Abstract
Inhibition of the apoptosis pathway controlled by opposing members of the Bcl-2 protein family plays a central role in cancer development and resistance to therapy. To investigate how pro-apoptotic Bcl-2 homology domain 3 (BH3)-only proteins impact on acute myeloid leukemia (AML), we generated mixed lineage leukemia (MLL)-AF9 and MLL-ENL AMLs from BH3-only gene knockout mice. Disease development was not accelerated by loss of Bim, Puma, Noxa, Bmf, or combinations thereof; hence these BH3-only proteins are apparently ineffectual as tumor suppressors in this model. We tested the sensitivity of MLL-AF9 AMLs of each genotype in vitro to standard chemotherapeutic drugs and to the proteasome inhibitor bortezomib, with or without the BH3 mimetic ABT-737. Loss of Puma and/or Noxa increased resistance to cytarabine, daunorubicin and etoposide, while loss of Bim protected against cytarabine and loss of Bmf had no impact. ABT-737 increased sensitivity to the genotoxic drugs but was not dependent on any BH3-only protein tested. The AML lines were very sensitive to bortezomib and loss of Noxa conveyed significant resistance. In vivo, several MLL-AF9 AMLs responded well to daunorubicin and this response was highly dependent on Puma and Noxa but not Bim. Combination therapy with ABT-737 provided little added benefit at the daunorubicin dose trialed. Bortezomib also extended survival of AML-bearing mice, albeit less than daunorubicin. In summary, our genetic studies reveal the importance of Puma and Noxa for the action of genotoxics currently used to treat MLL-driven AML and suggest that, while addition of ABT-737-like BH3 mimetics might enhance their efficacy, new Noxa-like BH3 mimetics targeting Mcl-1 might have greater potential.
Acute myeloid leukemia (AML) is a devastating disease primarily affecting children and older people. Although genetically diverse,1, 2 most AMLs are oligoclonal at presentation, with perhaps only two to four driver mutations.1, 3, 4, 5 Chromosomal translocations are common (~50% of cases) and those involving the mixed lineage leukemia (MLL) gene, the mammalian homolog of Drosophila trithorax gene located on chromosome 11 band q23, occur in ~10% of acute leukemias, including AML, acute lymphoblastic leukemia and leukemias of mixed or indeterminate lineage.6 MLL translocations are associated with poor prognosis.6, 7 MLL encodes a large multi-domain protein that activates transcription through its C-terminal histone H3 lysine 4 (H3K4) methyl transferase domain. MLL translocations create a fusion gene containing the 5′ portion of MLL and the 3′ portion of the partner gene.8 The DNA-binding MLL portion of the resulting fusion protein binds MLL target genes, including Hox genes, and the partner moiety enforces constitutive expression through interaction with a higher order transcriptional elongation complex.6, 7, 9 Nearly 80 different MLL fusion partners have been identified in AML,7 two of the most common being AF9 and ENL. Transgenic mice expressing Mll-AF9 and Mll-ENL under the control of the endogenous mll promoter are highly prone to AML, although the long latency indicates a requirement for additional genetic event(s) before the emergence of fully malignant cells.10, 11, 12
Major improvements in AML therapy have remained elusive. Current ‘standard of care' involves an initial phase of intense chemotherapy (remission induction therapy) followed by additional chemotherapy cycles and/or allogeneic stem cell transplantation. Most commonly, induction therapy involves administration of cytarabine with an anthracycline, usually daunorubicin or idarubicin, with etoposide sometimes also included. Because all these drugs act on DNA synthesis, they preferentially affect rapidly dividing cells. Cytarabine (cytosine arabinoside) is phosphorylated intracellularly and incorporated into DNA during S-phase, resulting in chain termination of the elongating nascent DNA chain.13 Anthracyclines and etoposide inhibit topoisomerase II, thereby increasing the frequency of double strand DNA breaks.14 Multiple additional activities have been ascribed to anthracyclines,15 including inhibition of DNA and RNA synthesis as a result of intercalation between base pairs and generation of damaging reactive oxygen species (ROS).16
By provoking DNA damage, ROS and other intracellular stresses, cytotoxic drugs kill cells (at least in part) by inducing the intrinsic (also known as the mitochondrial or stress-induced) apoptosis pathway, which is regulated by pro- and anti-apoptotic members of the Bcl-2 family (for reviews see refs 17, 18, 19). Bcl-2 and its closest relatives (Bcl-xL, Mcl-1, A1/BFL1, Bcl-w and, in humans, possibly also Bcl-B) promote cell survival by inhibiting apoptosis, whereas structurally similar relatives Bax and Bak (and possibly also Bok) instead promote apoptosis, as do the so-called ‘BH3-only proteins' (Bim, Puma, Noxa, Bad, Bid, Bmf, Bik and Hrk), which have only one of the four Bcl-2 homology (BH) domains. In healthy cells, the pro-survival proteins hold Bax and Bak in check. Stress signals – such as DNA damage or oncogene expression – up-regulate expression of Bcl-2 homology domain 3 (BH3)-only proteins, which bind tightly to the hydrophobic surface groove of pro-survival Bcl-2-like proteins, thereby neutralizing their capacity to inhibit activated Bax and Bak. The most potent BH3-only proteins, Bim, Puma and tBid, can bind all pro-survival proteins whereas others show more selective binding:20, 21 Noxa, for example, is specific for Mcl-1 and A1/BFL1, whereas Bad is specific for Bcl-2, Bcl-xL and Bcl-w. Certain BH3-only proteins can also bind transiently to the surface groove of Bax and Bak, inducing them to undergo conformational change and form homodimers on the outer mitochondrial membrane.22, 23 The homodimers then aggregate to form homo-oligomeric pores,24 through which cytochrome c egresses into the cytoplasm to initiate the cascade of caspase activation responsible for dismantling the cell.
A new class of small molecule that mimics BH3-only proteins is generating much clinical interest. BH3 mimetics bind to pro-survival Bcl-2-like proteins in a manner similar to BH3-only proteins, releasing previously sequestered BH3-only proteins to activate Bax and Bak.25, 26 Cancer cells have greater susceptibility to BH3 mimetic drugs than normal cells, in part because they often have higher levels of pro-survival proteins and hence higher ‘stores' of BH3-only proteins.26, 27 ABT-737, the first bonafide BH3 mimetic to be developed, binds Bcl-2, Bcl-xL and Bcl-w but not Mcl-1 or A1,28, 29 as does its orally bio-available derivative ABT-263 (Navitoclax),30 whereas the more recent ABT-199 (Venetoclax) is specific for Bcl-2 (ref. 31) and A-1155463 is specific for Bcl-xL.32 These BH3 mimetics have shown promising efficacy in a variety of preclinical models (eg refs 31, 33) and ABT-263 and ABT-199 are now in advanced clinical trials for chronic lymphocytic leukemia and certain other malignancies.34, 35, 36, 37 High levels of Mcl-1 (or A1/BFL1) are likely to cause resistance26, 29, 38 and BH3 mimetics specific for Mcl-1 are under development.
