Abstract
Context: Microcrystalline cellulose (MCC) is the most widely used excipient for the production of pellets but it retards the release of poorly water soluble drugs. Objective: The present investigation reports incorporation of camphor, cross carmellose sodium (CCS) and spray dried lactose (SDL) into MCC pellets to enhance the dissolution rate of telmisartan. Materials and methods: A full factorial design (32) was used in the study. Concentration of camphor and CCS was selected as independent variables whereas percentage porosity and percentage drug release at 60 min were selected as dependent variables. Pellets were produced by extrusion–spheronization technique and evaluated for percentage yield, particle size analysis, flow characteristics, percentage porosity, drug content and in vitro drug release. Contour plots and 3-D surface plots were presented for graphical expression of the results. Results and discussion: Pellet formulations exhibited acceptable morphological, flow and mechanical properties. As against to 38.54% drug release after 60 min with MCC pellets, pellets prepared with optimized formulation, composed of proper combination of MCC, SDL, camphor and CCS, released 100% drug after 60 min. Conclusion: Our study underlines the fact that dissolution of telmisartan from MCC pellets can be successfully enhanced by incorporating water soluble excipient, disintegrant and pore formers.
Keywords: Microcrystalline cellulose, Crosscarmellose sodium, Spray dried lactose, Camphor, Factorial design
1. Introduction
Telmisartan is angiotensin II receptor antagonist, which is used in the prevention and treatment of hypertension. The solubility of telmisartan in aqueous solutions is strongly pH-dependent, with maximum solubility observed at high and low pH values. In the physiological pH range its aqueous solubility is 0.078 mg/ml. Its poor dissolution characteristics result into variable absorption and suboptimal bioavailability (Yanzhuo et al., 2010, Masaaki et al., 1998, Kaura et al., 2014). Thus, improving the dissolution behaviour of telmisartan is of therapeutic importance (Wienen et al., 2000, Bala and Sayyad, 2013).
Pellets are small, free-flowing, spherical particulates prepared by the agglomeration of fine powders of drugs and excipients (Sellassie and Knoch, 2002, Benchawan and Pornsak, 2013, Gothi et al., 2010). Microcrystalline cellulose (MCC) is the most widely used excipient for the production of pellets due to plasticity and cohesiveness of its wet masses (Kranz et al., 2009, Alvaro et al., 2011a). However, its applicability is limited particularly in case of poorly water soluble drugs due to its non-disintegration behaviour of the MCC pellets. This, in turn, decreases the release rate of drugs from the pellets (Alvaro et al., 2011b, Jorg et al., 2010). This limitation of MCC pellets has been addressed using a variety of pharmaceutical approaches. They include the use of 40% of isopropanol/water mixture as a granulating binder (Schroder and Kleinebudde, 1995), use of pectinic acid (Tho et al., 2003) and carrageenan (Kranz et al., 2009) instead of MCC and incorporation of disintegrants into MCC pellets (Alvaro et al., 2011a). The present research describes enhancement of dissolution rate of telmisartan from MCC pellets by incorporating a varied proportion of superdisintegrant (crosscarmellose sodium, CCS), pore former (camphor) and water soluble components (spray dried lactose, SDL). To our knowledge, such an approach has not been investigated for dissolution-improvement of telmisartan till date.
Factorial design is an efficient tool for estimating the influence of individual variables and studying their interactions using minimum number of experiments (Singh and Ahuja, 2002). A full factorial design (32) was used in the present study. The concentrations of camphor and crosscarmellose sodium (CCS) were selected as independent variables whereas; percentage porosity and percentage drug release at 60 min were selected as dependent variables in the design. Pellets were prepared using extrusion–spheronization method and evaluated for percentage yield, particle size analysis, flow characteristics, percentage porosity, drug content and in vitro drug release. Contour plots and 3-D surface plots were presented for graphical expression of the results.
2. Materials and methods
2.1. Materials
Microcrystalline cellulose (MCC) PH 101 (Balaji Drugs, Surat, India); spray dried lactose (Signet Chemicals, Mumbai, India); camphor (Su-lab Chemicals, Mumbai, India); crosscarmellose sodium (Yarrow Chem. Products, Mumbai, India) were purchased in present study. Telmisartan was received as a gift sample from Vapi Care Pvt. Ltd. (Vapi, India). All reagents were used of analytical grade and were used as received.
2.2. Methods
2.2.1. Preformulation studies
2.2.1.1. Flow property and compressibility of drug
Flow property and compressibility of telmisartan were established by determining parameters like Hausner’s ratio, Carr’s index and angle of repose (United States Pharmacopoeial Convention, 2008).
2.2.1.2. Drug excipient compatibility studies
2.2.1.2.1. Fourier transform infrared spectroscopy (FT-IR)
Chemical interaction between drug and selected excipients was checked using FTIR studies (Bruker Optics Alpha, Germany). IR spectra of individual sample of telmisartan, MCC PH101 and CCS; and physical mixture of drug with MCC PH 101 and drug with CCS were recorded in the range of 4000–500 cm−1.
