Abstract
Diet is a modifiable factor associated with the risk of several cancers, with convincing evidence showing a link between diet and breast cancer. The role of bioactive compounds of food origin, including those found in cruciferous vegetables, is an active area of research in cancer chemoprevention. This review focuses on 3,3′-diindolylmethane (DIM), the major bioactive indole in crucifers. Research of the cancer-preventive activity of DIM has yielded basic mechanistic, animal, and human trial data. Further, this body of evidence is largely supported by observational studies. Bioactive DIM has demonstrated chemopreventive activity in all stages of breast cancer carcinogenesis. This review describes current evidence related to the metabolism and mechanisms of DIM involved in the prevention of breast cancer. Importantly, this review also focuses on current evidence from human observational and intervention trials that have contributed to a greater understanding of exposure estimates that will inform recommendations for DIM intake.
Keywords: breast cancer chemoprevention, 3-3′-diindolylmethane, glucosinolates.
INTRODUCTION
Cruciferous vegetables have been shown to be protective against breast cancer in some,1–8 but not all,9–11 epidemiological studies. Discrepancies in current findings are thought to be explained in part by variances in exposure to multiple bioactive constituents found in this unique classification of vegetables. In fact, the chemopreventive roles of multiple phytochemicals found in crucifers have been described previously.12 The argument for the focus on one bioactive constituent in particular, 3,3′-diindolylmethane (DIM), as a relevant bioactive food compound in breast cancer chemoprevention is based on the extensive evaluation of DIM in relation to its chemopreventive potential, particularly for breast cancer. DIM is one of the best-characterized and most abundant bioactive compounds found in commonly consumed crucifers. The purpose of this review is to describe the metabolism of DIM, the mechanisms of action of DIM against breast cancer, and the current state of human observational and intervention trials with DIM to introduce the next steps toward advancing understanding and developing guidance on DIM intake for public health.
METABOLISM OF DIINDOLYLMETHANE FROM INDOLE-3-CARBINOL
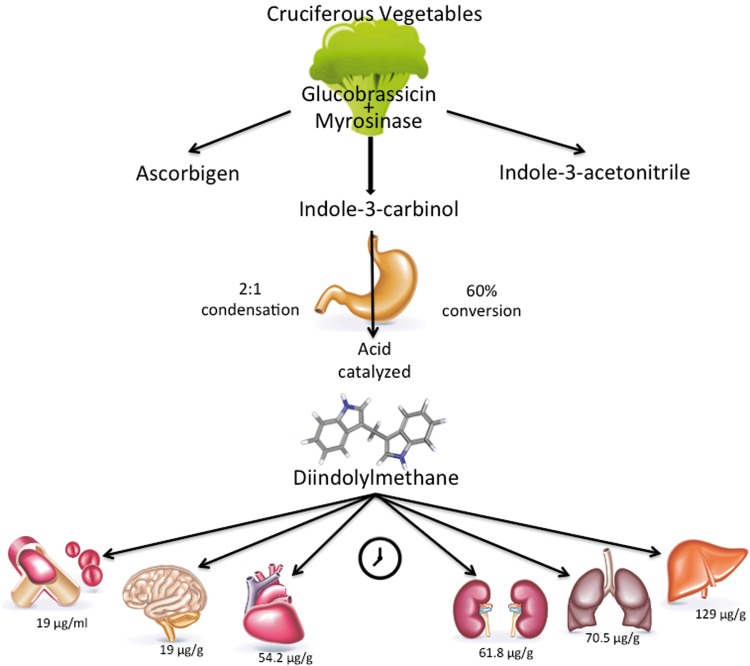
DIM is readily metabolized in cruciferous vegetables (Figure 1). Cruciferous vegetables are within the mustard family, or the family Brassicaceae (Cruciferae). Commonly consumed cruciferous vegetables include broccoli, bok choy, cabbage, cauliflower, collards, kale, Brussels sprouts, and kohlrabi. Once consumed, bioactive constituents are rapidly metabolized into several intermediate and end products. The bioactive content and end products vary and are dependent on the specific vegetable consumed. Other factors that influence DIM content in cruciferous vegetables include plant age, cultivar, and vegetable storage and preparation methods.13,14 The spectrum of effects from cooking and storage on DIM concentrations remains relatively unknown and warrants further exploration.
Figure 1.
Metabolism of diindolylmethane from cruciferous vegetables. Diindolylmethane is an end product of the pH-dependent metabolism of indole-3-carbinol. The concentration of diindolylmethane is highest in liver, followed by lung, kidney, and heart and, to a lesser extent, brain and plasma. Concentrations are time dependent, as demonstrated in a mouse model after supplementation with pure crystalline DIM at a dosage of 250 mg/kg.26
Cruciferous vegetables contain bioactive precursor compounds known as glucosinolates (Table 1).15–19 Major glucosinolates are glucobrassicin and glucoraphanin, the latter of which is a derivative of isothiocyanates,20 including sulforaphane. The average human consumption of glucosinolates from food sources is estimated at 0.5μM/kg/d.12 US dietary intake estimates for cruciferous vegetables are low10 and are currently not classified by specific vegetable type, limiting the availability of intake estimates of glucosinolates. The average consumption of glucosinolates from vegetable sources is nonspecific and approximated,21 and estimates for US intakes are generally lower than those for European and Asian nations. Chemopreventive roles of phytochemicals have been described previously, but only limited data about the specific types of vegetables contributing to the overall glucosinolate intake in the United States are available.
Table 1.
