Abstract
Human body is continuously exposed to different types of agents that results in the production of reactive species called as free radicals (ROS/RNS) which by the transfer of their free unpaired electron causes the oxidation of cellular machinery. In order to encounter the deleterious effects of such species, body has got endogenous antioxidant systems or it obtains exogenous antioxidants from diet that neutralizes such species and keeps the homeostasis of body. Any imbalance between the RS and antioxidants leads to produce a condition known as “oxidative stress” that results in the development of pathological condition among which one is diabetes. Most of the studies reveal the inference of oxidative stress in diabetes pathogenesis by the alteration in enzymatic systems, lipid peroxidation, impaired Glutathione metabolism and decreased Vitamin C levels. Lipids, proteins, DNA damage, Glutathione, catalane and superoxide dismutase are various biomarkers of oxidative stress in diabetes mellitus. Oxidative stress induced complications of diabetes may include stroke, neuropathy, retinopathy and nephropathy. The basic aim of this review was to summarize the basics of oxidative stress in diabetes mellitus.
1. Diabetes mellitus
Likewise Osteoporosis, Cushing’s syndrome and Scleroderma, Diabetes mellitus is a group of metabolic disorders that is characterized by elevated levels of glucose in blood (hyperglycemia) and insufficiency in production or action of insulin produced by the pancreas inside the body (Maritim et al., 2003). Insulin is a protein (hormone) synthesized in beta cells of pancreas in response to various stimuli such as glucose, sulphonylureas, and arginine however glucose is the major determinant (Joshi et al., 2007). Long term elevation in blood glucose levels is associated with macro- and micro-vascular complications leading to heart diseases, stroke, blindness and kidney diseases (Loghmani, 2005). Sidewise to hyperglycemia, there are several other factors that play great role in pathogenesis of diabetes such as hyperlipidemia and oxidative stress leading to high risk of complications (Kangralkar et al., 2010).
2. Types of diabetes mellitus
Diabetes mellitus can be classified in different ways but one form of classification is as follow (American Diabetes Association, 2004):
-
1.
Type I diabetes (Insulin dependent) is due to immune mediated beta-cells destruction, leading to insulin deficiency.
-
2.
Idiopathic diabetes is the type 1 diabetes with no known etiologies and is strongly inherited.
-
3.
Type II diabetes (Non-Insulin dependent) is due to insulin secretory defect and insulin resistance.
-
4.
Gestational diabetes mellitus is any form of intolerance to glucose with onset or first recognition of pregnancy.
However diabetes is mostly classified basically into TWO major types: Type I Diabetes (IDDM) and Type II Diabetes (NIDDM).
3. Pathophysiology of diabetes
Whenever somebody takes the meal, there is rise in blood glucose levels that stimulates insulin secretion resulting in an increase in transportation, biotransformation and storage in muscles and fat tissues. In fasting conditions, the glucose in blood is provided by liver that is used by the brain, without any dependency on insulin. Besides the storage of glucose, insulin also inhibits the secretion of glucagon and lowers the concentration of serum fatty acids leading to a decline in liver glucose production (Kangralkar et al., 2010). Insufficient insulin or resistance to insulin in the body results in reduced tissue uptake of glucose that results in intracellular hypoglycemia and extracellular hyperglycemia. The intracellular hypoglycemia causes glucogenesis and gluconeogenesis that leads to fats breakdown (causing diabetic ketoacidosis) and decreases protein synthesis and gamma globulins (causing cachexia, polyphagia, and impaired wound healing), while the extracellular hyperglycemia leads to hyperglycemic coma and osmotic dieresis (Ozougwu et al., 2013) (see Fig. 1).
Figure 1.
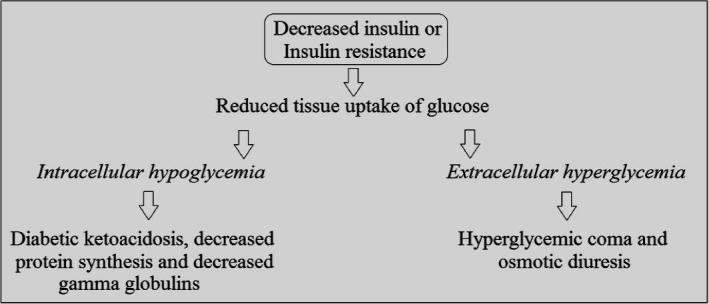
Pathophysiology of diabetes mellitus.