2.2.1.2.2. Differential scanning calorimetry (DSC)
Pure drug and physical mixture of drug with MCC were subjected to DSC study. 10 mg sample was heated in an aluminium pan under nitrogen (50 mL/min) at 10 °C/min from 30 to 300 °C (Mettler-Toledo STARSW 9.20, USA). An empty aluminium pan was used as a reference.
2.2.2. Formulation of immediate release pellets (preliminary batches)
Three batches of placebo MCC pellets were prepared for screening of ratio of ethanol to water (40:60, 50:50 and 60:40) as granulating fluid. Pellets were successfully extruded and spheronized and there was no significant difference in % morphology, yield and pellet size of pellets of the three batches. Therefore, 60% ethanol and 40% water was selected (as granulating liquid) for further studies. Higher concentration of ethanol would help for dissolution rate enhancement of drug in further studies.
Five batches of placebo MCC pellets having 10%, 15%, 20%, 25% and 50% of lactose were tried but pellets were not produced in batch with 25% and 50% of lactose due to insufficient plasticity of wet mass. Thus, 20% of spray dried lactose was incorporated for further studies. Higher amount of spray dried lactose would help in dissolution rate enhancement of drug in further studies.
2.2.2.1. Formulation of immediate release pellets (preliminary batches P1–P4)
The required quantities of drug and excipients were weighed according to the batch composition (Table 1) and mixed for 10 min. The dry mixture was moistened with ethanol: water mixture (60:40). The wet mass, thus formed, was extruded through screen with perforations 1 mm in diameter and 1.5 mm in thickness. The extrudates were subjected to spheronization at 700 rpm until spherical pellets were obtained (Cronimach, India). The resulting pellets were dried to constant weight at 50 °C and evaluated for morphology, flow property, compressibility, particle size analysis, % porosity, drug content, and in vitro drug release.
Table 1.
Composition of preliminary batches (P1–P4).
| Batches | Composition of pellets |
||||
|---|---|---|---|---|---|
| Drug (mg) | MCC (mg) | Spray dried lactose | Camphor (mg) | CCS (mg) | |
| N9 | 20 | 280 | – | – | – |
| N10 | 20 | 244 | 56(20%) | ||
| N11 | 20 | 220 | – | 30(10%) | |
| N12 | 20 | 244 | – | – | 36(12%) |
2.2.3. Formulation of pellets prepared using 32 full factorial design
Quantity of drug, MCC and other excipients was selected as per 32 full factorial design. Concentrations of camphor and CCS were varied at three different levels (Table 2) (Vangala et al., 2014, Putta et al., 2012).
Table 2.
Coding of the actual values for 32 full factorial design.
| Factors (Independent variables) | Levels used |
||
|---|---|---|---|
| Low (−1) (%) | Medium (0) (%) | High (+1) (%) | |
| X1 = percentage of camphor | 2 | 6 | 10 |
| X2 = percentage of cross carmellose sodium | 4 | 8 | 12 |
Dependent variables: Y1 = % porosity; Y2 = % drug release at 60 min.
Experiments were conducted using all 9 possible combinations (Table 3). Pellets were prepared in a similar manner as of preliminary batches and evaluated for the same set of parameters.
Table 3.
Composition of factorial batches (F1–F9).
| Batch | Telmisartan (mg) | MCC:Lactose | Camphor (%) | CCS (%) | Ethanol:Water |
|---|---|---|---|---|---|
| F1 | 20 | 80:20 | 2 | 4 | 60:40 |
| F2 | 20 | 80:20 | 2 | 8 | 60:40 |
| F3 | 20 | 80:20 | 2 | 12 | 60:40 |
| F4 | 20 | 80:20 | 6 | 4 | 60:40 |
| F5 | 20 | 80:20 | 6 | 8 | 60:40 |
| F6 | 20 | 80:20 | 6 | 12 | 60:40 |
| F7 | 20 | 80:20 | 10 | 4 | 60:40 |
| F8 | 20 | 80:20 | 10 | 8 | 60:40 |
| F9 | 20 | 80:20 | 10 | 12 | 60:40 |
2.2.4. Evaluation of pellets
2.2.4.1. Morphological characterization of pellets
The pellet shape and surface morphology were observed by optical microscope. Mean particle size and particle size distribution were estimated by sieving method which directly gives weight distribution. Sieves were arranged in a nest with the coarsest at the top. 50 g sample was placed on the top sieve and subjected to mechanical agitation for 10 min. The pellets retained on each sieve were weighed. The pellets were assigned the mesh number of the screen through which it passed to consider undersize. It was expressed in terms of arithmetic mean of the two sieves (Rashid, 2001). Mean particle size was calculated using formula given in Eq. (1).
| (1) |
ΣXiFi = Weight size.
ΣFi = Percentage weight retained.
2.2.4.2. Flow property, compressibility and % porosity
Percentage porosity of pellets was determined by liquid displacement method (Subrahmanyam, 2002). Flow property and compressibility of telmisartan pellets were established by determining parameters like Hausner’s ratio, Carr’s index and angle of repose (United States Pharmacopoeial Convention, 2008).