Sources and bioactive concentrations of glucosinolates
| Source | Glucosinolate content | Glucosinolate content | Glucobrassicin content | 3-Indolylmethyl content | Isothiocyanate yield |
|---|---|---|---|---|---|
| (mg/100 g, raw)15 | (mg/100 g, cooked)15 | (mg/100 g)16 | (μmol/100 g, fresh)19 | (μmol/100 g, fresh)20 | |
| Broccoli | 61.7 | 37.2 | – | 71.7 | 6.9 |
| Brussels sprouts | 236.6 | 135.9 | 29.02 | 443.3 | 9.6 |
| Cabbage | 58.9 | 78.6 | 35.84 | – | 31.7 |
| Cauliflower | 43.2 | 42.0 | 18.29 | 75.9 | 1.5 |
| Collard greens | 200.67 | – | – | 150.4 | 5.8 |
| Kale | 89.4 | 69.10 | 92.13 | 69.5 | 3.7 |
| Kohlrabi | 45.9 | 73.4 | 5.38 | 27.7 | – |
| Radish | 92.5 | – | 1.31 | 6.018 | – |
| Turnip | 93.0 | – | – | 62.017 | 9.0 |
Glucobrassicin is the most abundant glucosinolate in vegetables within the family Brassicaceae. Enzymatic breakdown of glucobrassicin by the plant-derived enzyme myrosinase during plant storage, preparation, and/or chewing22 yields various indoles. Included in the various indoles is indole-3-carbinol (I3C), which is a relatively unstable compound. In fact, a pH-dependent, acid-catalyzed condensation rapidly converts I3C to oligomers that include DIM, which is the major indole bioactive compound, accounting for an estimated 60% of the I3C end product.23 As the conversion is pH dependent, exposure to stomach acid is necessary for the conversion of I3C into DIM22 and other acid condensation products. In experimental models, I3C has been shown to self-condense to produce DIM at a ratio of 2:1.24
DIM concentrations rise during cooking,25 in part because of the thermal activation of myrosinase. This is evidenced by a 6-fold increase in DIM concentrations in boiled cabbage compared with uncooked cabbage.25 After ingestion, DIM concentrations in different tissues vary. The highest concentration of DIM is found in the liver. Postprandial concentrations are also elevated in kidney, lung, and heart and, to a lesser extent, in blood plasma, and brain. However, after 24 hours, DIM is no longer measurable in brain tissue.26 Metabolites of DIM are found in both human serum and urine but demonstrate significant clearance within 24 hours.27,28
MECHANISMS OF ACTION IN CELL LINES
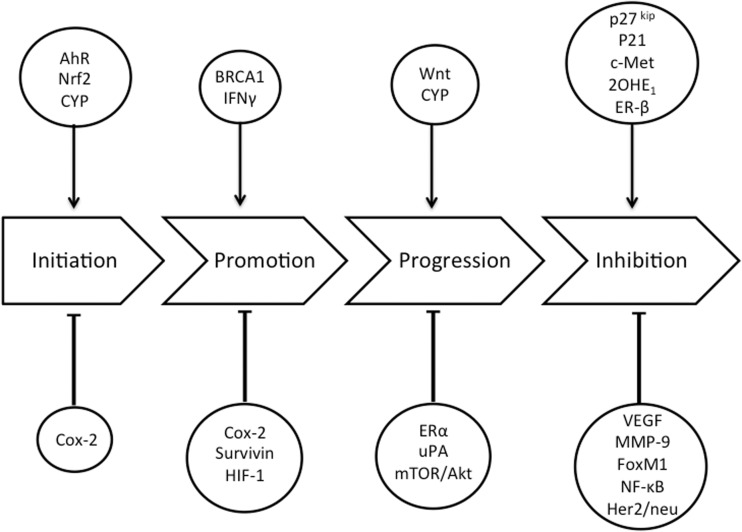
In the 1970s, Wattenberg and Loub29 first described the presence of DIM in crucifers, the cancer-preventive activity of freeze-dried broccoli, and the bioactivity of supplemental DIM in the prevention of carcinogen-induced breast cancer in animals. More recently, a wide array of mechanisms of cancer-related bioactivity of DIM have been described,30 including the specific efficacy of DIM in modulating carcinogenesis at all stages of breast tumor development, including initiation, promotion, and progression (Figure 2).
Figure 2.
Biological targets of diindolylmethane in breast carcinogenesis. Abbreviations: AhR, aryl hydrocarbon receptor; Akt, protein kinase B; Cox-2, cyclooxygenase 2; CYP, cytochrome P450; ER-β, estrogen receptor β; FoxM1, Forkhead box M1; Her2/neu, human epidermal growth factor receptor 2; HIF-1, hypoxia-inducible factor 1; IFN-γ, interferon-γ; MMP-9, matrix metalloproteinase-9; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor-κB; 2OHE1, 2-hydroxyestrone; uPA, urokinase-type plasminogen activator; VEGF, vascular epithelial growth factor.
Initiation of breast tumors
First, DIM can be considered an anti-initiating agent through its ability to stimulate cellular detoxification pathways. DIM is reported to modulate aryl hydrocarbon receptor (AhR), as evidenced in multiple breast cancer cell lines.31 Aryl hydrocarbon receptor is a ubiquitous cytoplasmic receptor that, when activated and transported to the nucleus, promotes transcription of genes that stimulate the expression of detoxification enzymes, including the phase I cytochrome P450 (CYP) family. Cellular responses to AhR signaling can either promote or diminish inflammation. DIM has an affinity to bind to the AhR.32 Modulation of AhR by DIM treatment has also been shown to stimulate the Nrf2-mediated phase II response, which enhances excretion of genotoxins and induces a significant antioxidant response.30,33 Through the activation of AhR and Nrf2 signaling pathways, DIM effectively increases detoxification and reduces inflammatory signaling, blocking what could otherwise be cancer-initiating events. Furthermore, modulation of AhR by DIM inhibited the growth of mammary gland cell cancer, an action suggested as a mechanism of crosstalk between estrogen receptor α and AhR.34
Promotion of breast tumors
The influence of DIM on the AhR results in a change in gene activity that reduces the induction and activity of the enzyme cyclooxygenase-2.35 Evidence from mammary cell lines has demonstrated the role of DIM in reducing oxidative stress by stimulating the phosphorylation of BRCA1.36 Further, DIM has a demonstrated role in reducing cyclooxygenase-2–induced inflammation in mammary cell lines.35 In advanced stages of tumor development, DIM has been shown in tumor cell line models to inhibit the expression of genes involved in angiogenesis and energy metabolism, including those involved in the induction of survivin37 and hypoxia-inducible factor-1.38 The interferon-γ signaling pathway also is activated by DIM through the interferon-γ receptor and the interferon-γ-responsive proteins p56- and p69-oligoadenylate synthase, which inhibit growth of human breast cancer cells, resulting in inhibition of cell proliferation.39 Additionally, DIM has been proposed to have an epigenetic effect on breast cancer. Modulation of noncoding RNA such as microRNA 21 by DIM resulted in the downregulation of CdC25A, an important protein regulating the cell cycle, and resulted in the inhibition of cell proliferation.40
Progression of breast tumors
The effects of DIM on transcription and proliferation are mediated by estrogen receptor α and are evident at a concentration of 1μM.41 However, in the notable absence of estradiol, concentrations of DIM at 10μM have been shown to activate estrogen receptor α signaling pathways in human breast cancer cell lines in vitro, increasing cellular proliferation in an estradiol-independent manner, yet an opposite effect (growth arrest) can be demonstrated when higher concentrations of DIM (50μM) are provided.42 These dose-dependent studies suggest that protective associations between DIM and breast cancer may require exposures well above what would be possible with human dietary modulation. In estrogen-dependent and estrogen-independent breast cancer cell lines (MCF-7 and MDA-MB-231, respectively), DIM has been observed to arrest proliferation, possibly through arrest of de novo cell lipogenesis or induction of Wnt signaling pathways.43 DIM may also act as an aromatase inhibitor. It was efficient at decreasing aromatase expression in MCF-7 cells and also upregulated CYP19 expression, which encodes aromatase and synthesizes estrogens in MDA-MB-231 cells. It has also been shown to have greater antiproliferative activity than I3C or cabbage juices in MDA-MB-231 cell lines.44
DIM may reduce the invasive and metastatic potential of breast tumors. In one study, MDA-MB-231 cells exposed to DIM showed a downregulation of urokinase plasminogen activator, resulting in stabilization of the membrane. The urokinase plasminogen activator–independent effects on tumor growth potential were demonstrated through possible downregulation of vascular endothelial growth factor and metalloproteinase-9, leading to inhibition of both cell growth and migration of breast cancer cells.45 DIM has been shown to reduce the expression of both vascular endothelial growth factor and metalloproteinase-9 via downregulation of transcription factor Forkhead box M1 (FoxM1), further supporting a role for DIM in reducing breast cancer metastatic events.46 Additional support for an antimetastatic role of DIM was demonstrated in MDA-MB-231 and MCF-7 cell lines. Administration of DIM was associated with a marked reduction in the chemokine receptor CXCR4 and its ligand, CXCL12, thus reducing signaling from breast tissue to promote metastatic growth.47 DIM has been demonstrated to induce apoptosis in breast cancer cells MCF-7, MDA-MB-231, and MDA-MB-468 in vitro.48
DIM alters cancer growth through modulation of protein kinase B (Akt)-dependent bioactivity. An increase in Akt activity allows cells to evade death. In breast cancer, Akt is activated in situ,49 and breast cancer cells rely on this pathway as a survival factor. Growth factors, including epidermal growth factor, insulin-like growth factor 1, and hepatocyte growth factor activate Akt in cells. Nicastro et al.50 found that, after 4 hours, a concentration of 25μM DIM optimally inhibited the activation of Akt in MDA-MB-231 cells but did not inhibit the activation of Akt in nontumorigenic cells. DIM did not inhibit activation of Akt by epidermal growth factor or insulin-like growth factor 1 but did reduce activation of hepatocyte growth factor. The mechanism of this inhibition is thought to be through decreased phosphorylation and, therefore, decreased activation of c-Met, a hepatocyte growth factor receptor, at tyrosines 1234 and 1235. DIM also had inhibitory effects on a substrate of Akt, GSK-3α/β.