3.1. Pathogenesis of Type I diabetes mellitus (IDDM)
In Insulin dependent diabetes mellitus (IDDM) there is a deficiency of insulin secretion due to the autoimmune destruction of beta pancreatic cells that leads to metabolic disturbances associated with IDDM (Ozougwu et al., 2013). The end stage of β-cell destruction represents the onset of clinical disease leading to type 1 diabetes mellitus in which there are infiltrating monocytes, lymphocytes and a mixture of pseudoatrophic islets with some cells secreting somatostatin, glycogen and pancreatic polypeptide which then, consequently through immunogenic process, induces the disease (Al Homsi and Lukic, 1992, Gill and Haskins, 1993, Yagi et al., 1992). Autoimmunity, genetic makeup and environmental factors are responsible for islets cell destruction (Michael et al., 2000).
3.2. Pathogenesis of Type II diabetes mellitus (NIDDM)
In Non-Insulin dependent diabetes mellitus (NIDDM) there are certain mechanisms broken that keep regulation between tissue sensitivity to insulin which consequently leads to impaired insulin secretion by the pancreatic beta cells and impaired insulin action through insulin resistance (Defronzo and Lily, 1987). In this type of diabetes, multiple genetic defects, and certain environmental factors especially obesity are responsible for beta cell defects and peripheral tissue insulin resistance respectively (Michael et al., 2000).
4. Complications of diabetes
Diabetes is such a sort of disorder in which the patients are at all the time on risk of complications. Complications may be macrovascular (coronary heart disease, peripheral vascular disease and stroke), microvascular (neuropathy, retinopathy and nephropathy) and both micro- and macrovascular (diabetic foot). The mortality and morbidity of diabetes are associated more with macrovascular degeneration as compared to the risks of microvascular complications in older people (Wallace, 2004). In general, complications of diabetes mellitus can be categorized into two groups (Wallace, 2004, Mohan, 2002) (see Table 1):
-
a.
Metabolic acute complications: These are short term and include hypoglycemia, ketoacidosis and hyperosmolar non-ketonic coma.
-
b.
Systemic late complications: These are long term chronic sort of complications that include diabetic nephropathy, microangiopathy, diabetic neuro- and retinopathy, atherosclerosis and infections.
Table 1.
Complications of diabetes.
| Acute complications (Metabolic) | Chronic complications (Systemic) |
|---|---|
| Infection (s) | Blindness, retinopathy |
| Diabetic ketoacidosis (DKA) | Neuropathy |
| Hyperglycemic, hyperosmolar, non-ketonic coma | Atherosclerosis |
| Polydipsia, polyuria, fatigue, blurred vision | Peripheral vascular disease |
| Infection, amputation | |
| Cerebrovascular disease | |
| Macrovascular complications | Microvascular complications |
| Stroke | Retinopathy and cataracts |
| Heart disease and hypertension | Renal disease |
| Peripheral vascular disease | Neuropathy |
| Foot problems | Foot problems |
5. Overview of free radicals
5.1. Free radicals
Free radicals are reactive chemical entities that are short lived species containing one or more unpaired electrons. They can also be considered as necessary evil for signaling involved in normal process of differentiation and migration. The free radicals induce damage to cells by passing the unpaired electron resulting in oxidation of cell components and molecules (Bansal and Bilaspuri, 2011). They are generally very unstable and very much reactive.
5.2. Types of free radicals
Free radicals can be classified into following three types:
-
1.
Reactive oxygen species (ROS).
-
2.
Reactive Nitrogen species (RNS) (Droge, 2011).
-
3.
Reactive chlorine species (RCS) (Freidovich, 1999).
5.3. Biological roles of free radicals
As discussed earlier, free radicals are said to be necessary evil, as they play role in origin and evolution of life. These are important for activating different signaling pathways inside the cell, such as the Mitogen activated protein kinase (MAPK) and extracellular-signal-regulated kinase (ERK) pathways that alter gene expression, as well as in coordination with superoxide dismutase initiates cell death (Cho and Wolkenhauer, 2003). For instance, RNS produced by neurons act as neurotransmitters and those generated by macrophages act as mediators of immunity. These are also responsible for leukocyte adhesion, thrombosis, angiogenesis and vascular tone. Similarly ROS is involved in gene transcription, single transduction and regulation of other activities in cell (Fang et al., 2002).