2.2.4.3. Drug content
Pellets were crushed and powder equivalent to the 20 mg of drug was dissolved in 50 ml of methanol. It was sonicated for 20 min. The volume was adjusted up to mark with methanol. The solution was filtered through Whatmann filter paper and absorbance was recorded at 296 nm after suitable dilution. Percentage drug content was calculated using calibration curve of drug prepared previously.
2.2.4.4. In vitro drug release study
Pellets equivalent to 20 mg of telmisartan were used for the in vitro dissolution studies. Dissolution was performed in 900 ml 0.1 N HCl using USP dissolution apparatus I equipped with a basket operating at the speed of 100 rpm. Temperature of dissolution medium was maintained at 37 ± 0.5 °C. The amount of drug released was measured at suitable time intervals using UV spectrophotometer (UV-3092, Lab India, India).
2.2.5. Statistical analysis
Various response surface methodology (RSM) computations were performed using Design Expert 9.0.2 software (Trial version). Polynomial models, including interactions and quadratic terms, were generated for all the response variables of using multiple linear regression analysis. In order to assess the reliability of the developed mathematical model, three formulations corresponding to random compositions covering entire range of experimental domain were prepared and referred as check-point batches. For each of these formulations, the responses were estimated by the use of generated mathematical models and by the experimental procedures. The linear correlation plots between observed and predicted values of the response properties were plotted using MS Excel 2010. The % Error in prognosis (%PE) was calculated using Eq. (2):
| (2) |
2.2.6. SEM study
The size, shape and surface characteristics of pellets of optimized batch were determined by scanning electron microscopy. Aspect ratio and roundness of pellets were determined using image analysis software (Image J).
2.2.7. Stability studies
Samples of the optimized batch were kept at 40 °C/75% RH and at room temperature for three months in high-density polyethylene (HDPE) bottle. They were analysed for physical characteristics, drug content, disintegration and in vitro drug release at regular interval of 1 month till 3 months (ICH Guideline, 2013).
3. Results and discussion
3.1. Preformulation studies
3.1.1. Flow property and compressibility of drug
Carr’s index, Hausner’s ratio and angle of repose of drug powder were 43.24%, 1.761 and 43°, respectively which indicated poor flow and compressibility of drug.
3.1.2. Drug excipient compatibility studies
3.1.2.1. Differential scanning calorimetry (DSC)
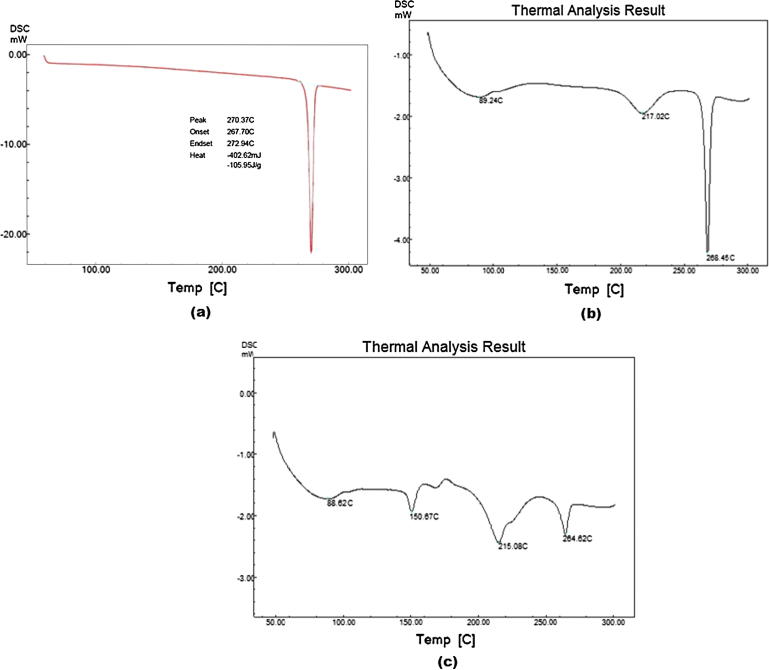
A strong endothermic peak, observed at 78 °C, was indicative of temperature-induced phase transition and melting of telmisartan. Phase transition started at 267 °C and ended at 272 °C (Fig. 1(a)). Endotherm of drug was well-preserved in its physical mixture with MCC PH 101 (Fig. 1(b)) which shows no incompatibility between MCC and telmisartan.
Figure 1.
DSC thermogram of: (a) telmisartan, (b) telmisartan + MCC, and (c) telmisartan + optimized formulation.