There are a limited number of studies describing the role of DIM in targeting mammalian target of rapamycin (mTOR), a key regulatory molecule in cell growth. Cancers with overexpression of mTOR exhibit a 3 times greater risk of recurrence.49 One study showed DIM significantly inhibited mTOR and Akt activity in cancer cells expressing platelet-derived growth factor-D (PDGF-D).51 This is important because inhibition of mTOR and Akt activity is correlated with decreased cell proliferation and invasion. Previous work has shown that breast cancer cell lines expressing PDGF-D, including the MDA-MB-231 and SUM-149 lines, are more invasive than those that do not express PDGF-D.52 Inhibition of PDGF-D in these cells resulted in decreased cell proliferation and increased apoptosis. The inhibition of the mTOR pathway without activation of Akt in PDGF-D–expressing cancer cell lines further suggests the therapeutic potential of DIM. This area warrants additional research and discovery.
Inhibition of breast tumors
In combination with Taxotere, a concentration of 40μM DIM resulted in a 78% inhibition of growth and a decreased invasive capacity of the aggressive breast cancer cell line MDA-MB-231; these findings were associated with decreased activation of FoxM1. MDA-MB-231 cells express higher levels of FoxM1 than MCF-7 breast cancer cells. Cells treated with DIM showed reduced FoxM1 mRNA levels. Downregulation of FoxM1 expression induced the growth-inhibitory effect of DIM, suggesting a mechanistic role of FoxM1 and a regulatory role of DIM.46
DIM has been shown to induce select tumor-suppressing proteins, including p21 and p27kip, in cell culture.53,54 In breast cancer cell lines that overexpress both human epidermal growth factor receptor 2 (Her2) and activated Akt, DIM exposure resulted in inhibition of activated Akt expression as well as independent induction of both p27kip transcript expression and nuclear localization of p27kip, ultimately resulting in apoptosis.53 Apoptosis was also evident in Her2/neu-positive human breast cancer cells treated with a combination of DIM and paclitaxel, resulting in G2 phase cell cycle arrest. Moreover, treatment with DIM alone decreased activation of the Her2/neu receptor, affecting cell growth and differentiation.55
More recently, DIM has been demonstrated to protect against ionizing radiation through activation of the protein kinase ataxia telangiectasia mutated (ATM), which regulates responses to DNA damage and oxidative stress as well as cell survival signaling through nuclear factor-κB (NF-κB).56 Overexpression of PDGF-D is linked to increased DNA-binding activity of NF-κB in aggressive breast tumors.52 Conversely, DIM did not protect human breast cancer xenograft tumors against radiation, suggesting its potential use to mitigate undesirable side effects of cancer treatment.56
Modulation of estrogen
Metabolites of sex hormones, particularly estrogens, have shown an important role in breast cancer initiation and progression. Thus, modulation of sex hormones is an active area of research. The effects of DIM on estrogen activity are thought to be primarily the result of altered CYP enzyme metabolism by DIM. Changes in steroid hormone metabolism and estrogen metabolite profiles are consequences of enhanced CYP expression.57 In turn, changes in CYP enzyme activity and the resulting alterations in hormone metabolite concentrations modify oxidation and reduction reactions as well as the activity of estrogen, thus altering breast cancer risk. More specifically, CYP enzymes convert estrone to hydroxyestrones.58 Of the estrogen metabolites, 2-hydroxyestrone (2OHE1) has been suggested to have a protective effect against breast cancer.59
Expression of CYP1 genes is low in some breast cancer cell lines. CYP1A1 is responsible for promoting the metabolism of estrogen toward greater 2OHE1 production; transcripts of CYP1A1 and CYP1A2 are increased when exposed to DIM.58 Importantly, DIM supplementation has been shown to enhance the 2-hydroxlyation of estrogen, resulting in selective activation of estrogen receptor β target genes, which is thought to contribute to anti-inflammatory effects in hormone-responsive cell lines.60 The influence of DIM on AhR, as described above, results in reduced production of the carcinogenic 4-hydroxyesterone (4OHE1).61 Increased 4OHE1 has been associated with breast tumor formation and related to initiating mutations through formation of depurinating DNA adducts.62 DIM also induces expression of CYP3A4,63 which, similar to CYP1A1, has been shown to influence total production of the mitogenic metabolites 4OHE1 and 16α-hydroxyesterone (16αOHE1)64 by 2-hydroxylation of estrogens.
The efficacy of DIM has been demonstrated in vitro in breast tumor subtypes. DIM selectively induced cell cycle arrest and apoptosis in both estrogen receptor–positive and estrogen receptor–negative breast cancer cells, without producing evidence of antiproliferative activity in normal breast epithelial cells.53 The chemopreventive activity of DIM may have clinical applications in both hormone-dependent and hormone-independent disease. This may expand therapeutic options for triple-negative breast cancer.