5.4. Production and scavenging of free radicals
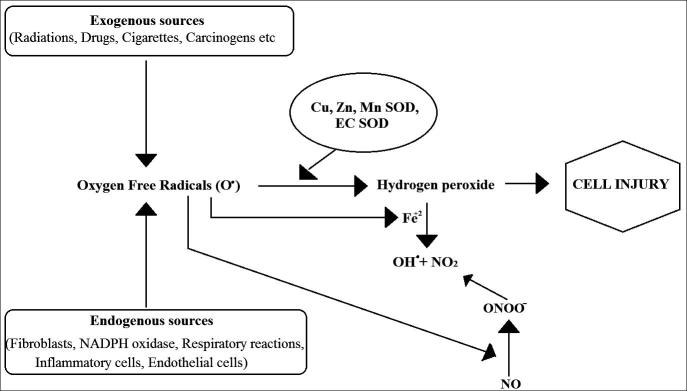
Both exogenous and endogenous substances produce free radicals in cells and its surroundings. They can be produced from non-enzymatic reactions of organic compounds with oxygen as well as those initiated by ionizing radiations (Pham-Huy et al., 2008). This process may also occur in mitochondrion by oxidative phosphorylation. Different sources include radiations, ROS, RNS, Neutrophils and macrophages production, chemicals, smoking of cigarettes, beedi, cigars and industrial effluents (Sen et al., 2010). Now in order to scavenge the deleterious effects of these free radicals, the body has different mechanisms to produce antioxidants, endogenous or exogenous, that will neutralize the elevated amount of free radicals and keep the cells protected against their toxic effects and contributing toward the prevention of diseases (Pham-Huy et al., 2008) (see Fig. 2).
Figure 2.
Free radicals induced cell injury.
6. Oxidative stress and antioxidants
It is a universal truth that oxygen is the major factor that has made the life finite. It is one of the important components of aerobic life. However in some circumstances, this oxygen may be a killer of cells when it generates reactive species that causes necrosis and ultimately the cell death. RNS and RCS also cause oxidation by the generation of certain mechanism that interferes with the normal physiological processes inside the cell (Weseler and Bast, 2010). “Oxidative stress” can be defined as any disturbance in the balance of antioxidants and pro-oxidants in favor of the later due to different factors such as aging, drug actions and toxicity, inflammation and/or addiction (Sies, 1985). It is in general, excess formation or/and insufficient removal of highly reactive molecules such as reactive nitrogen species (RNS) and reactive oxygen species (ROS) (Johansen et al., 2005) Oxygen is highly reactive specie that has the ability to become part of potentially harmful and damaging molecules (Free Radicals). Oxidative stress causes healthy cells of the body to lose their function and structure by attacking them. Up until now, pathogenesis of about more than 50 diseases has been implicated by free radicals (see Table 2). It is when the antioxidant level is limited that this damage can become debilitating and cumulative (Mark Percival, 1996). Damage to DNA, proteins, and other macromolecules due to oxidation has been implicated in the pathogenesis of a wide variety of diseases, most notably cancer and heart disease (Halliwell, 1994).
Table 2.
Oxidative stress induced organ damage (Nosratola et al., 2003).
| Lungs | Asthma, chronic bronchitis |
| Kidneys | Glomerulonephritis, chronic renal failure |
| Joints | Arthritis, rheumatism |
| Brain | Alzheimer’s disease, Parkinson’s disease, memory loss, depression, stroke |
| Eyes | Cataract, retinal diseases |
| Fetus | Preeclampsia, IU growth restriction |
| Heart vessels | Arteriosclerosis, hypertension, ischemia, cardiomyopathy, heart failure |
| Multi-organs | Cancer, diabetes, inflammation infection, aging |
The term “antioxidant” can be labeled for any substance whose availability, even in minute concentration inhibits or delays the oxidation of a substrate. There are several species or molecules, endogenous (internally synthesized) or exogenous (consumed), that play a role in antioxidant defense and may be considered as biomarkers of oxidative stress. Antioxidants can be divided as either chain breaking antioxidants or preventive antioxidants, based on their mechanism of action (Somogyi et al., 2007). Different types of biological antioxidants include, for instance, Glutathione (oxidized/reduced), Vitamin C & E, cystine, etc. (Savita Khanna 2000).