3.1.2.2. Fourier transform infrared spectroscopic (FT-IR) analysis
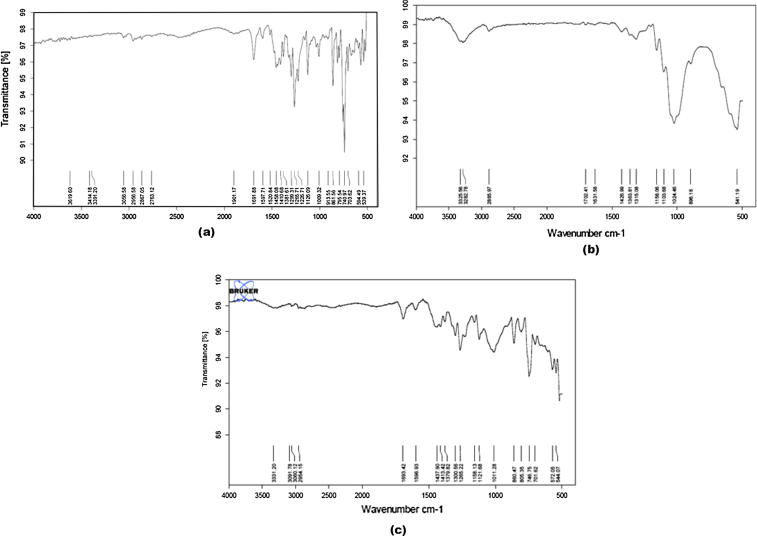
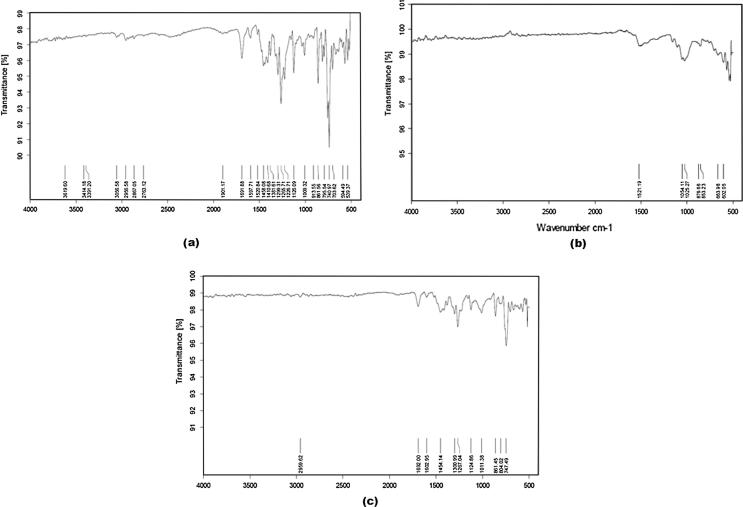
IR spectra of telmisartan showed a characteristic peak at 740 and 757 cm−1 (ring vibration due to 1, 2-disubstituted benzene), 1350–1000 cm−1 (C—N stretching vibrations), 1455 and 1381 cm−1 (CH3 bending vibrations), 1599 cm−1 (C—C aromatic band and stretching), 1460 cm−1 (C—H bend) and 1695 cm−1 (C O stretching vibrations). Characteristic peaks of telmisartan seemed to be preserved in both physical mixtures (drug with MCC and drug with CCS) which proved no chemical interaction of telmisartan with MCC and CCS (Figure 2, Figure 3).
Figure 2.
IR spectra of: (a) telmisartan, (b) MCC, and (c) physical mixture of telmisartan and MCC.
Figure 3.
IR spectra of: (a) telmisartan, (b) CCS, and (c) physical mixture of telmisartan and CCS.
3.2. Evaluation of immediate release pellets (preliminary batches P1–P4)
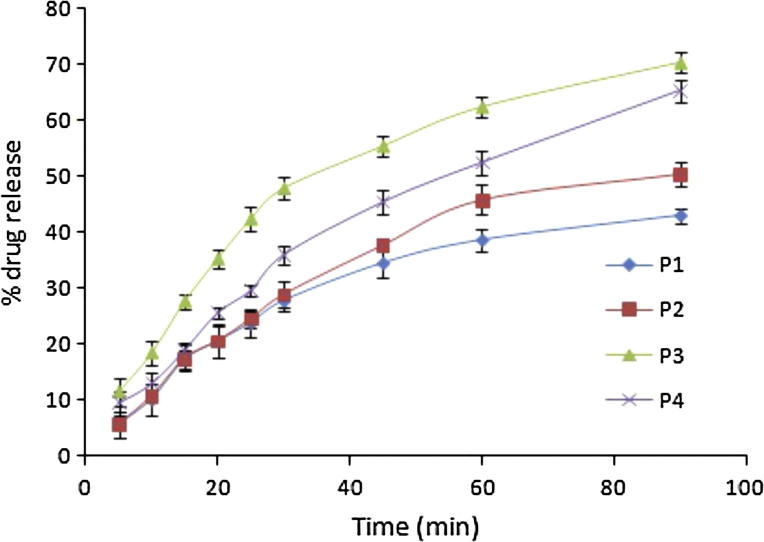
Spherical pellets having smooth surface were observed in optical microscope. Mean pellet size for all batches were observed to be in the range of 927–954 μm. Percentage porosity of batch P1, P2, P3 and P4 was 12.01%, 12.60%, 31.54% and 15.56%, respectively. There was no significant difference in % porosity of pellets of batches P2 and P4 with respect to P1 (p > 0.005). Pellets of batch P3 exhibited maximum (31.54%) porosity which could be due to the presence of camphor in the formulation.