MECHANISMS OF ACTION IN RODENTS
While I3C has been demonstrated to a play role in breast cancer prevention in animal models,65 the conversion of I3C to DIM in cell culture24 suggests that the growth-inhibitory effects of I3C on breast cancer cells are likely attributable to DIM, rather than to I3C alone. In fact, the bioavailability of supplemental DIM has been evaluated in Sprague Dawley rats. DIM was administered either at 200 mg/kg by atraumatic gavage or as a single morning dose of 0.1 mg/kg suspended in 200 μL of cod liver oil. Concentrations of DIM were present in plasma circulation within 15 minutes of administration and gradually decreased 12 hours after administration. The formulation of DIM suspended in liquid oil had the highest bioavailability. The liquid formulation was stable under acidic conditions and provided virtually 100% bioavailability in the animal model. In contrast, crystalline DIM did not show significant bioavailability.66
Evidence of the role of DIM in vivo as a chemopreventive agent in animal models of breast cancer substantiates the findings of cell culture experiments. Oral administration of DIM at 5 mg/kg on alternating days was associated with inhibition in the growth of 7,12-dimethylbenzanthracene–induced mammary tumors as well as inhibition of 17β estradiol–induced proliferation of MCF-7 cells in rats.67 DIM (orally at 5 mg/kg) had an apoptotic effect on 7,12-dimethylbenzanthracene–induced Sprague Dawley rat mammary tumor by negatively regulating the activity of epidermal growth factor receptor and downstream molecules, including Akt.48 A study in mice has shown evidence that DIM produced a concentration-dependent reduction in the proliferation, migration, invasion, and capillary tube formation of xenograft-transplanted human breast carcinoma.68 At concentration of 5μM DIM, G1 cell cycle arrest was demonstrated along with an upregulation in the expression of p27kip. The study also showed DIM at a dose of 5 mg/kg inhibited the growth of human MCF-7 cell tumor xenografts by up to 64%. Another study of HER-2/neu transgenic mice, DIM has been shown to reduce mammary tumor formation.69 These data support a role for DIM in inhibiting the invasive capacity of tumor cells, which is a common concern in premenopausal breast cancer45 and may also play a role in triple-negative disease.70
Murine models have also shown evidence of a favorable effect of DIM on estrogen metabolism in relation to breast cancer risk. Sepkovic et al.71 exposed wild-type mice to standard chow or standard chow plus 0.2% (2000 ppm) DIM for 12 weeks and found enhanced interferon-γ response and lower estradiol concentrations in those fed DIM compared with those fed control chow. It should be noted that the DIM supplementation approximated a 1000-mg single dose in humans. Additionally, the same study showed significantly higher C-2 hydroxylation of estrogen in the DIM-fed mice, reflected by an increased 2OHE1:16αOHE1 ratio, further supporting the favorable estrogenic effects of DIM. DIM was more effective than I3C in inducing estradiol-2-hydroxylase in rats, increasing synthesis of 2OHE1 in the competing pathways and, consequentially, decreasing 16αOHE1.32 Earlier work has suggested that the 2OHE1:16αOHE1 ratio may have prognostic value in breast cancer,59,72 although the evidence is inconsistent.
EPIDEMIOLOGICAL EVIDENCE
Cruciferous vegetables are the primary source of DIM in the human diet. Data from studies evaluating the association between cruciferous vegetable intake and cancer risk or prognosis are therefore of value in estimating the role of DIM in cancer prevention and control. Table 2 summarizes the epidemiological evidence published since 2000 regarding consumption of cruciferous vegetables and breast cancer risk and recurrence. Overall, point estimates of the odds ratio and relative risk suggest a protective role, though less so in US studies.1,3,4,9 This may be due to the overall lower intakes in the United States,11 particularly in comparison with intakes in Asian countries, where the risk of breast cancer is lower among those with greater intakes.5,7,8 A recent meta-analysis of 13 case–control and prospective cohort studies and 18 673 individual cases suggested that overall high intake of cruciferous vegetables was significantly associated with a 15% lower risk of breast cancer.2
Table 2.
Epidemiological evidence of cruciferous vegetable intake and breast cancer risk and recurrence since 2000
| Reference | Study population, geographic location (sample size) | Exposure measure | Mean intake (SD) of cruciferous vegetable | OR, RR, or HR (95%CI) for comparison group vs reference group |
|---|---|---|---|---|
| Studies of breast cancer risk | ||||
| Frazier et al. (2003)3 | Nurses’ Health Study, USA (n = 843) | Cabbage, broccoli |
|
|
| Ambrosone et al. (2004)1 | Erie County and Niagara County hospitals, USA (n = 740) | Broccoli, Brussels sprouts, sauerkraut, coleslaw, cauliflower, cabbage |
|
|
| Adebamowo et al. (2005)9 |
|
Broccoli | 5–6 servings/wk | RR: 0.99 (0.59–1.65), 5–6 servings/wk vs <1 serving/mo |
| Zhang et al. (2009)8 |
|
Chinese cabbage, cabbage, broccoli, cauliflower | 52.96 (58.23) g/d | Adjusted OR: 0.49 (0.32–0.74), Q4 vs Q1 |
| Boggs et al. (2010)4 |
|
Broccoli, collard or mustard greens, cabbage, or coleslaw |
|
|
| Butler et al. (2010)5 |
|
Total cruciferous vegetables |
|
|
| Suzuki et al. (2013)7 |
|
Cabbage, Japanese radish, Chinese cabbage, komatsuna, broccoli, leaf mustard, qing-geng-cai (bok choy), and chard |
|
|
| Studies of breast cancer recurrence | ||||
| Thomson et al. (2011)6 |
|
Broccoli, broccolini, broccoflower, Bok choy, Brussels sprouts, cauliflower, cabbage, kale, radicchio, mustard/collard/turnip greens, rutabaga, sauerkraut, kohlrabi, watercress, radish, horseradish | 0.5 (0.02) servings/d |
|
| Nechuta et al. (2013)11 |
|
Total cruciferous vegetables (g/d) |
|
HR, pooled: 1.10 (0.95–1.28), Q4 vs Q1 |
Abbreviations: OR, odds ratio; RR, relative risk; HR, hazard ratio; Q, quartile; SD, standard deviation.
With regard to cruciferous vegetable intake and breast cancer recurrence, one study by Thomson et al.6 suggested that total baseline intake of cruciferous vegetables was associated with a nonsignificant 15% decrease in the hazard rate of recurrence in women with stage I, II, or III invasive breast cancer after an average study duration of 7.3 years. The protective effect reached a significant 35% decrease in women on adjuvant tamoxifen therapy. A pooling of 4 cohorts of women with stages I–III breast cancer by Nechuta et al.11 showed no significant associations between intake of cruciferous vegetables and breast cancer recurrence or total mortality after a median follow-up of 9 years. In addition to variations in intake across studies, particularly within the highest quartiles, measurement errors in dietary assessment limit the interpretation of the epidemiological evidence.73
MECHANISMS OF ACTION AND SURROGATE ENDPOINTS IN HUMANS
In sum, the current evidence is mixed but compelling enough to validate the initiation of several human intervention studies to further ascertain the cancer-preventive and therapeutic potential of cruciferous vegetables and their bioactive compounds. While few intervention trials have evaluated the role of cruciferous vegetables or DIM in relation to modification of breast cancer risk, several studies have evaluated the effects of cruciferous vegetables or DIM on surrogate breast cancer endpoint biomarkers such as modulation of oxidative stress or metabolism of estrogen.
A cross-sectional analysis of 1005 middle-aged Chinese women found the inflammatory markers tumor necrosis factor α, interleukin 1β, and interleukin 6 to be inversely associated with higher intakes of cruciferous vegetables.74 Inflammation as well as oxidative stress may contribute to irreparable DNA damage, thereby increasing the risk of cancer. Studies evaluating the role of cruciferous vegetables in modifying oxidative stress include a crossover study by Fowke et al.,75 who randomized 20 healthy adults to a Brassica-rich diet or a vitamin/mineral fiber supplement for 4 weeks, with an intermediate 2-week washout period. The Brassica-rich intervention was associated with a 22% reduction in lipid peroxidation as assessed by urinary F2-isoprostane levels, a stable biomarker of systemic oxidative stress. A larger randomized, placebo-controlled clinical trial76 of 200 Chinese adults showed that consumption of glucoraphanin-rich beverages (broccoli sprouts) nightly for 2 weeks was not associated with lower aflatoxin-DNA adduct formation but did increase the excretion of these adducts in a subject with high dithiocarbamate levels. In a small trial in 16 healthy adults who consumed 500 g of broccoli per day, the average 2OHE1:16αOHE1 ratio increased, and results indicated that CYP enzymes involved in hydroxylation are induced by dietary broccoli intake.64
Studying the physiological responses to the intake of selected bioactive compounds is difficult, due in part to the variations in DIM content of different food sources. On average, 100 g of cruciferous vegetables contains up to 30 mg of glucobrassicin, which is estimated to convert to approximately 2 mg of DIM. However, the variation in DIM content between different cruciferous vegetables is considerable, with differences ranging from 5- to 8-fold.15 To achieve a biologically relevant exposure, it is suggested that intake would need to be upwards of 600 g/d77 and sustained for several years for to achieve an anticancer benefit. An intake this high is difficult to attain or maintain through diet alone.
