, et al. Human placental ferritin receptor. Biochim Biophys Acta. 1986;884:31–38. [DOI] [PubMed] [Google Scholar]
- 91.Liao QK, Kong PA, Gao J, et al. Expression of ferritin receptor in placental microvilli membrane in pregnant women with different iron status at mid-term gestation. Eur J Clin Nutr. 2001;55:651–656. [DOI] [PubMed] [Google Scholar]
- 92.Li L, Fang CJ, Ryan JC, et al. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc Natl Acad Sci U S A. 2010;107:3505–3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li JY, Paragas N, Ned RM, et al. Scara5 is a ferritin receptor mediating non-transferrin iron delivery. Dev Cell. 2009;16:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee LK, Ueno M, Van Handel B, et al. Placenta as a newly identified source of hematopoietic stem cells. Curr Opin Hematol. 2010;17:313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chhabra A, Lechner AJ, Ueno M, et al. Trophoblasts regulate the placental hematopoietic niche through PDGF-B signaling. Dev Cell. 2012;22:651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr. 2000;71(5 suppl):1280S–1284S. [DOI] [PubMed] [Google Scholar]
- 97.Young MF, Pressman E, Foehr ML, et al. Impact of maternal and neonatal iron status on placental transferrin receptor expression in pregnant adolescents. Placenta. 2010;31:1010–1014. [DOI] [PubMed] [Google Scholar]
- 98.Georgieff MK, Berry SA, Wobken JD, et al. Increased placental iron regulatory protein-1 expression in diabetic pregnancies complicated by fetal iron deficiency. Placenta. 1999;20:87–93. [DOI] [PubMed] [Google Scholar]
- 99.Bradley J, Leibold EA, Harris ZL, et al. Influence of gestational age and fetal iron status on IRP activity and iron transporter protein expression in third-trimester human placenta. Am J Physiol Regul Integr Comp Physiol. 2004;287:R894–R901. [DOI] [PubMed] [Google Scholar]
- 100.Nicolas G, Bennoun M, Porteu A, et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci U S A. 2002;99:4596–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Martin ME, Nicolas G, Hetet G, et al. Transferrin receptor 1 mRNA is downregulated in placenta of hepcidin transgenic embryos. FEBS Lett. 2004;574:187–191. [DOI] [PubMed] [Google Scholar]
- 102.Gambling L, Czopek A, Andersen HS, et al. Fetal iron status regulates maternal iron metabolism during pregnancy in the rat. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1063–R1070. [DOI] [PubMed] [Google Scholar]
- 103.Garcia-Valdes L, Campoy C, Hayes H, et al. The impact of maternal obesity on iron status, placental transferrin receptor expression and hepcidin expression in human pregnancy. Int J Obes (London). 2015;39:571–578. [DOI] [PubMed] [Google Scholar]
- 104.Young MF, Griffin I, Pressman E, et al. Maternal hepcidin is associated with placental transfer of iron derived from dietary heme and nonheme sources. J Nutr. 2012;142:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.O’Brien KO, Zavaleta N, Abrams SA, et al. Maternal iron status influences iron transfer to the fetus during the third trimester of pregnancy. Am J Clin Nutr. 2003;77:924–930. [DOI] [PubMed] [Google Scholar]
- 106.Gambling L, Danzeisen R, Gair S, et al. Effect of iron deficiency on placental transfer of iron and expression of iron transport proteins in vivo and in vitro. Biochem J. 2001;356:883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cornock R, Gambling L, Langley-Evans SC, et al. The effect of feeding a low iron diet prior to and during gestation on fetal and maternal iron homeostasis in two strains of rat. Reprod Biol Endocrinol. 2013;11:32 doi:10.1186/1477-7827-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li Y-Q, Yan H, Bai B. Change in iron transporter expression in human term placenta with different maternal iron status. Eur J Obstet Gynecol Reprod Biol. 2008;140:48–54. [DOI] [PubMed] [Google Scholar]
- 109.Kumar A, Rai AK, Basu S, et al. Cord blood and breast milk iron status in maternal anemia. Pediatrics. 2008;121:e673–e677. [DOI] [PubMed] [Google Scholar]
- 110.Singla P, Tyagi M, Shankar R, et al. Fetal iron status in maternal anemia. Acta Paediatr. 1996;85:1327–1330. [DOI] [PubMed] [Google Scholar]
- 111.Ali R, Ismail EAR, Nada AS. Cord blood iron profile and breast milk micronutrients in maternal iron deficiency anemia. Pediatr Blood Cancer. 2012;58:233–238. [DOI] [PubMed] [Google Scholar]
- 112.Singla PN, Chand S, Khanna S, et al. Effect of maternal anaemia on the placenta and the newborn infant. Acta Paediatr Scand. 1978;67:645–648. [DOI] [PubMed] [Google Scholar]
- 113.Golub MS, Hogrefe CE, Tarantal AF, et al. Diet-induced iron deficiency anemia and pregnancy outcome in rhesus monkeys. Am J Clin Nutr. 2006;83:647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Preziosi P, Prual A, Galan P, et al. Effect of iron supplementation on the iron status of pregnant women: consequences for newborns. Am J Clin Nutr. 1997;66:1178–1182. [DOI] [PubMed] [Google Scholar]
- 115.Zhao G, Xu G, Zhou M, et al. Prenatal iron supplementation reduces maternal anemia, iron deficiency, and iron deficiency anemia in a randomized clinical trial in rural China, but iron deficiency remains widespread in mothers and neonates. J Nutr. 2015;145:1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hubbard AC, Bandyopadhyay S, Wojczyk BS, et al. Effect of dietary iron on fetal growth in pregnant mice. Comp Med. 2013;63:127–135. [PMC free article] [PubMed] [Google Scholar]
- 117.Balesaria S, Hanif R, Salama MF, et al. Fetal iron levels are regulated by maternal and fetal Hfe genotype and dietary iron. Haematologica. 2012;97:661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shao J, Lou J, Rao R, et al. Maternal serum ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J Nutr. 2012;142:2004–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Siddappa AM, Rao R, Long JD, et al. The assessment of newborn iron stores at birth: a review of the literature and standards for ferritin concentrations. Neonatology. 2007;92:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Georgieff MK, Petry CD, Mills MM, et al. Increased N-glycosylation and reduced transferrin-binding capacity of transferrin receptor isolated from placentae of diabetic women. Placenta. 1997;18:563–568. [DOI] [PubMed] [Google Scholar]
- 121.World Health Organization. The Global Prevalence of Anaemia in 2011. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 122.Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Am J Clin Nutr. 2011;93:1312–1320. [DOI] [PubMed] [Google Scholar]
- 123.Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72(1 suppl):257S–264S. [DOI] [PubMed] [Google Scholar]
- 124.Blumfield ML, Hure AJ, Macdonald-Wicks L, et al. A systematic review and meta-analysis of micronutrient intakes during pregnancy in developed countries. Nutr Rev. 2013;71:118–132. [DOI] [PubMed] [Google Scholar]
- 125.McArdle HJ, Morgan EH. Transferrin and iron movements in the rat conceptus during gestation. J Reprod Fertil. 1982;66:529–536. [DOI] [PubMed] [Google Scholar]
- 126.Baron J, Ben-David G, Hallak M. Changes in non-transferrin-bound iron (NTBI) in pregnant women on iron supplements. Eur J Obstet Gynecol Reprod Biol. 2008;140:281–282. [DOI] [PubMed] [Google Scholar]