In vitro drug release of batch P1–P4 is displayed in Fig. 4. Only 38.54% drug was released at 60 min in case of pellets prepared with MCC alone. Drug release rate exhibited the following order: MCC + camphor > MCC + CCS > MCC + spray dried lactose > MCC. Hence, it was considered worthwhile to investigate the effect of incorporation of CCS, camphor and MCC into the pellets using factorial design.
Figure 4.
In vitro drug release of preliminary batches (P1–P4).
3.3. Evaluation of pellets prepared using 32 full factorial design
The results of percentage yield, bulk density, tapped density; Hausner’s ratio, Carr’s index and angle of repose of pellets are given in Table 4. Values of Hausner’s ratio, Carr’s index and angle of repose were suggestive of excellent flow properties and compressibility of pellets of all batches. Average pellet size remained within acceptable range. Thus, incorporation of CCS, SDL and camphor to the MCC pellets did not deteriorate any of these characteristics appreciably.
Table 4.
Results of flow properties and particle size of pellets of batches F1–F9.
| Batches | % Yield | Bulk densitya (gm/cm3) | Tapped densitya (gm/cm3) | Carr′s index (%) | Hausner’s ratio | Angle of repose (°)a | Avg. particle size (μm) |
|---|---|---|---|---|---|---|---|
| F1 | 83.64 | 0.701 ± 0.023 | 0.723 ± 0.045 | 1.98 | 1.03 | 18.43 ± 1.25 | 950.06 |
| F2 | 88.24 | 0.817 ± 0.019 | 0.825 ± 0.068 | 1.01 | 1.01 | 18.26 ± 2.35 | 955.84 |
| F3 | 86.32 | 0.649 ± 0.026 | 0.656 ± 0.48 | 1.02 | 1.01 | 18.72 ± 3.15 | 948.07 |
| F4 | 81.42 | 0.656 ± 0.098 | 0.67 ± 0.015 | 2.04 | 1.02 | 16.47 ± 1.87 | 935.67 |
| F5 | 81.67 | 0.618 ± 0.064 | 0.625 ± 0.027 | 1.02 | 1.01 | 18.14 ± 1.54 | 940.45 |
| F6 | 86.9 | 0.670 ± 0.078 | 0.698 ± 0.059 | 3.99 | 1.04 | 18.26 ± 3.25 | 947.72 |
| F7 | 71.99 | 0.798 ± 0.098 | 0.831 ± 0.014 | 3.99 | 1.04 | 18.43 ± 1.53 | 948.88 |
| F8 | 83.56 | 0.659 ± 0.056 | 0.68 ± 0.049 | 3 | 1.03 | 19.87 ± 1.49 | 991.42 |
| F9 | 89.44 | 0.701 ± 0.065 | 0.716 ± 0.020 | 1.99 | 1.02 | 18.43 ± 1.66 | 913.50 |
Mean (n = 3) ± SD.
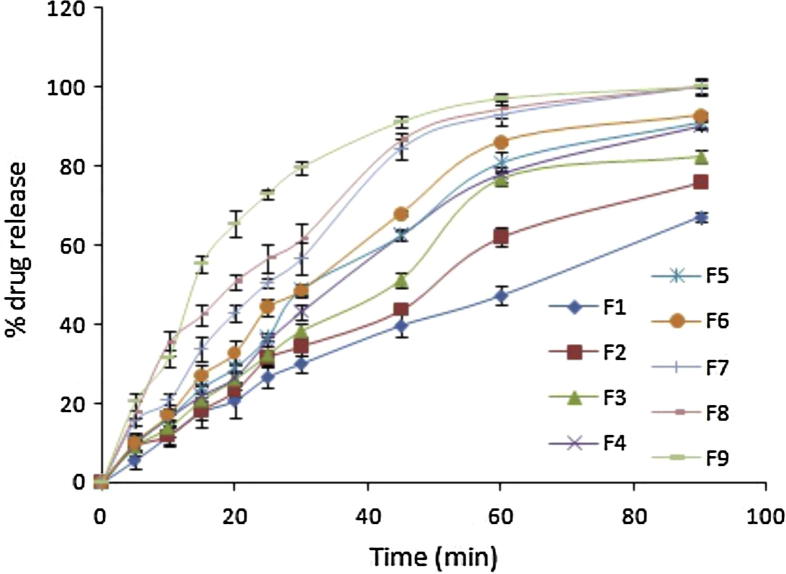
The results of % porosity and % drug content are provided in Table 5 and % drug release is displayed in Fig. 5. More than 80% of drug was released at 60 min in batches F5–F9. As the concentration of camphor and CCS was increased, % porosity and % drug release was increased. It corroborates to the findings of Benchawan and Pornsak (2013) who reported improvement in dissolution and disintegration behaviour of MCC pellets through inclusion of polyethylene glycol, polysorbate and crosscarmellose sodium. In our case, desired results were obtained in batch F9 which contained the highest concentration of camphor and CCS. Percentage drug release of batch F9 was significantly higher compared to preliminary batches P3 and P4. Souto C (Souto et al., 2013) and co-workers studied the effect of crosscarmellose sodium and sodium starch glycolate for increasing the dissolution rate of pellets containing hydrochlorothiazide. However, only slight increment in drug release was observed. Water wicking and swelling are the two possible mechanisms of disintegrant action. The exposure to water causes ingredients to swell and exerts pressure against surrounding of formulation ingredients, which, in turn, results into disintegration of the formulation. Tablets prepared with camphor exhibited rapid disintegration due to an improvement in the ability of water to penetrate into the tablets subsequent to generation of high porosity after sublimation of camphor (Oh et al., 2013, Keiichi et al., 1997). We attributed the improvement drug release characteristics of our formulation to the synergistic effect of camphor and CCS. Bhardwaj et al. (2010) reported this fact for fast dissolving tablet dosage form.