Using retroviruses expressing MLL-fusion genes,39, 40, 41 we are generating murine AMLs with a variety of apoptotic lesions. Here we report genetic studies designed to clarify whether BH3-only proteins act as tumor suppressors during AML development and which of them are critical for the response of MLL fusion gene-driven AMLs to standard chemotherapeutics (cytarabine, daunorubicin and etoposide) or to the proteasome inhibitor bortezomib, which is being trialed clinically for AML.42, 43 We also tested whether the MLL AMLs are sensitive to ABT-737, and whether combination therapy with ABT-737 improves sensitivity.
Results
Generation of murine AMLs lacking specific BH3-only proteins
MLL-AF9 and MLL-ENL driven AMLs were generated using retroviral transduction of fetal liver cells from wild type (WT) or BH3-only gene knock out (KO) mice and hematopoietic reconstitution of sub-lethally irradiated mice,39, 40, 41 as described in Figure 1a. The reconstituted mice are designated hereafter according to the virus and the genotype of the fetal liver cells (e.g., WT/green fluorescent protein (GFP) indicates mice reconstituted with WT fetal liver cells exposed to control GFP virus and noxa−/−/MLL-AF9 indicates mice reconstituted with noxa−/− fetal liver cells exposed to MLL-AF9 GFP virus).
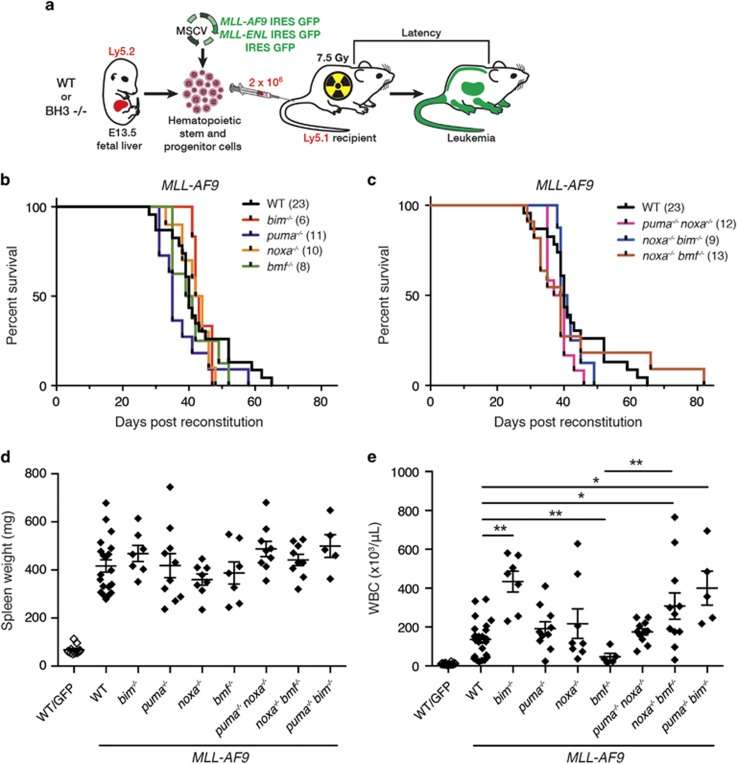
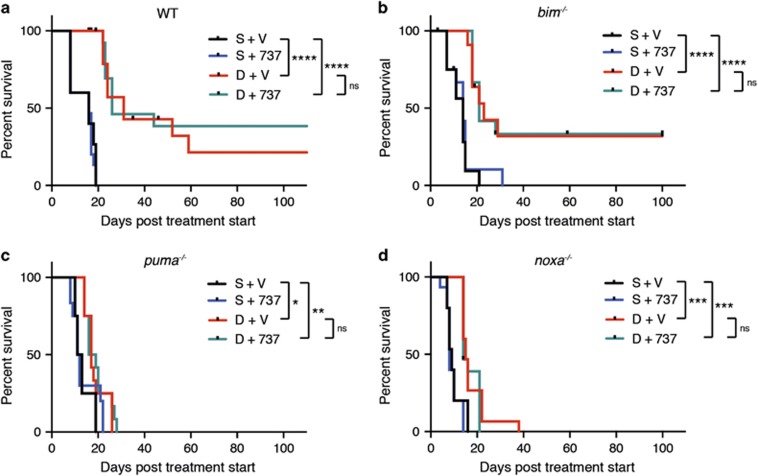
Figure 1.
Generation of murine AMLs. (a) E13.5 fetal liver cells from WT or BH3-only gene KO Ly5.2 C57BL/6 mice were infected with MLL-AF9/GFP, MLL-ENL/GFP or GFP MSCV retrovirus (Materials and methods section) and injected into sub-lethally irradiated (7.5 Gy) C57BL/6-Ly5.1 mice (2 × 106 cells/mouse). (b,c) Kaplan–Meier survival analysis of mice transplanted with fetal liver cells of the indicated genotype after infection with MLL-AF9 virus; number of recipient mice is indicated in brackets. Mice were monitored regularly and euthanized humanely when showing signs of AML-induced stress. (d) Spleen weight and (e) WBC count of individual mice at autopsy. Data points represent individual mice with mean and S.E.M. indicated. P-values were determined by unpaired t test with Welsh's correction for differences in variance. *P<0.05, **P<0.01. Comparable data for mice transplanted with MLL-ENL virus-exposed cells is shown in Supplementary Figure S1
Irrespective of the genotype of the donor stem/progenitor cells, AML developed in all mice reconstituted with MLL-AF9 or MLL-ENL virus-infected cells, most needing to be euthanized within a period of 30–65 days (Figures 1b and c and Supplementary Figure S1a). The sick mice had an enlarged spleen and elevated blood leukocytes (Figures 1d and e,Supplementary Figures S1b and c and Supplementary Table S1), as well as thrombocytopenia and anemia (Supplementary Figure S2). In contrast, GFP mice of all genotypes remained healthy until culled, usually after 120 days.
Loss of Bim, Puma, Noxa, Bmf or combinations thereof, made no significant difference to the kinetics of morbidity (Figures 1b and c and Supplementary Figure S1a) or degree of splenomegaly (Figure 1d,Supplementary Figure S1b), although circulating leukocytes were significantly elevated in sick bim−/−/MLL-AF9 and bim−/−/MLL-ENL mice compared with their WT/MLL-AF9 and WT/MLL-ENL counterparts, principally due to a greater increase in myeloid cells (Mac1+Gr1+; see Supplementary Table S1). Curiously, blood leukocytes were not as elevated in sick bmf −/−/MLL-AF9 mice as in sick WT MLL-AF9 mice but were comparable in bmf−/−/MLL-ENL mice (Figure 1e and Supplementary Figure S1c).