DIM has limited bioavailability because of its extreme insolubility in water and oil. Pure, crystalline DIM is poorly soluble and poorly absorbed upon ingestion. Supplementation with specialized formulations of DIM is necessary to achieve these higher exposures and, in turn, to evaluate the potential chemopreventive activity of DIM in humans. While there are several different DIM formulas available, the majority of monitored and placebo-controlled trials have used BioResponse-DIM (BR-DIM), a dietary supplement containing microencapsulated DIM. Compared with a pure crystalline formulation, BR-DIM is suggested to have 50% higher bioavailability.26 The half-life of DIM ranges from 2.6 to 4.5 hours, and there is a significant increase in the maximum concentration of plasma DIM at supplementation levels between 100 and 200 mg/d.78 The recommendation for a tolerable single dose of DIM from BR-DIM has been established as 300 mg (4.3 mg/kg/d).79 After a single dose of 300 mg of BR-DIM, peak plasma levels of DIM were 236 ng/ml, equivalent to a concentration of 0.94μM DIM.78 The bioavailability and plasma levels of DIM following oral doses of BR-DIM have been published previously in placebo-controlled human studies.78–80 Thus far, most clinical research on DIM is based on the consumption of cruciferous vegetables, but current clinical trials are examining the effects of DIM, specifically the BR-DIM formulation, on breast cancer risk.
The number of intervention studies with DIM supplementation remains limited. In a study of BRCA1-inherited mutation, a mutation associated with a high lifetime risk of breast cancer,81 BR-DIM providing DIM at 300 mg/d (150 mg twice daily) showed a borderline significant (P = 0.05) increase in BRCA1 expression in healthy women aged 25 to 63 years. In the 13 women with the inherited BRCA1 mutation, 4 to 6 weeks of DIM supplementation resulted in an average 34% (range −24% to 194%) increase in BRCA1 mRNA expression.82 In a study of 20 healthy women with a BRCA1 mutation, Nikitina et al.83 evaluated the effect of 4 to 6 weeks of BR-DIM supplementation on the urinary 2OHE1:16αOHE1 ratio. This short-term intervention showed no significant change in the 2OHE1:16αOHE1 ratio (P = 0.35), regardless of menopausal status.
A placebo-controlled, double-blind trial on BR-DIM was conducted in 19 postmenopausal women with early-stage breast cancer. Women were randomized to receive 108 mg of BR-DIM or placebo daily for 30 days. When compared with placebo, BR-DIM resulted in a significant increase in 2OHE1 (P = 0.02) and a modestly protective shift in the 2OHE1:16αOHE1 ratio (P = 0.059).84 The ability of DIM to induce CYP enzymes responsible for catalyzing hydroxylation could be responsible for the enhanced production of 2OHE1 and the limited production of 16αOHE1. Additionally, administration of BR-DIM to premenopausal women improves symptoms of cyclical mastalgia,85 a potential indicator of increased breast cancer risk.86 To date, many trials evaluating DIM and breast cancer have been conducted in samples of less than 50 subjects, limiting the interpretation of study findings. Several trials evaluating the role of DIM in breast cancer prevention are currently under way (Table 3).
Table 3.
Completed and ongoing clinical trials testing the efficacy of diindolylmethane in breast cancer preventiona
| Study title | Clinicaltrial.gov identifier | Principal investigator | Purpose | Intervention | Date of study completion | Publication |
|---|---|---|---|---|---|---|
| Phase I ascending single dose pharmacokinetics and safety study of 3,3′ diindolylmethane | NCT00784394 | A. Hurwitz | Study the side effects and best dose of DIM in preventing cancer in healthy volunteers | Single-dose DIM (BR-DIM); response observed over 24 h | October 2009 | Reed et al. (2008)79 |
| Phase 1 multiple-dose safety, pharmacokinetic, and drug interaction clinical study of nutritional-grade, absorption-enhanced DIM (BR-DIM) | NCT00392652 | G. Reed | Study the side effects and best dose of DIM in healthy volunteers | Low- and higher-dose BR-DIM twice daily for 4 wk | October 2009 | N/A |
| The potential for oral DIM supplementation to increase the production of the BRCA1 protein in BRCA1 mutation carriers | NCT01022333 | S.A. Narod | Determine whether there is potential for oral DIM supplementation to result in increased production of BRCA1 protein in BRCA1 mutation carriers | 300 mg of DIM (BR-DIM) daily for 6 wk | July 2010 | Nikitina et al. (2015)83 |
| Effect of cruciferous vegetables or cruciferous supplement on urinary estrogen metabolites in premenopausal women | NCT01726127 | S.A. Tanumihardjo | Evaluate ratio of urinary estrogen metabolites in healthy premenopausal women | Consumption of 40 g of broccoli or Brussels sprouts daily for 8 wk, followed by Cruciferous Complete supplement for 8 wk | July 2015 | N/A |
| Evaluation of diindolylmethane supplementation to modulate tamoxifen efficacy in breast cancer: the diindolylmethane efficacy study | NCT01391689 | C. Thomson | Phase II/III trial to study how well DIM works and to compare DIM with placebo in treating patients with breast cancer | DIM (BR-DIM) orally twice daily for ≈36 mo | September 2016 (estimated) | N/A |
| A nutritional intervention to decrease breast density among female BRCA carriers – a prospective clinical trial | NCT02197000 | D. Margel | Determine whether adding DIM supplement will decrease breast density among female BRCA mutation carriers over a period of 2 y | 100 mg of DIM-Avail (nutritional supplement) once daily for 24 mo | January 2018 (estimated) | N/A |
Abbreviations: BR-DIM, BioResponse diindolylmethane; DIM, diindolylmethane; Q, quartiles; N/A, not available.
aInformation obtained from clinicaltrials.gov on February 4, 2016.
CONCLUSION
DIM and its precursor I3C are among the most commonly evaluated indoles found in cruciferous vegetables. These compounds have been widely studied in relation to breast cancer chemoprevention.87 Numerous mechanisms by which dietary exposure to these compounds may modulate breast cancer have been reported, including apoptosis, modulation of response to oxidative stress, estrogen metabolism, and cell cycle modulation, and other antiproliferative activities have been evaluated, largely in cell culture and animal studies.24,30 The evidence for a protective role of DIM against breast cancer continues to grow, but additional research is needed to further identify and refine the mechanistic targets of this compound, particularly in humans. DIM is available to consumers in a generic crystalline formulation (low bioavailability) and in a microencapsulated form as BR-DIM (higher bioavailability). Patient inquiries regarding the possible use of DIM as protective or adjuvant therapy during chemotherapy are mounting, in part because of the increasing availability of and information on DIM. Nevertheless, information about the specific dosing of DIM and the corresponding intakes of cruciferous vegetables is currently lacking. Before any recommendations can be developed, clinical trials must be completed to determine the evidence-driven basis for a dietary recommendation.