Table 5.
% Porosity and % drug content of batches (F1–F9).
| Batches | % Porositya (n = 3) | % Drug contenta (n = 20) |
|---|---|---|
| F1 | 15.01 ± 2.05 | 86.71 ± 1.69 |
| F2 | 15.13 ± 1.05 | 88.25 ± 2.56 |
| F3 | 16.69 ± 1.45 | 82 ± 1.98 |
| F4 | 20.98 ± 1.98 | 83.98 ± 3.56 |
| F5 | 22.49 ± 2.45 | 90.2 ± 2.05 |
| F6 | 23.49 ± 1.98 | 76.55 ± 2.06 |
| F7 | 25.1 ± 1.58 | 87.1 ± 3.45 |
| F8 | 30.26 ± 2.36 | 80.86 ± 2.15 |
| F9 | 32.33 ± 1.69 | 79.29 ± 2.27 |
Mean ± SD.
Figure 5.
In vitro drug release of factorial batches (F1–F9).
3.4. Statistical analysis
Results of Y1 and Y2 for all batches (F1–F9) showed a wide variation; which indicated that the values of dependent variables were strongly dependent on the independent variables. There was not much difference between the actual and predicted values of Y1 and Y2 which indicated good predictability of the selected model. Responses observed for each of the formulations (F1–F9) were simultaneously fitted to quadratic model using Design Expert 9.0.2.
3.4.1. Data analysis of Y1 (% Porosity)
| (3) |
The observed value for % porosity for all 9 batches (F1–F9) varied from 15.01% to 32.33% among the batches. Correlation coefficient was found to be 0.9904 which showed best fit to the model. Positive sign of X1 and X2 in regression equation (3) indicated agonistic effect of independent variables on response. Out of two independent variables, X1 had higher value of co-efficient (6.81) compared to X2 (1.90) which indicated that X1 had prominent effect on Y1.
3.4.2. Data analysis of Y2 (%drug release at 60 min)
| (4) |
The observed value for % drug release at 60 min varied from 47.19% to 96.95%. The value of correlation coefficient was found to be 0.9885 which showed best fit to the model. Positive sign of X1, and X2 in regression equation (4) indicated agonistic effect of independent variables on response. X1 with higher value of co-efficient (16.36 as against to 6.903 with X2) showed much pronounced effect upon Y2.
3.4.3. Contour plots, 3D-response surface plots and validation of response surface methodology
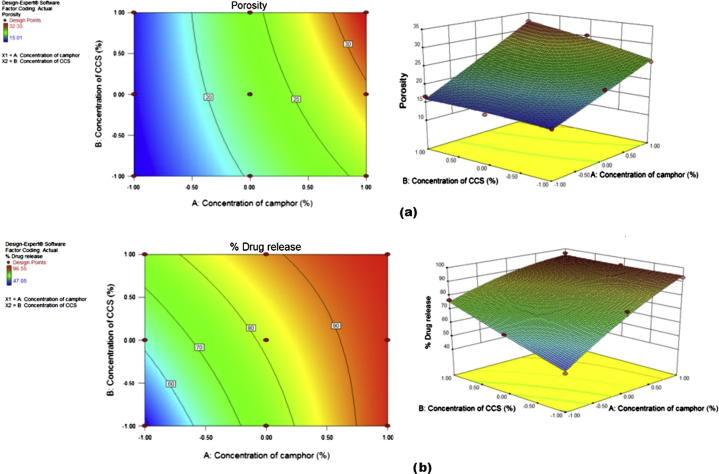
The response surface and contour plots are the graphical representation of the regression equation used to visualize the relationship between the response and experimental levels of each factor. 2-D contour plots and 3-D surface plots were drawn using the design expert 9.0.2 software (Fig. 6). Non-linear relationship was observed between two factors with two responses (% porosity and % drug release at 60 min).
Figure 6.
Contour plots and 3D response surface plots for responses (a) Y1 and (b) Y2.
Pellet composition of the check-point batches, their predicted values and experimental values of all the response variables, and % Error is mentioned in Table 6. There was not much difference between actual and predicted value which indicated reliability of the optimization procedure.
Table 6.