7. Oxidative stress in diabetes mellitus
It is believed that oxidative stress plays important role in the development of vascular complications in diabetes particularly type 2 diabetes (Pham-Huy, 2008). ROS level elevation in diabetes may be due to decrease in destruction or/and increase in the production by catalase (CAT—enzymatic/non-enzymatic), superoxide dismutase (SOD) and glutathione peroxidase (GSH–Px) antioxidants. The variation in the levels of these enzymes makes the tissues susceptible to oxidative stress leading to the development of diabetic complications (Lipinski, 2001). According to epidemiological studies, diabetic mortalities can be explained notably by an increase in vascular diseases other than hyperglycemia (Pham-Huy, 2008).
7.1. Pathophysiology of oxidative stress in diabetes
Nowadays, evidences have been reported that support the role of oxidative stress in the pathogenesis of both type 1 and type 2 diabetes. Free radical formation in diabetes by non-enzymatic glycation of proteins, glucose oxidation and increased lipid peroxidation leads to damage of enzymes, cellular machinery and also increased insulin resistance due to oxidative stress (Maritim et al., 2003). According to latest research, lipid is not only but also the apolipoprotein component of LDL that forms insoluble aggregates oxidatively due to hydroxyl radical-induced cross-linkage between apo-B monomers that is responsible for oxidative damage in diabetic complications (Pham-Huy, 2008). In diabetes mellitus, main sources of oxidative stress are mitochondria. During oxidative metabolism in mitochondria, a component of the utilized oxygen is reduced to water, and the remaining oxygen is transformed to oxygen free radical (O•) which is an important ROS that is converted to other RS such as ONOO−, OH and H2O2 (Moussa, 2008). Insulin signaling is modulated by ROS/RNS by two ways. On one side, in response to insulin, the ROS/RNS are produced to exert its full physiological function and on the other side, the ROS and RNS have got negative regulation on insulin signaling, interpreting them to develop insulin resistance which is a risk factor for diabetes type 2 (Erejuwa, 2012).
7.2. Oxidative stress and diabetic complications
Many evidences from experiments have given link between diabetes and oxidative stress by measuring various biomarkers that include DNA damage biomarkers and lipid peroxidation products. It is believed that in the onset and progression of late diabetic complication, free radicals have got a major role due to their ability to damage lipids, proteins and DNA (Ayepola, 2014). A variety of pathological conditions are induced by oxidative stress such as Rheumatoid arthritis, Diabetes mellitus and cancer (El Faramawy and Rizk, 2011). Free radical and oxidative stress induced complications from DM include coronary artery disease, Neuropathy, nephropathy, retinopathy (Phillips et al., 2004) and stroke (Asfandiyarova et al., 2007). In-vivo studies support the role of hyperglycemia in the generation of oxidative stress leading to endothelial dysfunction in blood vessels of diabetic patients (Ceriello, 2006). Increase in the levels of glucose and insulin along with dyslipidemia in patients suffering from diabetes develops macroangiopathies that cause oxidative stress leading to atherosclerosis (Giugliano et al., 1995).
7.3. Biomarkers of oxidative stress in diabetes mellitus
7.3.1. Proteins
ROS reacts with some amino acid in vitro, producing anything from modified, denatured and non-functioning proteins that in further may be responsible for oxidative stress (Nishigaki et al., 1981) Diabetic hyperglycemia, by the process of free radical production, causes protein glycation and oxidative degeneration. The degree of such protein glycation is estimated by using some biomarkers such as glycated hemoglobin and fructosamine levels. Alteration in function and structure of antioxidant protein enzymes may also be due to nonenzymatic glycation such that detoxification of free radicals is effected enhancing oxidative stress in diabetes (Maritim et al., 2003) According to in vitro studies myeloperoxidase catalyzes the conversion of l-tyrosine to 3,3-dityrosine which serves as a crosslink between polypeptide chains of the same or different proteins making it a convenient biomarker for protein oxidation (Ylä-Herttuala, 1999).