Sick mice were autopsied and tissues subjected to histological and flow cytometric analysis (Supplementary Figure S3,Supplementary Table S1 and data not shown). As reported by others,39, 40, 41 the bone marrow and blood were replete with differentiated myeloid (Mac1+/Gr1+/−) cells. Normal splenic architecture was effaced by these abundant malignant myeloid cells, which also infiltrated the liver, kidney and other tissues. WT and BH3-only gene KO MLL-AF9 and MLL-ENL AMLs had comparable pathology (data not shown).
Expression of apoptosis regulators
To further characterize the MLL-AF9 AMLs, we ascertained the expression pattern of key apoptosis regulators by western blot and quantitative PCR analysis of bone marrow cells from sick primary mice (4–10 per genotype; Figure 2 and Supplementary Figure S4). In addition, western blots were performed on spleen cells (Supplementary Figure S5).
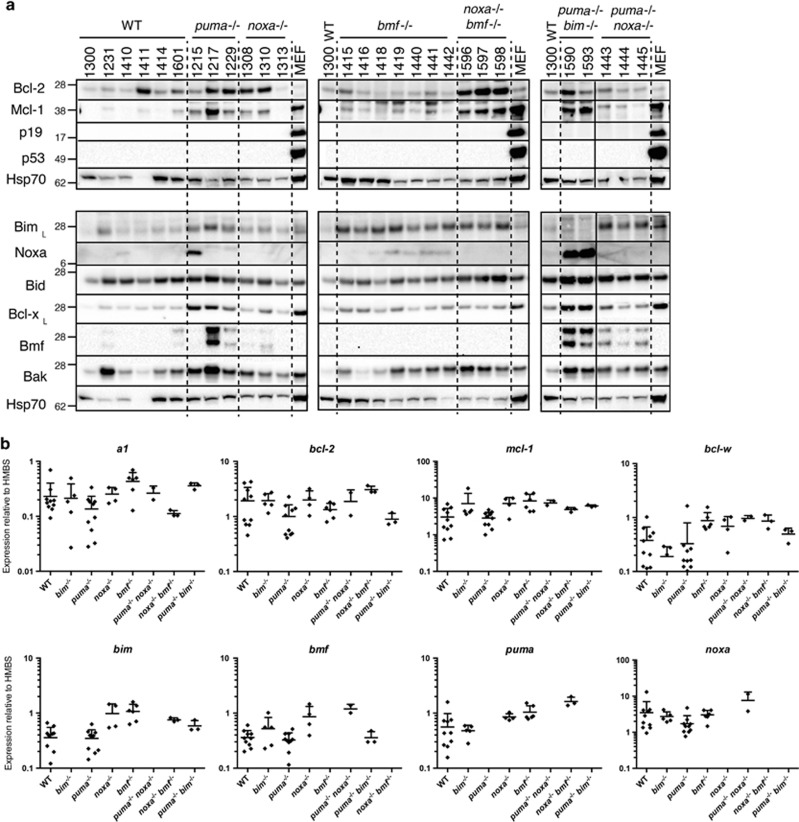
Figure 2.
Expression of apoptosis regulatory genes in MLL-AF9 AMLs. (a) Western blot and (b) qPCR analysis of expression of p53, p19Arf and bcl-2 gene family members in bone marrow cells of sick primary MLL-AF9 AML mice (Supplementary Methods). Protein and RNA were prepared from bone marrow taken at autopsy. Panels in a indicate six separate gels, each of which included WT AML #1300, with dotted lines separating different genotypes (the solid line in panel 3 indicates electronic removal of a lane). SV40 transformed mouse embryonic fibroblasts (MEF) served as positive controls for p53 and p19Arf expression; Hsp70 served as a loading control. Molecular weight (kD) markers are indicated. The following AMLs were analyzed by qPCR: WT #1223, 1224, 1155, 1156, 1231, 1232, 1601, 1410, 1411, 1414; bim−/− #1249, 1250, 1213, 1157, 1158; puma−/− #1230, 1214, 1215, 1216, 1217, 1218, 1225, 1226, 1228, 1229; noxa−/− #1259, 1313, 1308, 1310; bmf−/− #1440, 1441, 1442, 1416, 1419, 1418; puma−/− bim−/− # 1590, 1593; noxa−/− bmf −/− #1596; 1597; 1598; puma−/− noxa−/− #1443; 1444, 1445. qPCR analysis was relative to that of hydroxymethylbilane synthase (HMBS). Error bars indicate S.D. Data for additional Bcl-2 family members is provided in Supplementary Figure S4
Pro-survival Bcl-2 family proteins Bcl-2, Bcl-xL, Mcl-1 and A1 were detected in all AML genotypes analyzed at variable levels. No suitable antibody was available for Bcl-w protein but bcl-w transcript levels were comparable in the WT, bim−/− and puma−/− MLL-AF9 AMLs and somewhat higher in AMLs of other genotypes.
Pro-apoptotic Bax and Bak proteins were readily detected in all AMLs examined. BH3-only proteins Bim, Puma, Bmf and Bad were apparent in most and Bid was seen in all (except those lacking the corresponding gene). Noxa protein was detectable in some bim−/−, puma−/−, bmf −/− and puma−/−bim−/− AMLs but not in any of the WT AMLs, although noxa transcripts were detectable in the WT AMLs.
In general, the AMLs appeared to have higher expression of Bcl-2 family members than Mac1+Gr1+ cells isolated by flow cytometry from normal bone marrow (not shown).
Expression of p53 or p19Arf protein in the absence of an apoptotic stimulus is indicative of mutation or loss of p53 respectively.44 However, none of 27 AMLs tested expressed detectable levels of either of these proteins (Figure 2a), suggesting that mutations affecting the p53 pathway were rare, as is also the situation in human AML.2
Drug sensitivity of WT and BH3-only gene KO AMLs
To ascertain the in vitro drug sensitivity of the AMLs, short-term cell lines were established from multiple primary tumors of each genotype by culturing bone marrow or spleen cells from sick mice (Materials and methods section). The drugs tested included the genotoxic agents cytarabine (cytosine arabinoside), daunorubicin and etoposide as well as ABT-737 and the proteasome inhibitor bortezomib. Drug doses were guided by published data2, 45, 46 and preliminary dose response tests.