Acknowledgments
The authors would like to acknowledge Nicole Bergier for her editorial support and her help with formatting and submission of the article, as well as Vernon Hartz for the procurement of literature on plant sources of glucosinolates.
Funding. This review has been supported by the National Institutes of Health (NIH) National Cancer Institute (NCI) funding under 1R01CA149417-01A1 (C.A. Thomson, principal investigator) and the University of Arizona Comprehensive Cancer Center support grant funded by NIH-NCI grant no. CCSG-CA023074 (D.S. Alberts, principal investigator). The authors had full access to the literature included in this review; the funding agencies provide financial support to cover a percentage of the lead author’s effort used to author this review.
Declaration of interest. The authors have no relevant interests to declare.
References
- 1.Ambrosone CB, McCann SE, Freudenheim JL, et al. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr. 2004;134:1134–1138. [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Lv K. Cruciferous vegetables intake is inversely associated with risk of breast cancer: a meta-analysis. Breast. 2013;22:309–313. [DOI] [PubMed] [Google Scholar]
- 3.Frazier AL, Ryan CT, Rockett H, et al. Adolescent diet and risk of breast cancer. Breast Cancer Res. 2003;5:R59–R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boggs DA, Palmer JR, Wise LA, et al. Fruit and vegetable intake in relation to risk of breast cancer in the Black Women's Health Study. Am J Epidemiol. 2010;172:1268–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler LM, Wu AH, Wang R, et al. A vegetable-fruit-soy dietary pattern protects against breast cancer among postmenopausal Singapore Chinese women. Am J Clin Nutr. 2010;91:1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson CA, Rock CL, Thompson PA, et al. Vegetable intake is associated with reduced breast cancer recurrence in tamoxifen users: a secondary analysis from the Women's Healthy Eating and Living Study. Breast Cancer Res Treat. 2011;125:519–527. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki R, Iwasaki M, Hara A, et al. Fruit and vegetable intake and breast cancer risk defined by estrogen and progesterone receptor status: the Japan Public Health Center-based Prospective Study. Cancer Causes Control. 2013;24:2117–2128. [DOI] [PubMed] [Google Scholar]
- 8.Zhang CX, Ho SC, Chen YM, et al. Greater vegetable and fruit intake is associated with a lower risk of breast cancer among Chinese women. Int J Cancer. 2009;125:181–188. [DOI] [PubMed] [Google Scholar]
- 9.Adebamowo CA, Cho E, Sampson L, et al. Dietary flavonols and flavonol-rich foods intake and the risk of breast cancer. Int J Cancer. 2005;114:628–633. [DOI] [PubMed] [Google Scholar]
- 10.Smith-Warner SA, Spiegelman D, Yaun SS, et al. Intake of fruits and vegetables and risk of breast cancer: a pooled analysis of cohort studies. JAMA. 2001;285:769–776. [DOI] [PubMed] [Google Scholar]
- 11.Nechuta S, Caan BJ, Chen WY, et al. Postdiagnosis cruciferous vegetable consumption and breast cancer outcomes: a report from the After Breast Cancer Pooling Project. Cancer Epidemiol Biomarkers Prev. 2013;22:1451–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdull Razis AF, Noor NM. Cruciferous vegetables: dietary phytochemicals for cancer prevention. Asian Pac J Cancer Prev. 2013;14:1565–1570. [DOI] [PubMed] [Google Scholar]
- 13.Johnson IT. Glucosinolates: bioavailability and importance to health. Int J Vitam Nutr Res. 2002;72:26–31. [DOI] [PubMed] [Google Scholar]
- 14.Verkerk R, van der Gaag MS, Dekker M, et al. Effects of processing conditions on glucosinolates in cruciferous vegetables. Cancer Lett. 1997;114:193–194. [DOI] [PubMed] [Google Scholar]
- 15.McNaughton SA, Marks GC. Development of a food composition database for the estimation of dietary intakes of glucosinolates, the biologically active constituents of cruciferous vegetables. Br J Nutr. 2003;90:687–697. [DOI] [PubMed] [Google Scholar]
- 16.Ciska E, Martyniak-Przybyszewska B, Kozlowska H. Content of glucosinolates in cruciferous vegetables grown at the same site for two years under different climatic conditions. J Agric Food Chem. 2000;48:2862–2867. [DOI] [PubMed] [Google Scholar]
- 17.Carlson DG, Daxenbichler ME, VanEtten CH, et al. Glucosinolates in crucifer vegetables: turnips and rutabagas. J Agric Food Chem. 1981;29:1235–1239. [DOI] [PubMed] [Google Scholar]
- 18.Carlson DG, Daxenbichler ME, VanEtte CH, et al. Glucosinolates in radish cultivars. J Am Soc Hortic Sci. 1985;110:634–638. [Google Scholar]
- 19.Carlson DG, Daxenbichler ME, VanEtten CH, et al. Glucosinolates in crucifer vegetables: broccoli, brussels sprouts, cauliflower, collards, kale, mustard greens and kohlrabi. J Am Soc Hortic Sci. 1987;112:173–178. [Google Scholar]
- 20.Tang L, Paonessa JD, Zhang Y, et al. Total isothiocyanate yield from raw cruciferous vegetables commonly consumed in the United States. J Funct Foods. 2013;5:1996–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International Agency for Research on Cancer. Cruciferous Vegetables, Isothiocyanates and Indoles. Lyon, France: IARC Press; 2004. [Google Scholar]
- 22.Chevolleau S, Debrauwer L, Boyer G, et al. Isolation and structure elucidation of a new thermal breakdown product of glucobrassicin, the parent indole glucosinolate. J Agric Food Chem. 2002;50:5185–5190. [DOI] [PubMed] [Google Scholar]
- 23.De Kruif CA, Marsman JW, Venekamp JC, et al. Structure elucidation of acid reaction products of indole-3-carbinol: detection in vivo and enzyme induction in vitro. Chem Biol Interact. 1991;80:303–315. [DOI] [PubMed] [Google Scholar]
- 24.Bradlow HL, Zeligs MA. Diindolylmethane (DIM) spontaneously forms from indole-3-carbinol (I3C) during cell culture experiments. In Vivo. 2010;24:387–391. [PubMed] [Google Scholar]
- 25.Ciska E, Verkerk R, Honke J. Effect of boiling on the content of ascorbigen, indole-3-carbinol, indole-3-acetonitrile, and 3,3'-diindolylmethane in fermented cabbage. J Agric Food Chem. 2009;57:2334–2338. [DOI] [PubMed] [Google Scholar]
- 26.Anderton MJ, Manson MM, Verschoyle R, et al. Physiological modeling of formulated and crystalline 3,3'-diindolylmethane pharmacokinetics following oral administration in mice. Drug Metab Dispos. 2004;32:632–638. [DOI] [PubMed] [Google Scholar]
- 27.Fujioka N, Ainslie-Waldman CE, Upadhyaya P, et al. Urinary 3,3'-diindolylmethane: a biomarker of glucobrassicin exposure and indole-3-carbinol uptake in humans. Cancer Epidemiol Biomarkers Prev. 2014;23:282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reed GA, Arneson DW, Putnam WC, et al. Single-dose and multiple-dose administration of indole-3-carbinol to women: pharmacokinetics based on 3,3'-diindolylmethane. Cancer Epidemiol Biomarkers Prev. 2006;15:2477–2481. [DOI] [PubMed] [Google Scholar]