7.3.2. Lipids
Diabetes mellitus produces disturbances in the lipid profile of body making the cells more susceptible to lipid peroxidation (Patricia, 2009). Experimental studies show that polyunsaturated fatty acids in cell membrane are extremely prone to attack by free radicals due to the presence of multiple bonds (Butterfiel et al., 1998). Lipid hyperperoxides (LHP) through intermediate radical reactions produce such fatty acids that generate highly reactive and toxic lipid radicals that form new LHP (Matough et al., 2012). A critical biomarker of oxidative stress is Lipid peroxidation which is the most explored area of research when it comes to ROS (Hatice et al., 2004). Malondialdehyde (MDA) is formed as a result of lipid peroxidation that can be used to measure lipid peroxides after reacting it with thiobarbituric acid (Esterbauer et al., 1991).
7.3.3. Vitamins
Vitamins are very important part of biological system as they play important role in different biochemical processes. Among such vitamins, Vitamin A, C and E act as antioxidants by detoxifying the free radicals. Any alteration in their levels is significant biomarkers of oxidative stress. These vitamins also promote toxicity by producing pro-oxidants in certain conditions. Body levels of vitamin E have been reported to be either increased or decreased by diabetes. However conflicting reports present the deleterious effects of vitamin E on diabetes induced vascular changes (Maritim et al., 2003).
7.3.4. Glutathione
Diabetes induces alterations in activity of enzymes glutathione peroxidase and glutathione reductase. These enzymes are found in cell that metabolizes peroxide to water and converting glutathione disulfide back into glutathione (Maritim et al., 2003). Any alteration in their levels will make the cells prone to oxidative stress and hence cell injury.
7.3.5. Catalase (CAT)
Catalase is regulator of hydrogen peroxide metabolism that can, in excess, cause serious damage to lipids, RNA and DNA. CAT converts H2O2 catalytically into water and oxygen and thus neutralizes it. In case of catalase deficiency, beta cell of pancreas that contain large amount of mitochondria, undergoes oxidative stress by producing excess ROS that leads to β-cells dysfunction and ultimately diabetes (Dana Jamieson, 1986). While investigating hyperglycemia-induced functional changes, hydrogen peroxide production, superoxide, mitochondrial membrane polarization, and gene expression fingerprints of related enzymes in endothelial cells suggest that hyperglycemia increased hydrogen peroxide production and down-regulated CAT gene expression (Patel et al., 2013).
7.3.6. Superoxide dismutase (SOD)
Superoxide dismutase provides first line defense against ROS mediated cell injury by catalyzing the proportion of superoxide, the primary ROS in oxygen metabolism, to molecular oxygen and peroxide. We can say that superoxide is dismutated to other compounds that are less toxic by SODs (Tiwari et al., 2013).
8. Conclusion
Oxidative stress has been demonstrated in many studies to participate in the progression of diabetes which plays important role during diabetes, including impairment of insulin action and elevation of the complication incidence. Antioxidants have already shown to be prospective in the treatment of diabetes both type 1 and type 2. Increase in the levels of oxygen and nitrogen free radicals (ROS/RNS) has been linked with lipid peroxidation, non-enzymatic glycation of proteins and oxidation of glucose which contributes toward diabetes mellitus and its complications. Most of the studies have shown relationship between oxidative stress and diabetes along with their complications related to heart, liver kidney and eye. Thus, oxidative stress seems to be more worrying in metabolic disorders specially diabetes type 2.
Acknowledgment
The authors would like to acknowledge Mrs. Syeda Faiza Asmat for her support while writing this review.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al Homsi M.F., Lukic M.L. Department of Pathology and Medical Microbiology (Immunology Unit), Faculty of Medicine and Health Sciences, UAE University; Al Ain, United Arab Emirates: 1992. An Update on the pathogenesis of Diabetes Mellitus. [Google Scholar]
- American Diabetes Association Diagnosis and classification of diabetes. Diabetes Care. 2004;27(1):S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- Asfandiyarova N., Kolcheva N., Ryazantsev I., Ryazantsev V. Risk factors for stroke in type 2 diabetes mellitus. Diab. Vasc. Dis. Res. 2007;3:57–60. doi: 10.3132/dvdr.2006.009. [DOI] [PubMed] [Google Scholar]
- Ayepola, O.R., Brooks, N.L., Oguntibeju, O.O. 2014. Oxidative Stress and Diabetic Complications: The Role of Antioxidant Vitamins and Flavonoids. <http://dx.doi.org/10.5772/57282>.