Figure 3a shows the kinetics of cell death obtained for WT/MLL-AF9 AMLs (n=4) exposed to each of these drugs. Killing with ABT-737 was more rapid than with the other agents, particularly cytarabine, but then plateaued. For subsequent studies, a 16 h time point was chosen and the cytarabine concentration was increased to 600 ng/ml to increase its efficacy.
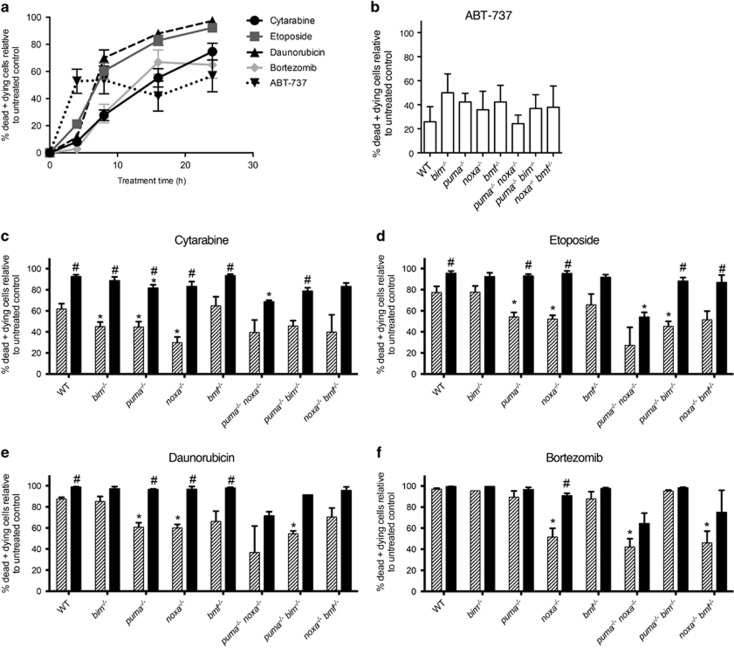
Figure 3.
In vitro sensitivity of MLL-AF9 AML cell lines to cytotoxic agents. Drug sensitivity tests were performed using short-term cell lines established from bone marrow of sick primary MLL-AF9 AML mice (Materials and methods section). (a) WT/MLL-AF9 AMLs (n=4) were treated with standard chemotherapeutic drugs cytarabine (300 ng/ml), etoposide (300 ng/ml), daunorubicin (50 ng/ml), bortezomib (5 nM) or BH3 mimetic ABT-737 (1 μg/ml), harvested at 4, 8, 16 and 24 h and analyzed by flow cytometry following staining with annexin V-Alexa Fluor 647 and propidium iodide (dying and dead cells are positive for annexin V or both markers). Results are expressed as percentage of dead and dying cells relative to that of cells cultured in parallel in medium alone. Value shown is mean±S.E.M. (b–f) AMLs of the indicated genotypes were cultured for 16 h in the presence of ABT-737 alone (b); or with cytarabine (c), etoposide (d), daunorubicin (e) or the proteasome inhibitor bortezomib (f), each alone (gray) or in combination with ABT-737 (black). The percentage of dead and dying cells is expressed relative to cells of the same genotype incubated in the absence of drug(s). Values represent mean±S.E.M. Data plotted for ABT-737 alone were pooled from all experiments (n=8 independent lines for WT and n=2–6 independent lines for other genotypes). The number of independent lines used for other drugs±ABT-737 were: WT n=5; bim−/− n=3, except with bortezomib where n=1; puma−/− n=3; noxa−/− n=3; bmf−/− n=4; puma−/−noxa−/− n=2; puma−/−bim−/− n=2; noxa−/−bmf−/− n=3. P-values were calculated using an unpaired t test with Welch's correction. *P<0.05 between WT AMLs and the indicated BH3-only gene KO AMLs in response to a particular drug or combination; #P<0.05 for drug in combination with ABT-737 versus just the single drug alone for the same genotype
To assess which BH3-only proteins might be critical for individual drug regimens, we compared the viability of the various BH3-only gene KO/MLL-AF9 AMLs with those of WT/MLL-AF9 AMLs treated in parallel. Three or more independent lines were tested for each genotype, except the puma−/−noxa−/− and puma−/−bim−/− lines for which only two were available. Figure 3b shows the results for treatment with ABT-737 alone and Figure 3c–f for treatment with cytarabine, etoposide, daunorubicin or bortezomib, either alone (gray) or in combination with ABT-737 (black). An asterisk above a column indicates significantly greater resistance for specific BH3-only gene KO AMLs than WT AMLs and a hash indicates significantly better response of the indicated genotype to the combination therapy than to the single drug.
Neither Bim, Puma, Noxa or Bmf was essential for ABT-737 cytotoxic activity, since none of the corresponding gene KO AMLs displayed significant resistance to this BH3 mimetic, and neither did any of the double BH3-only gene KO AML lines tested (Figure 3b).
Loss of Puma and/or Noxa increased resistance to the DNA damaging agents cytarabine, etoposide and daunorubicin. Loss of Bim increased resistance to cytarabine but not etoposide or daunorubicin and loss of Bmf had no significant impact for any of the drugs (Figures 3c–e).
Encouragingly, WT AMLs were highly sensitive to treatment with bortezomib, as were bim−/−, puma−/− and bmf−/− AMLs (Figure 3f). Only loss of Noxa conferred significant resistance to bortezomib, suggesting that, for this type of leukemia, Noxa is the primary trigger for apoptosis mediated via bortezomib.
ABT-737 improved the response of WT/MLL-AF9 AMLs to cytarabine, etoposide and daunorubicin (P<0.05). It also enhanced the sensitivity of puma−/−, noxa−/− and bmf−/− AMLs to each of these drugs and of bim−/− AMLs to cytarabine. Furthermore, despite their resistance to bortezomib as a single agent, noxa−/−/MLL-AF9 AMLs appeared sensitive to the combination.