Composition of checkpoint formulations, the predicted and experimental values of response variables and percentage error.
| Checkpoint batches composition (X1:X2) | Response variables | Experimental value | Predicted value | % Error |
|---|---|---|---|---|
| 0.5:0.5 | % Porosity (Y1) % Drug release at 60 min (Y2) |
28.52 92.35 |
27.17 90.57 |
4.73 1.92 |
| −0.5:−0.5 | % Porosity (Y1) % Drug release at 60 min (Y2) |
17.79 65.38 |
18.54 67.32 |
−4.21 −2.96 |
| 0.5:−0.5 | % Porosity (Y1) % Drug release at 60 min (Y2) |
23.06 84.25 |
24.57 86.94 |
−5.16 −3.19 |
3.5. Formulation and evaluation of optimized batch
Based on the inferences derived from Design Expert, optimized formulation (Batch F10) consisted of 9.248% Camphor, 12% CCS, MCC to spray dried lactose ratio of 80:20 and mixture of ethanol–water (60:40) as granulating liquid was prepared and evaluated. The desirable ranges of responses were restricted to maximum porosity and 100% drug release in design expert software. The target range of these responses was restricted to a stricter range than the USP specification, considering the dissolution profiles of the commercial product. Considering the values of Hausner’s ratio (1.01) and Carr’s index (1.28), optimized batch showed excellent flow property and compressibility. Average particle size and drug content of pellets were in acceptable range. In DSC study, no shifting of melting endotherm of drug was conclusive of no incompatibility of drug with any of the excipients (Fig. 1). Though, intensity of the melting peak was reduced which could possibly be due to dilution effect exerted by the excipients. Percentage porosity and percentage drug release of batch F10 were 29.98 and 94.25, respectively. There was not much difference between observed and predicted response for the optimized batch. Keeping in view aforementioned findings, batch F10, was successfully developed to improve dissolution rate of telmisartan from MCC pellets.
3.6. SEM study
SEM photomicrograph (Fig. 7) showed that the pellets were spherical in nature and had a smooth surface. From the photomicrograph image analysis, calculated Aspect Ratio (AR) and Roundness of pellets were found to be 1.006 ± 0.0005 and 0.9812 ± 0.0041, respectively. The obtained AR and roundness of the pellets were closer to the value of 1, which confirmed that the prepared pellets were spherical in nature (Gupta et al., 2011).
Figure 7.

SEM image of pellets of the optimized batch (F10).
3.7. Stability study
No significant changes were observed in appearance, Carr’s index, Hausner’s ratio, average pellets size, drug content and in vitro drug release of optimized pellets at room temperature and at accelerated condition of 40 °C/75% RH for 3 months, indicating stability of batch F10.
4. Conclusion
In the present research work, novel formulations of MCC pellets with faster drug release were successfully developed. The optimized batch (F10) consisted of 9.248% Camphor, 12% CCS, MCC to spray dried lactose ratio of 80:20 and mixture of ethanol–water (60:40) as granulating liquid. Independent variables, concentration of camphor (X1) and concentration of CCS (X2), exhibited a positive effect upon % porosity (Y1) and percentage drug release at 60 min (Y2). As per co-efficient of factorial design, camphor had prominent effect on percentage drug release and percentage porosity. Combination of camphor and CCS into MCC pellets exhibited synergistic effect on responses. Such an approach can be exploited for improving the dissolution rate of other poorly water soluble drugs.
Acknowledgement
The authors are thankful to Vapi Care Pvt. Ltd. (Vapi, India) for providing gift sample of telmisartan.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alvaro G., Consuelo S., Ramon M. A comparison of chitosan-silica and sodium starch glycolate as disintegrants for spheronized extruded microcrystalline cellulose pellets. Drug Dev. Ind. Pharm. 2011;37:825–831. doi: 10.3109/03639045.2010.545415. [DOI] [PubMed] [Google Scholar]
- Alvaro G., Consuelo S., Ramon M. Co-processed MCC-Eudragit E excipients for extrusion–spheronization. Eur. J. Pharm. Biopharm. 2011;79:658–663. doi: 10.1016/j.ejpb.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Bala J., Sayyad F. Enhancement of solubility and dissolution rate of telmisartan by spray drying technique. Indo Am. J. Pharm. Res. 2013;3:1732–1745. [Google Scholar]
- Benchawan C., Pornsak S. Novel disintegrating microcrystalline cellulose pellets with improved drug dissolution performance. Powder Technol. 2013;233:278–285. [Google Scholar]
- Bhardwaj V., Bansal M., Sharma P. Formulation and evaluation of fast dissolving tablets of amlodipine besylate using different super disintegrants and camphor as sublimating agent. Am. Eur. J. Sci. Res. 2010;5:264–269. [Google Scholar]
- Gothi G., Parikh B., Patel T., Patel B., Patel C., Patel D. Pelletization. J. Glob. Pharma. Technol. 2010;2:45–57. [Google Scholar]
- Gupta N.V., Gowda D.V., Balamuralidhara V., Khan S.M. Formulation and evaluation of olanzapine matrix pellets for controlled release. Daru. 2011;19:249–256. [PMC free article] [PubMed] [Google Scholar]
- ICH Guideline. <http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q1C/Step4/Q1C_Guideline.pdf> (Accessed 5th June 2013).