- Bansal A.K., Bilaspuri G.S. Impacts of oxidative stress and antioxidants on semen functions (review article) Vet. Med. Int. 2011 doi: 10.4061/2011/686137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfiel Structural and functional changes in proteins induced by free radical-mediated oxidative stress and protective action of the antioxidants N-tert-butyl-alpha-phenylnitrone and vitamin E. Ann. N.Y. Acad. Sci. 1998;854:448–462. doi: 10.1111/j.1749-6632.1998.tb09924.x. [DOI] [PubMed] [Google Scholar]
- Ceriello A. Oxidative stress and diabetes-associated complications. Endocr. Pract. 2006;12(l):60–62. doi: 10.4158/EP.12.S1.60. [DOI] [PubMed] [Google Scholar]
- Cho K.-H., Wolkenhauer O. Analysis and modelling of signal transduction pathways in systems biology. Biochem. Soc. Trans. 2003;31(6):1503–1509. doi: 10.1042/bst0311503. [DOI] [PubMed] [Google Scholar]
- Defronzo R.A., Lily Ferrannini E. Lecture 1987, the triumvirate: beta cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37:667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- Droge Wulf. Free radicals in the physiological control of cell function. Physiol. Rev. 2011;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- El Faramawy S.M., Rizk R.A. Spectrophotometric studies on antioxidants-doped liposomes. J. Am. Sci. 2011;7:363–369. [Google Scholar]
- Erejuwa O.O. Oxidative stress in diabetes mellitus: is there a role for hypoglycemic drugs and/or antioxidants. Oxid. Stress Dis. 2012:217–246. [Google Scholar]
- Esterbauer H., Schaur R.J.r., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Rad. Biol. Med. 1991;11(1):81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Fang Yun-Zhong, Yang Sheng, Wu Guoyao. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- Freidovich I. Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen? Ann. N.Y. Acad. Sci. 1999;893:13. doi: 10.1111/j.1749-6632.1999.tb07814.x. [DOI] [PubMed] [Google Scholar]
- Gill R.G., Haskins K. Molecular mechanisms underlying diabetes and other autoimmune diseases. Immunol. Today. 1993:49–51. doi: 10.1016/0167-5699(93)90056-Q. [DOI] [PubMed] [Google Scholar]
- Giugliano D., Ceriello A., Paolisso G. Diabetes mellitus, hypertension, and cardiovascular disease: which role for oxidative stress? Metabolism. 1995;44(3):363–368. doi: 10.1016/0026-0495(95)90167-1. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. 1994;344:721–724. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- Hatice P. Lipid peroxidation and resistance to oxidation in patients with type 2 diabetes mellitus. Tohuku J. Exp. Med. 2004;203:211–218. doi: 10.1620/tjem.203.211. [DOI] [PubMed] [Google Scholar]
- Jamieson Dana. The relation of free radical production to hyperoxia. Ann. Rev. Physiol. 1986;48:703–719. doi: 10.1146/annurev.ph.48.030186.003415. [DOI] [PubMed] [Google Scholar]
- Johansen Jeanette Schultz. Oxidative stress and the use of antioxidants in diabetes. Cardiovas. Diabetol. 2005;4:5. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S.R., Parikh R.M., Das A.K. Insulin-history, biochemistry, physiology and pharmacology. J. Assoc. Phys. India. 2007;55(L):19. [PubMed] [Google Scholar]
- Kangralkar V.A., Patil S.D., Bandivadekar R.M. Oxidative stress and diabetes: a review. Int. J. Pharm. Appl. 2010;1(1):38–45. [Google Scholar]
- Lipinski B. Pathophysiology of oxidative stress in diabetes mellitus. J. Diabetes its Complications. 2001;15(4):203–210. doi: 10.1016/s1056-8727(01)00143-x. [DOI] [PubMed] [Google Scholar]
- Loghmani, E., 2005. Diabetes Mellitus: Type 1 and Type 2. In: Stang, J., Story, M. (Eds)., Guidelines for Adolescent Nutrition Services 2005.