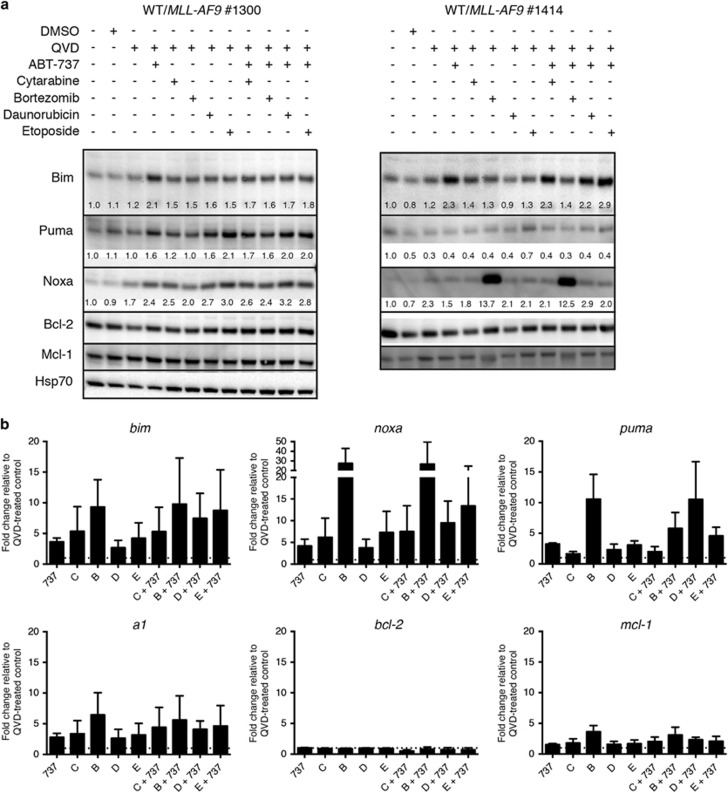
In an effort to better understand the responses triggered by the various drugs, three WT/MLL-AF9 cell lines (#1300, #1414 and #1411) and one bmf −/−/MLL-AF9 line (#1415) were treated in the presence of the pan-caspase inhibitor QVD-OPH and analyzed after 6 h by western blot (Figure 4a and Supplementary Figure S6). Exposure to bortezomib markedly increased the levels of Noxa protein in two lines (#1414 and #1415) but not the others. Puma levels modestly increased in response to etoposide and Bim increased with ABT-737. Bcl-2 and Mcl-1 protein levels remained relatively constant in the face of all agents.
Figure 4.
Expression of Bcl-2 family members following treatment. Expression of Bcl-2 family members in cultured primary WT/MLL-AF9 AMLs following treatment with cytotoxic agents. (a) Western blot analysis of protein expression in AMLs #1300 and #1414 after 6 h treatment with the indicated drugs. Numbers below bands indicate the intensity of each lane relative to the untreated control for each antibody. Quantitation was performed using Image Lab software. (b) qPCR analysis of RNA expression in AMLs #1300, #1411 and #1601 after 3 h treatment. ΔΔCT values normalized to HMBS control and made relative to cells treated with QVD only to determine drug-induced fold change. Data represents mean fold change±S.E.M., with dashed line indicating a value of 1; note the different axis for noxa expression. Comparable PCR analyzes of BH3-only gene KO/MLL-AF9 AMLs are summarized in Supplementary Figure S7. AML cell lines maintained in culture in IMDM with 10% FCS and supplemental IL-3 (Materials and methods section) were treated with 600 ng/ml cytarabine [C], 25 nM bortezomib [B], 200 ng/ml daunorubicin [D], 300 ng/ml etoposide [E], either alone or in combination with 1 μg/ml ABT-737 [737] as indicated, in presence of the pan-caspase inhibitor QVD-OPH (25 μM)
Comparable qPCR studies were performed 3 h after treatment for three WT/MLL-AF9 (Figure 4b) and various BH3-only gene KO/MLL-AF9 lines (Supplementary Figure S7 and data not shown). Noxa transcripts increased markedly in the WT AMLs following bortezomib treatment (Figure 4b) and also in the bim−/− AMLs (Supplementary Figure S7) but only modestly in the puma−/− AMLs. Puma RNA was elevated in WT AMLs in response to bortezomib (Figure 4b) and in Bim-deficient AMLs in response to most agents, particularly cytarabine, bortezomib and the various drug combinations. Smaller, more variable increases in bim, a1 and mcl-1 RNAs were noted in response to most agents but there was no significant change in bad, bid, bmf, bcl-2, bcl-w, bcl-xLRNAs, for either WT or BH3-only gene KO AMLs.
In vivo treatment of MLL-AF9 AMLs
We next embarked on in vivo trials comparing the efficacy of traditional and novel therapeutic regimens for treating AML-bearing mice. Figure 5 presents results obtained for daunorubicin, either alone or in combination with ABT-737 (see Supplementary Figure S8 for results obtained for individual AMLs). Healthy mice were injected with 0.5 × 106 bone marrow cells from sick secondary MLL-AF9 mice (3 recipients per AML per treatment arm) and treatment was started 3 days later. Mice received 5 mg/kg body weight daunorubicin intravenously on days 1, 4 and 9 and either ABT-737 (75 mg/kg body weight) or vehicle intraperitoneally on days 1–5 and 8–12.
Figure 5.
In vivo treatment of MLL-AF9 AMLs with daunorubicin in combination with ABT-737. Kaplan–Meier survival curves for mice transplanted with MLL-AF9 AMLs. 8–10-week-old Ly5.1 C57BL/6 mice (non-irradiated) were injected with 0.5 × 106 bone marrow cells from sick secondary WT/MLL-AF9 or BH3-only gene KO/MLL-AF9 mice (three recipients per tumor per treatment arm) and treatment was started 3 days later: 5 mg/kg daunorubicin [D] intravenously on days 1, 4 and 9 and/or 75 mg/kg ABT-737 [737] intraperitoneally on days 1–5 and 8–12; controls received saline [S] and ABT-737 vehicle [V]. Transplanted mice were monitored daily for symptoms of AML and euthanized if morbidly ill or at the end of experiment (100 days post treatment start). A total of (a) 5 WT/MLL-AF9 AMLs (#1211, 1223, 1224, 1411 and 1414), (b) 4 bim−/− MLL-AF9 AMLs (#1158, 1213, 1249 and 1250), (c) 4 puma−/− MLL-AF9 AMLs (#1218, 1225, 1226 and 1229) and (d) 5 noxa−/− MLL-AF9 AMLs (#1306,1308, 1309, 1310 and 1311) were tested (see also Supplementary Figure S8 for individual tumor results). Statistical significance was determined by Log-rank (Mantle-Cox) test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Daunorubicin treatment alone, or in combination with ABT-737, significantly prolonged survival of WT/MLL-AF9 and bim−/−/MLL-AF9 AMLs compared with saline treatment (P<0.0001), puma−/−/MLL-AF9 had significantly prolonged survival when treated with daunorubicin alone (P=0.0173) or with daunorubicin in combination with ABT-737 (P=0.0026), as did noxa−/−/MLL-AF9 when treated with daunorubicin alone (P=0.0006) or in combination with ABT-737 (P=0.0001). For all genotypes there was no significant difference (P>0.5) in survival between daunorubicin treatment and treatment with the combination of daunorubicin and ABT-737
For the WT/MLL-AF9 AMLs (Figure 5a), controls receiving saline and vehicle (black) all died within 20 days and those treated with ABT-737 alone (blue) fared no better. In contrast, daunorubicin (red) significantly extended lifespan; of the 15 mice transplanted with five different WT/MLL-AF9 AMLs, almost 50% survived for more than 30 days, and two were still alive at the end of the experiment (100 days; Figure 5a).