- 29.Wattenberg LW, Loub WD. Inhibition of polycyclic aromatic hydrocarbon-induced neoplasia by naturally occurring indoles. Cancer Res. 1978;38:1410–1413. [PubMed] [Google Scholar]
- 30.Banerjee S, Kong D, Wang Z, et al. Attenuation of multi-targeted proliferation-linked signaling by 3,3'-diindolylmethane (DIM): from bench to clinic. Mutat Res. 2011;728:47–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen I, McDougal A, Wang F, et al. Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis. 1998;19:1631–1639. [DOI] [PubMed] [Google Scholar]
- 32.Jellinck PH, Forkert PG, Riddick DS, et al. Ah receptor binding properties of indole carbinols and induction of hepatic estradiol hydroxylation. Biochem Pharmacol. 1993;45:1129–1136. [DOI] [PubMed] [Google Scholar]
- 33.Bradshaw TD, Bell DR. Relevance of the aryl hydrocarbon receptor (AhR) for clinical toxicology. Clin Toxicol (Philadelphia). 2009;47:632–642. [DOI] [PubMed] [Google Scholar]
- 34.Schlezinger JJ, Liu D, Farago M, et al. A role for the aryl hydrocarbon receptor in mammary gland tumorigenesis. Biol Chem. 2006;387:1175–1187. [DOI] [PubMed] [Google Scholar]
- 35.Degner SC, Papoutsis AJ, Selmin O, et al. Targeting of aryl hydrocarbon receptor-mediated activation of cyclooxygenase-2 expression by the indole-3-carbinol metabolite 3,3'-diindolylmethane in breast cancer cells. J Nutr. 2009;139:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan S, Meng Q, Saha T, et al. Low concentrations of diindolylmethane, a metabolite of indole-3-carbinol, protect against oxidative stress in a BRCA1-dependent manner. Cancer Res. 2009;69:6083–6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahman KW, Li Y, Wang Z, et al. Gene expression profiling revealed survivin as a target of 3,3'-diindolylmethane-induced cell growth inhibition and apoptosis in breast cancer cells. Cancer Res. 2006;66:4952–4960. [DOI] [PubMed] [Google Scholar]
- 38.Riby JE, Firestone GL, Bjeldanes LF. 3,3'-Diindolylmethane reduces levels of HIF-1α and HIF-1 activity in hypoxic cultured human cancer cells. Biochem Pharmacol. 2008;75:1858–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riby JE, Xue L, Chatterji U, et al. Activation and potentiation of interferon-γ signaling by 3,3'-diindolylmethane in MCF-7 breast cancer cells. Mol Pharmacol. 2006;69:430–439. [DOI] [PubMed] [Google Scholar]
- 40.Jin Y. 3,3'-Diindolylmethane inhibits breast cancer cell growth via miR-21-mediated Cdc25A degradation. Mol Cell Biochem. 2011;358:345–354. [DOI] [PubMed] [Google Scholar]
- 41.Wang TT, Milner MJ, Milner JA, et al. Estrogen receptor α as a target for indole-3-carbinol. J Nutr Biochem. 2006;17:659–664. [DOI] [PubMed] [Google Scholar]
- 42.Marques M, Laflamme L, Benassou I, et al. Low levels of 3,3'-diindolylmethane activate estrogen receptor α and induce proliferation of breast cancer cells in the absence of estradiol. BMC Cancer. 2014;14:524 doi:10.1186/1471-2407-14-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saati GE, Archer MC. Inhibition of fatty acid synthase and Sp1 expression by 3,3'-diindolylmethane in human breast cancer cells. Nutr Cancer. 2011;63:790–794. [DOI] [PubMed] [Google Scholar]
- 44.Licznerska BE, Szaefer H, Murias M, et al. Modulation of CYP19 expression by cabbage juices and their active components: indole-3-carbinol and 3,3'-diindolylmethene in human breast epithelial cell lines. Eur J Nutr. 2013;52:1483–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmad A, Kong D, Wang Z, et al. Down-regulation of uPA and uPAR by 3,3'-diindolylmethane contributes to the inhibition of cell growth and migration of breast cancer cells. J Cell Biochem. 2009;108:916–925. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Ahmad A, Ali S, Wang Z, et al. 3,3'-Diindolylmethane enhances taxotere-induced growth inhibition of breast cancer cells through downregulation of FoxM1. Int J Cancer. 2011;129:1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsu EL, Chen N, Westbrook A, et al. CXCR4 and CXCL12 down-regulation: a novel mechanism for the chemoprotection of 3,3'-diindolylmethane for breast and ovarian cancers. Cancer Lett. 2008;265:113–123. [DOI] [PubMed] [Google Scholar]
- 48.Bhowmik A, Das N, Pal U, et al. 2,2'-Diphenyl-3,3'-diindolylmethane: a potent compound induces apoptosis in breast cancer cells by inhibiting EGFR pathway. PLoS One. 2013;8:e59798 doi:10.1371/journal.pone.0059798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bose S, Chandran S, Mirocha JM, et al. The Akt pathway in human breast cancer: a tissue-array-based analysis. Mod Pathol. 2006;19:238–245. [DOI] [PubMed] [Google Scholar]
- 50.Nicastro HL, Firestone GL, Bjeldanes LF. 3,3'-Diindolylmethane rapidly and selectively inhibits hepatocyte growth factor/c-Met signaling in breast cancer cells. J Nutr Biochem. 2013;24:1882–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kong D, Banerjee S, Huang W, et al. Mammalian target of rapamycin repression by 3,3'-diindolylmethane inhibits invasion and angiogenesis in platelet-derived growth factor-D-overexpressing PC3 cells. Cancer Res. 2008;68:1927–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahmad A, Wang Z, Kong D, et al. Platelet-derived growth factor-D contributes to aggressiveness of breast cancer cells by up-regulating Notch and NF-κB signaling pathways. Breast Cancer Res Treat. 2011;126:15–25. [DOI] [PubMed] [Google Scholar]
- 53.Wang Z, Yu BW, Rahman KM, et al. Induction of growth arrest and apoptosis in human breast cancer cells by 3,3-diindolylmethane is associated with induction and nuclear localization of p27kip. Mol Cancer Ther. 2008;7:341–349. [DOI] [PubMed] [Google Scholar]
- 54.Gong Y, Sohn H, Xue L, et al. 3,3'-Diindolylmethane is a novel mitochondrial H+-ATP synthase inhibitor that can induce p21Cip1/Waf1 expression by induction of oxidative stress in human breast cancer cells. Cancer Res. 2006;66:4880–4887. [DOI] [PubMed] [Google Scholar]
- 55.McGuire KP, Ngoubilly N, Neavyn M, et al. 3,3'-Diindolylmethane and paclitaxel act synergistically to promote apoptosis in HER2/Neu human breast cancer cells. J Surg Res. 2006;132:208–213. [DOI] [PubMed] [Google Scholar]
- 56.Fan S, Meng Q, Xu J, et al. DIM (3,3'-diindolylmethane) confers protection against ionizing radiation by a unique mechanism. Proc Natl Acad Sci U S A. 2013;110:18650–18655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henry NL, Rae JM, Li L, et al. Association between CYP2D6 genotype and tamoxifen-induced hot flashes in a prospective cohort. Breast Cancer Res Treat. 2009;117:571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szaefer H, Licznerska B, Krajka-Kuźniak V, et al. Modulation of CYP1A1, CYP1A2 and CYP1B1 expression by cabbage juices and indoles in human breast cell lines. Nutr Cancer. 2012;64:879–888. [DOI] [PubMed] [Google Scholar]