- Maritim A.C., Sanders R.A., Watkins J.B. Diabetes, oxidative stress, and antioxidants: a review. J. Biochem. Mol. Toxicol. 2003;17(1):24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- Mark Percival, 1996. Antioxidants, Clinical Nutrition insights. NUT031 1/96 Rev. 10/98.
- Matough F.A., Budin S.B., Hamid Z.A., Alwahaibi N., Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ. Med. J. 2012;12(1):5–18. doi: 10.12816/0003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael J.C., James M.C., Robbins VinayK. Pathalogic Basis of Disease. sixth ed. Harcourt Publisher; 2000. The pancreas; pp. 902–929. [Google Scholar]
- Mohan Harsh. fourth ed. Jaypee publishers; 2002. Textbook of Pathology. [Google Scholar]
- Moussa S.A. Oxidative stress in diabetes mellitus. Romanian J. Biophys. 2008;18(3):225–236. [Google Scholar]
- Nishigaki I., Hagihara M., Tsunekawa H., Maseki M., Yagi K. Lipid peroxide levels of serum lipoprotein fractions of diabetic patients. Biochem. Med. 198. 1981;25(3):373–378. doi: 10.1016/0006-2944(81)90096-x. [DOI] [PubMed] [Google Scholar]
- Nosratola D. Oxidative stress and dysregulation of superoxide dismutase and NADPH oxidase in renal insufficiency. Kidney Int. 2003;63:179–185. doi: 10.1046/j.1523-1755.2003.00702.x. [DOI] [PubMed] [Google Scholar]
- Ozougwu J.C., Obimba K.C., Belonwu C.D., Unakalamba C.B. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. J. Physiol. Pathophysiol. 2013;4(4):46–57. [Google Scholar]
- Patel H., Chen J., Das K.C., Kavdia M. Hyperglycemia induces differential change in oxidative stress at gene expression and functional levels in HUVEC and HMVEC. Cardiovasc. Dialectol. 2013;12(1):142–146. doi: 10.1186/1475-2840-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patricia P.M. Reactive species and diabetes: counteracting oxidative stress to improve health. Curr. Opin. Pharmacol. 2009;9:771–779. doi: 10.1016/j.coph.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Pham-Huy L.A., He H., Pham-Huy C. Free radicals, antioxidants in disease and health. IJBS. 2008;4(2):89–96. [PMC free article] [PubMed] [Google Scholar]
- Phillips M., Cataneo R.N., Cheema T., Greenberg J. Increased breath biomarkers of oxidative stress in diabetes mellitus. Clin. Chim. Acta. 2004;344(1-2):189–194. doi: 10.1016/j.cccn.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Savita Khanna, 2000. Thiol Antioxidants, Ph.D. Dissertation. Department of Physiology University of Kuopio, Kuopio, Finland.
- Sen S. Free radicals, antioxidants, diseases and phytomedicines: current status and future prospect. Int. J. Pharm. Sci. Rev. Res. 2010;3(1):91–100. [Google Scholar]
- Sies H. Elsevier; Florida: 1985. Oxidative Stress. [Google Scholar]
- Somogyi Aniḱo. Antioxidant measurements. Physiol. Meas. 2007;28:R41–R55. doi: 10.1088/0967-3334/28/4/R01. [DOI] [PubMed] [Google Scholar]
- Tiwari B.K. Markers of oxidative stress during diabetes mellitus. J. Biomarkers. 2013 doi: 10.1155/2013/378790. (Article ID 378790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J.I. Management of diabetes in elderly. Clin. Diabetes. 2004;17:1. [Google Scholar]
- Weseler A.R., Bast A. Oxidative stress and vascular function: implications for pharmacologic treatments. Curr. Hypertension Rep. 2010;12(3):154–161. doi: 10.1007/s11906-010-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi H., Matsumoto M., Kunimoto K., Kawaguchi J., Makino S., Harada M. Analysis of the roles of CD4+ T cells in autoimmune diabetes of NOD mice using transfer to NOD male mice. Eur. J. Immunol. 1992;22:2387–2393. doi: 10.1002/eji.1830220931. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S. Oxidized LDL and atherogenesis. Ann. N.Y. Acad. Sci. 1999;874:134–137. doi: 10.1111/j.1749-6632.1999.tb09231.x. [DOI] [PubMed] [Google Scholar]