Treatment with ABT-737 as well as daunorubicin did not significantly increase survival over daunorubicin alone (compare aqua and red lines), although more mice achieved long-term survival (5/15 versus 2/15). No overt correlation was evident between the degree of responsiveness of individual AMLs (Supplementary Figure S8) and their pattern of expression of pro-survival Bcl-2 proteins or BH3-only proteins (Figure 2 and Supplementary Figure S5). RNASeq analysis is being undertaken to attempt to account for the differences in responsiveness.
Of note, while MLL-AF9 AMLs lacking Bim (Figure 5b) responded similarly to WT/MLL-AF9 AMLs, those lacking either Puma (Figure 5c) or Noxa (Figure 5d), were very resistant to daunorubicin, even when it was combined with ABT-737. Thus, Puma and Noxa are critical for the cytotoxic action of daunorubicin.
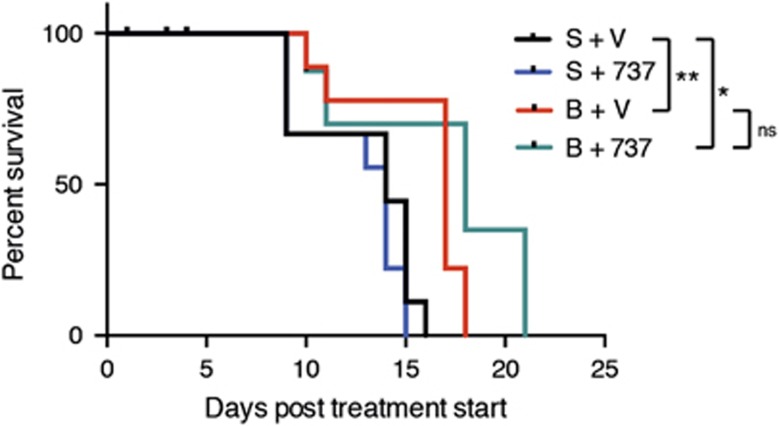
In view of the high sensitivity of the AML lines to bortezomib in vitro, we were keen to test its efficacy in vivo. Unfortunately, tests performed on healthy C57BL/6 mice indicated relatively poor tolerance of the drug, evidenced by severe weight loss. AML-bearing mice were given the maximum tolerated dose (0.75 mg/kg) and any mice euthanized early due to weight loss (usually before 7 days) were censored. Figure 6 summarizes the outcome for mice developing AML following transplantation with three different WT/MLL-AF9 AMLs. Treatment with bortezomib provided a significant extension of life although, at this dose, efficacy was not as great as with daunorubicin, and combination therapy with ABT-737 provided no additional benefit.
Figure 6.
In vivo treatment of WT/MLL-AF9 AMLs with bortezomib in combination with ABT-737. Kaplan–Meier survival curves for mice transplanted with WT/MLL-AF9 AMLs and treated with bortezomib with or without ABT-737. 8–10-week-old Ly5.2 C57BL/6 mice (non-irradiated) were injected with 0.5 × 106 bone marrow cells from sick secondary WT/MLL-AF9 mice (3–6 recipients per tumor per treatment arm) and treatment was started 3 days later: 0.75 mg/kg bortezomib [B] or saline [S] intravenously on days 1, 4, 8 and 11 of treatment and/or 75 mg/kg ABT-737 [737] or vehicle [V] intraperitoneally on days 1–5 and 8–12 of treatment. Transplanted mice were monitored daily and euthanized if morbidly ill, either from bortezomib toxicity within the first week (censored) or from typical AML. A total of 3 WT/MLL-AF9 AMLs (#1224, 1223 and 1414) were tested. Statistical significance was determined by Log-rank (Mantel–Cox) test. Treatment with bortezomib versus vehicle significantly prolonged survival (P=0.002), as did treatment with bortezomib plus ABT-737 (P=0.019). There was no significant difference between treatment with bortezomib alone compared with the combination of bortezomib and ABT-737 (P=0.116)
Discussion
In this study, we tested the impact of loss of individual BH3-only proteins on the development and treatment of AMLs driven by MLL-fusion genes. The AMLs were generated by transplanting WT or BH3-only gene KO fetal liver cells infected with MLL-AF9 or MLL-ENL virus into sub-lethally irradiated recipient mice. Of note, loss of Bim, Puma, Noxa, Bmf, or combinations thereof, made no significant difference to the kinetics of morbidity (Figure 1 and Supplementary Figure S1), all recipients developing florid AML within 30–65 days. These results differ markedly from those obtained for lymphomagenesis in Eμ-myc mice, where disease was accelerated by loss of Bim, Puma or Bmf, although not Noxa.47, 48, 49 Thus, in contrast to Myc-driven lymphomagenesis, none of the BH3-only proteins tested appear to serve as a critical tumor suppressor for the development of MLL-driven AML. This is suggestive of redundant roles. However, the faster morbidity of the retroviral AML model (median 6 weeks) compared with the Eμ-myc lymphoma model (median 15 weeks) may have masked a tumor suppressor role.
To ascertain the sensitivity of the MLL fusion protein-driven AMLs to conventional and targeted cytotoxics, we first tested primary cell lines established by culturing bone marrow cells from sick reconstituted mice in medium supplemented with IL-3. Each of the standard chemotherapeutic drugs was relatively effective at killing WT/MLL-AF9 AMLs, etoposide and daunorubicin more so than cytarabine at the concentrations tested. As might be predicted for DNA damaging agents such as these, loss of either Puma or Noxa, both p53 targets, increased resistance. Loss of Bim increased resistance to cytarabine but not to etoposide or daunorubicin, whereas loss of Bmf had no significant impact. ABT-737 was not very effective as a single agent but significantly improved the response to the standard agents suggesting that it may improve clinical response or allow lower concentrations of traditional cytotoxics to be used.
Bortezomib was highly effective at killing WT/MLL-AF9 AMLs in vitro. Significantly, whereas puma−/−, bim−/− and bmf−/− AMLs were as sensitive as WT/MLL-AF9 AMLs, Noxa-deficient AMLs displayed increased resistance. Noxa RNA levels increased markedly within 3 h in primary WT/MLL-AF9 lines exposed to bortezomib and high levels of Noxa protein were observed within 6 h in two bortezomib-treated lines (one WT and the other bmf−/−) but not in two other WT lines that were nevertheless highly sensitive to bortezomib (Figure 4a and Supplementary Figure S6). Differential kinetics of Noxa induction or breakdown may explain the varied results.