- Jorg C., Mont K., Pornsak S. Effect of drying technique and disintegrant on physical properties and drug release behavior of microcrystalline cellulose-based pellets prepared by extrusion/spheronization. Chem. Eng. Res. Des. 2010;88:100–108. [Google Scholar]
- Kaura M., Bhatiaa R., Raghuvir R., Evans C. Telmisartan complex augments solubility, dissolution and drug delivery in prostate cancer cells. Carbohydr. Polym. 2014;101:614–622. doi: 10.1016/j.carbpol.2013.09.077. [DOI] [PubMed] [Google Scholar]
- Keiichi K., Yoshiteru W., Kumiko M., Naoki U., Mitsuo M. New method of preparing high-porosity rapidly saliva soluble compressed tablets using mannitol with camphor, a subliming material. Int. J. Pharm. 1997;152:127–131. [Google Scholar]
- Kranz H., Jurgens K., Pinier M., Siepmann J. Drug release from MCC- and carrageenan based pellets: experiment and theory. Eur. J. Pharm. Biopharm. 2009;73:302–309. doi: 10.1016/j.ejpb.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Kranz H., Jurgens K., Pinier M., Siepmann J. Drug release from MCC- and carrageenan-based pellets: experiment and theory. Eur. J. Pharm. Biopharm. 2009;73:302–309. doi: 10.1016/j.ejpb.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Masaaki S., Takuya O., Shinji N., Yoshiyuki K., Kingo N. Improvement of dissolution characteristics and bioavailability of poorly water-soluble drugs by novel co-grinding method using water-soluble polymer. Int. J. Pharm. 1998;160:11–19. [Google Scholar]
- Oh T.O., Kim J.Y., Ha J.M., Chi S.C., Rhee Y.S., Park C.W., Park E.S. Preparation of highly porous gastroretentive metformin tablets using a sublimation method. Eur. J. Pharm. Biopharm. 2013;83:460–467. doi: 10.1016/j.ejpb.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Putta R.K., Sood V., Somashekar S., Syed A.A. Formulation and in vitro evaluation of Gemifloxacin fast dissolving tablets for bronchitis and pneumonia treatment in adult population. Novel Sci. Int. J. Pharm. Sci. 2012;1:595–599. [Google Scholar]
- Rashid, H., 2001. Academic Dissertation. Centrifugal Granulating Process for Preparing Drug-Layered Pellets Based on Microcrystalline Cellulose Beads, University of Helsinki Finland. <http://ethesis.helsinki.fi/julkaisut/mat/farma/vk/rashid/centrifu.pdf> (accessed 5th December 2012)
- Schroder M., Kleinebudde P. Structure of disintegrating pellets with regard to fractal geometry. Pharm. Res. 1995;12:1694–1700. doi: 10.1023/a:1016209620953. [DOI] [PubMed] [Google Scholar]
- Sellassie G., Knoch A. In: Encyclopedia of Pharmaceutical Technology. Swarbrick J., editor. Informa Healthcare; New York: 2002. Pelletization techniques; pp. 2651–2663. [Google Scholar]
- Singh B., Ahuja N. Development of controlled-release buccoadhesive hydrophilic matrices of diltiazem hydrochloride: optimization of bioadhesion, dissolution, and diffusion parameters. Drug Dev. Ind. Pharm. 2002;28:431–442. doi: 10.1081/ddc-120003004. [DOI] [PubMed] [Google Scholar]
- Souto C., Rodriguez A., Parajes S., Martinez-Pacheco R. A comparative study of the utility of two superdisintegrants in microcrystalline cellulose pellets prepared by extrusion-spheronization Powder Technology. Eur. J. Pharm. Biopharm. 2013;233:278–285. doi: 10.1016/j.ejpb.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Subrahmanyam C.V.S. first ed. Vallabh Prakashan; India: 2002. Laboratory Manual of Physical Pharmaceutics. [Google Scholar]
- Tho I., Sande S.A., Kleinebudde P. Disintegrating pellets from a water-insoluble pectin derivative produced by extrusion/spheronization. Eur. J. Pharm. Biopharm. 2003;56:371–380. doi: 10.1016/s0939-6411(03)00071-7. [DOI] [PubMed] [Google Scholar]
- United States Pharmacopoeial Convention, 2008. United State Pharmacopoeia 32-National Formulatory 27.
- Vangala M., Veerareddy P.R., Devadasu V.R., Vemula S.K. Meclizine hydrochloride fast dissolving tablets by sublimation method: formulation and evaluation. AJADD. 2014;2:133–144. [Google Scholar]
- Wienen W., Entzeroth M., Meel J., Stangier J., Busch U., Ebner T., Schmid J., Lehmann H., Matzek K., Kempthorne-Rawson J., Gladigau V., Hauel N.H. A review on telmisartan: a novel, long-acting angiotensin II-receptor antagonist. Cardiovasc. Drug Rev. 2000;18:127–154. [Google Scholar]
- Yanzhuo Z., Tongying J., Qiang Z., Siling W. Inclusion of telmisartan in mesocellular foam nanoparticles: drug loading and release property. Eur. J. Pharm. Biopharm. 2010;76:17–23. doi: 10.1016/j.ejpb.2010.05.010. [DOI] [PubMed] [Google Scholar]