- 59.Falk RT, Brinton LA, Dorgan JF, et al. Relationship of serum estrogens and estrogen metabolites to postmenopausal breast cancer risk: a nested case-control study. Breast Cancer Res. 2013;15:R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vivar OI, Saunier EF, Leitman DC, et al. Selective activation of estrogen receptor-β target genes by 3,3'-diindolylmethane. Endocrinology. 2010;151:1662–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parkin DR, Malejka-Giganti D. Differences in the hepatic P450-dependent metabolism of estrogen and tamoxifen in response to treatment of rats with 3,3'-diindolylmethane and its parent compound indole-3-carbinol. Cancer Detect Prev. 2004;28:72–79. [DOI] [PubMed] [Google Scholar]
- 62.Rogan EG, Badawi AF, Devanesan PD, et al. Relative imbalances in estrogen metabolism and conjugation in breast tissue of women with carcinoma: potential biomarkers of susceptibility to cancer. Carcinogenesis. 2003;24:697–702. [DOI] [PubMed] [Google Scholar]
- 63.Pondugula SR, Flannery PC, Abbott KL, et al. Diindolylmethane, a naturally occurring compound, induces CYP3A4 and MDR1 gene expression by activating human PXR. Toxicol Lett. 2015;232:580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kall MA, Vang O, Clausen J. Effects of dietary broccoli on human in vivo drug metabolizing enzymes: evaluation of caffeine, oestrone and chlorzoxazone metabolism. Carcinogenesis. 1996;17:793–799. [DOI] [PubMed] [Google Scholar]
- 65.Lubet RA, Heckman BM, De Flora SL, et al. Effects of 5,6-benzoflavone, indole-3-carbinol (I3C) and diindolylmethane (DIM) on chemically-induced mammary carcinogenesis: is DIM a substitute for I3C? Oncol Rep. 2011;26:731–736. [DOI] [PubMed] [Google Scholar]
- 66.Paltsev M, Kiselev V, Muyzhnek E, et al. Comparative preclinical pharmacokinetics study of 3,3'-diindolylmethane formulations: is personalized treatment and targeted chemoprevention in the horizon? EPMA J. 2013;4:25 doi:10.1186/1878-5085-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen I, Hsieh T, Thomas T, et al. Identification of estrogen-induced genes downregulated by AhR agonists in MCF-7 breast cancer cells using suppression subtractive hybridization. Gene. 2001;262:207–214. [DOI] [PubMed] [Google Scholar]
- 68.Chang X, Tou JC, Hong C, et al. 3,3'-Diindolylmethane inhibits angiogenesis and the growth of transplantable human breast carcinoma in athymic mice. Carcinogenesis. 2005;26:771–778. [DOI] [PubMed] [Google Scholar]
- 69.Lanza-Jacoby SM, McGuire K, Ngoubilly N. 3,3'-Diindolylmethane (DIM), a naturally occurring compound found in cruciferous vegetables, reduces HER-2/neu signaling and inhibits growth of HER-2/neu positive breast cancer cells. Supplement: International Research Conference on Food, Nutrition and Cancer [poster abstracts]. J Nutr. 2007;137(suppl):278S–295S. [Google Scholar]
- 70.Jiang J, Thyagarajan-Sahu A, Loganathan J, et al. BreastDefend™ prevents breast-to-lung cancer metastases in an orthotopic animal model of triple-negative human breast cancer. Oncol Rep. 2012;28:1139–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sepkovic DW, Stein J, Carlisle AD, et al. Diindolylmethane inhibits cervical dysplasia, alters estrogen metabolism, and enhances immune response in the K14-HPV16 transgenic mouse model. Cancer Epidemiol Biomarkers Prev. 2009;18:2957–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Osborne MP, Bradlow HL, Wong GY, et al. Upregulation of estradiol C16α-hydroxylation in human breast tissue: a potential biomarker of breast cancer risk. J Natl Cancer Inst. 1993;85:1917–1920. [DOI] [PubMed] [Google Scholar]
- 73.Prentice RL. Measurement error and results from analytic epidemiology: dietary fat and breast cancer. J Natl Cancer Inst. 1996;88:1738–1747. [DOI] [PubMed] [Google Scholar]
- 74.Jiang Y, Wu SH, Shu XO, et al. Cruciferous vegetable intake is inversely correlated with circulating levels of proinflammatory markers in women. J Acad Nutr Diet. 2014;114:700.e2–708.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fowke JH, Morrow JD, Motley S, et al. Brassica vegetable consumption reduces urinary F2-isoprostane levels independent of micronutrient intake. Carcinogenesis. 2006;27:2096–2102. [DOI] [PubMed] [Google Scholar]
- 76.Kensler TW, Chen JG, Egner PA, et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 2005;14:2605–2613. [DOI] [PubMed] [Google Scholar]
- 77.Greenlee H, Atkinson C, Stanczyk FZ, et al. A pilot and feasibility study on the effects of naturopathic botanical and dietary interventions on sex steroid hormone metabolism in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2007;16:1601–1609. [DOI] [PubMed] [Google Scholar]
- 78.Heath EI, Heilbrun LK, Li J, et al. A phase I dose-escalation study of oral BR-DIM (BioResponse 3,3'- Diindolylmethane) in castrate-resistant, non-metastatic prostate cancer. Am J Transl Res. 2010;2:402–411. [PMC free article] [PubMed] [Google Scholar]
- 79.Reed GA, Sunega JM, Sullivan DK, et al. Single-dose pharmacokinetics and tolerability of absorption-enhanced 3,3'-diindolylmethane in healthy subjects. Cancer Epidemiol Biomarkers Prev. 2008;17:2619–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rajoria S, Suriano R, Parmar PS, et al. 3,3'-Diindolylmethane modulates estrogen metabolism in patients with thyroid proliferative disease: a pilot study. Thyroid. 2011;21:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kotsopoulos J, Zhang S, Akbari M, et al. BRCA1 mRNA levels following a 4–6-week intervention with oral 3,3'-diindolylmethane. Br J Cancer. 2014;111:1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nikitina D, Llacuachaqui M, Sepkovic D, et al. The effect of oral 3,3'-diindolylmethane supplementation on the 2:16α-OHE ratio in BRCA1 mutation carriers. Fam Cancer. 2015;14:281–286. [DOI] [PubMed] [Google Scholar]
- 84.Dalessandri KM, Firestone GL, Fitch MD, et al. Pilot study: effect of 3,3'-diindolylmethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr Cancer. 2004;50:161–167. [DOI] [PubMed] [Google Scholar]
- 85.Zeligs M, Brownstone P, Sharp M, et al. Managing cyclical mastalgia with absorbable diindolylmethane:a randomized, placebo-controlled trial. J Am Nutraceutical Assoc. 2005;8:5–15. [Google Scholar]
- 86.Plu-Bureau G, Lê MG, Sitruk-Ware R, et al. Cyclical mastalgia and breast cancer risk: results of a French cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15:1229–1231. [DOI] [PubMed] [Google Scholar]
- 87.Higdon JV, Delage B, Williams DE, et al. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55:224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]