Several mechanisms have been implicated in the cytotoxicity induced by proteasome inhibitors, including stabilization of p53 and IκB, and induction of ER stress and the unfolded protein response (reviewed in refs 50, 51), all of which trigger apoptosis through the Bcl-2 family-regulated pathway. The apparent lack of Puma-dependence implies that Noxa induction by bortezomib in MLL-AF9 AML cells occurs through a p53-independent mechanism. Noxa transcription can be upregulated by c-myc in tumor cells in response to bortezomib.52
Noxa strongly binds to and inhibits anti-apoptotic Mcl-1,20, 21 thereby facilitating activation of Bak and Bax. Since Noxa promotes Mcl-1 degradation via the BH3-only E3 ubiquitin ligase Mule,53 Mcl-1 levels might be expected to rise in bortezomib-treated cells but this was not apparent for the AML lines (Figure 4a). Presumably bortezomib-mediated Noxa up-regulation titrates the level of Mcl-1 sufficiently to kill the cells.
We transplanted five independent WT/ MLL-AF9 AMLs into immunocompetent mice to test their sensitivity in vivo to daunorubicin and bortezomib, both alone or in combination with ABT-737. Daunorubicin treatment significantly prolonged the life of most AML-bearing mice, some of which (7/30) survived until the end of experiment (100 days post treatment; Figure 5). Although the addition of ABT-737 did not further improve survival significantly, given the in vitro results discussed above it would be worthwhile testing whether combination therapy enabled a lower dose of daunorubicin to be effective in vivo.
Importantly, our data clearly show, for the first time, that effective killing of MLL-AF9 AML cells by daunorubicin in vivo is dependent on expression of Puma and Noxa, as the AMLs lacking these BH3-only genes were more refractory to treatment (compare Figures 5c and d with a). Bim appears to play no role (Figure 5b), in contrast to the situation observed for Eμ-myc lymphomas where Bim, Puma and Noxa were all involved in killing by DNA damage-inducing drugs.54 Although not as efficacious as daunorubicin, bortezomib also extended the life of AML-transplanted mice (Figure 6) and the in vitro data suggests this response is highly Noxa-dependent.
In summary, our mouse genetic studies have revealed the importance of the BH3-only proteins Puma and Noxa for the efficacy of cytotoxic drugs currently used to treat MLL-AF9 AML. Our tests with the BH3 mimetic ABT-737, which is specific for Bcl-2, Bcl-xL and Bcl-w,28, 29 suggest that its orally available derivative, ABT-263 (ref. 30) or Bcl-2-specific ABT-199,31 may prove beneficial in combination therapy with either genotoxic or other drugs. However, given the widespread and robust expression of Mcl-1 and A1 in AMLs, their dependence on Mcl-1 (ref. 41) and the susceptibility of MLL-AF9 AMLs to Noxa-induced killing (this paper), Noxa-like BH3 mimetics currently being developed to target Mcl-1 (and/or A1/BFL1) may prove more efficacious.
Materials and Methods
Mice
The noxa−/−,55 puma−/−,55 bim−/− (ref. 56) and bmf−/− (ref. 57) mice have been described previously; all were maintained on a C57BL/6 J (Ly5.2) background at the Walter and Eliza Hall Institute (WEHI). C57BL/6-Ly5.1 mice were originally obtained from Jackson Laboratories. Experimental protocols were approved by WEHI's Animal Ethics Committee.
AML generation
MSCV retroviruses encoding internal ribosomal entry site (IRES)/GFP, MLL-AF9/IRES/GFP39 or MLL-ENL/IRES/GFP DNA58 were prepared by transfection of Phoenix cells.59 For infection, cells prepared from three cryopreserved fetal livers of each genotype in stem cell medium (SCM; Iscove's modified Dulbecco's medium (IMDM) containing 20% fetal bovine serum, 100 ng/ml stem cell factor, 50 ng/ml thrombopoietin and 50 ng/ml Flt-3 ligand, 10 ng/ml IL-6; all obtained from WEHI cytokine facility), were incubated at 37 °C in 10% CO2 for 24 h, plated at 1 × 106 viable cells/ml into 12-well plates coated with retronectin containing an equal volume (1 ml) of fresh virus-containing supernatant in SCM and then polybrene was added to 4 μg/ml. Following centrifugation at 2500 r.p.m. (1360g) for 1 h at 32 °C, the cells were incubated overnight at 37 °C. A second ‘spin infection' was performed the next day, using fresh virus. After incubation overnight, cells were detached using a rubber policeman, washed in PBS, resuspended at 107 viable cells/ml then injected into the tail vein of sub-lethally irradiated (7.5 Gy) Ly5.1 mice (200 μl/mouse). Mice were monitored regularly for AML symptoms: hunched stance, ruffled coat, lethargy, anemia and splenomegaly. Mouse survival analysis utilized GraphPad Prism and significance was determined using Log-rank (Mantel–Cox) test.
In vitro drug treatment
Bone marrow-derived primary AML cells were cultured in IMDM supplemented with 10% fetal bovine serum (FBS) and IL-3-containing culture supernatant (WEHI). Aliquots of 100 μl were plated into 96-well plates (1 × 105 cells/well) and incubated for 16 h with 600 ng/ml cytarabine, 300 ng/ml etoposide, 50 ng/ml daunorubicin or 5 nM bortezomib, either alone or in combination with 1 μg/ml ABT-737. Following treatment, cells were washed once with balanced salt solution (150 mM NaCl, 3.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 7.4 mM HEPES.NaOH, 1.2 mM KH2PO4 and 0.8 mM K2HPO4) containing 5% FBS and resuspended in the same medium with Annexin V-Alexa Fluor 647 (kindly provided by Daniel Gray, WEHI) and 4 μg/ml propidium iodide. Cell viability was determined on an LSR I flow cytometer using FlowJo software.
In vivo drug treatment
Primary tumors (T0) were expanded by transplanting 2 × 106 spleen cells via tail vein injection into C57BL/6 Ly5.1 mice. Bone marrow cells of sick recipients (T1) were cryopreserved. For treatment studies, non-irradiated mice (8–10 week female C57BL/6 Ly5.1) were injected intravenously with 0.5 × 106 bone marrow-derived T1 tumor cells and drug regimens commenced 3 days later: on days 1, 4 and 9, 5 mg/kg body weight daunorubicin or an equal volume (100 μl) of saline was injected intravenously followed by a flush of saline (700 μl) using a butterfly catheter; on days 1, 4, 8 and 11, 0.75 mg/kg body weight bortezomib or an equal volume (100 μl) of saline was injected intravenously; on days 1–5 and 8–12, 75 mg/kg body weight ABT-737 (dissolved in 10% dimethyl sulfoxide and 90% vehicle and titrated to pH 4.0 using 1 M Hepes) or an equal volume of vehicle (65% glucose-anhydrous BP 1.0, 30% propylene glycol, 5% Tween 80)33 was injected intraperitoneally. The daunorubicin and bortezomib doses were based on initial dose escalation toxicity tests in healthy C57BL/6 mice; tail necrosis, the limiting toxicity for daunorubicin, was subsequently overcome by flushing with saline; the ABT-737 dose was based on published data.60 Mice were euthanized when showing severe AML symptoms, significant weight loss (>15% of initial body weight), or after the experimental end point (100 days).
Molecular analysis
Procedures used for immunoblotting and RT-PCR are provided in the Supplementary Methods.
Acknowledgments
We thank our institute colleagues A Roberts, A Strasser and JM Adams for useful discussions and review of the manuscript; K Hughes, G Siciliano, J Corbin and J McManus for excellent technical assistance; and the institute's flow cytometry and histology facilities for skilled support. This work was supported by funding from the NHMRC (Australia; program grant 1016701 and project grant 1058746); US Leukemia and Lymphoma Society Specialized Center for Research Grant 7001-13; Leukemia Foundation of Australia; and infrastructure support to the institute from the NHMRC Independent Research Institute Infrastructure Support Scheme (IRISS 9000220) and the Victorian State Government Operational Infrastructure Support (OIS). The Walter and Eliza Hall Institute has received research funding from Genentech and Abbott Laboratories (now Abbvie) for the development of BH3 mimetics and as a consequence now receives commercial income.
Glossary
- BH3
Bcl-2 homology domain 3
- MLL
Mixed lineage leukemia
- MLL-ENL
Fusion gene combining MLL with MLLT1
- MLL-AF9
Fusion gene combining MLL with MLLT3
- AML
Acute myeloid leukemia
- KO
Knock out
- WT
Wild type
- GFP
Green fluorescent protein
- IRES
Internal ribosomal entry site
- SCM
Stem cell medium
- IMDM
Iscove's modified Dulbecco's medium
- FBS
Fetal bovine serum
- ROS
reactive oxygen species
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by G Raschella'
Supplementary Material
References
- Cancer Genome Atlas Research N., Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 2013; 368: 2059–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwade D, Ivey A, Huntly BJ. Molecular landscape of acute myeloid leukemia in younger adults and its clinical relevance. Blood 2016; 127: 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC et al. The origin and evolution of mutations in acute myeloid leukemia. Cell 2012; 150: 264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G, Luc S, Marco E, Lin TW, Peng C, Kerenyi MA et al. Mapping cellular hierarchy by single-cell analysis of the cell surface repertoire. Cell Stem Cell 2013; 13: 492–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman DH, Greystoke BF, Somervaille TC. The variety of leukemic stem cells in myeloid malignancy. Oncogene 2014; 33: 3091–3098. [DOI] [PubMed] [Google Scholar]
- Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nat Rev Cancer 2007; 7: 823–833. [DOI] [PubMed] [Google Scholar]
- Meyer C, Hofmann J, Burmeister T, Groger D, Park TS, Emerenciano M et al. The MLL recombinome of acute leukemias in 2013. Leukemia 2013; 27: 2165–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RL, Vaughan AT. A systematic description of MLL fusion gene formation. Crit Rev Oncol Hematol 2014; 91: 283–291. [DOI] [PubMed] [Google Scholar]
- Yokoyama A, Lin M, Naresh A, Kitabayashi I, Cleary ML. A higher-order complex containing AF4 and ENL family proteins with P-TEFb facilitates oncogenic and physiologic MLL-dependent transcription. Cancer Cell 2010; 17: 198–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral J, Lavenir I, Impey H, Warren AJ, Forster A, Larson TA et al. An Mll-AF9 fusion gene made by homologous recombination causes acute leukemia in chimeric mice: a method to create fusion oncogenes. Cell 1996; 85: 853–861. [DOI] [PubMed] [Google Scholar]
- Forster A, Pannell R, Drynan LF, McCormack M, Collins EC, Daser A et al. Engineering de novo reciprocal chromosomal translocations associated with Mll to replicate primary events of human cancer. Cancer Cell 2003; 3: 449–458. [DOI] [PubMed] [Google Scholar]
- Chen W, Kumar AR, Hudson WA, Li Q, Wu B, Staggs RA et al. Malignant transformation initiated by Mll-AF9: gene dosage and critical target cells. Cancer Cell 2008; 13: 432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese ND, Schiller GJ. High-dose cytarabine (HD araC) in the treatment of leukemias: a review. Curr Hematol Malig Rep 2013; 8: 141–148. [DOI] [PubMed] [Google Scholar]
- Burden DA, Osheroff N. Mechanism of action of eukaryotic topoisomerase II and drugs targeted to the enzyme. Biochim Biophys Acta 1998; 1400: 139–154. [DOI] [PubMed] [Google Scholar]
- Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol 1999; 57: 727–741. [DOI] [PubMed] [Google Scholar]
- Ferraro C, Quemeneur L, Prigent AF, Taverne C, Revillard JP, Bonnefoy-Berard N. Anthracyclines trigger apoptosis of both G0-G1 and cycling peripheral blood lymphocytes and induce massive deletion of mature T and B cells. Cancer Res 2000; 60: 1901–1907. [PubMed] [Google Scholar]
- Strasser A, Cory S, Adams JM. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J 2011; 30: 3667–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbridge AR, Strasser A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ 2015; 22: 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldoveanu T, Follis AV, Kriwacki RW, Green DR. Many players in BCL-2 family affairs. Trends Biochem Sci 2014; 39: 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG et al. Differential targeting of pro-survival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Molecular Cell 2005; 17: 393–403. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR et al. BH3 domains of BH3-only proteins differentially regulate bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell 2005; 17: 525–535. [DOI] [PubMed] [Google Scholar]
- Czabotar PE, Westphal D, Dewson G, Ma S, Hockings C, Fairlie WD et al. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell 2013; 152: 519–531. [DOI] [PubMed] [Google Scholar]
- Brouwer JM, Westphal D, Dewson G, Robin AY, Uren RT, Bartolo R et al. Bak core and latch domains separate during activation, and freed core domains form symmetric homodimers. Mol Cell 2014; 55: 938–946. [DOI] [PubMed] [Google Scholar]
- Westphal D, Kluck RM, Dewson G. Building blocks of the apoptotic pore: how Bax and Bak are activated and oligomerize during apoptosis. Cell Death Differ 2014; 21: 196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov 2008; 7: 989–1000. [DOI] [PubMed] [Google Scholar]
- Merino D, Khaw SL, Glaser SP, Anderson DJ, Belmont LD, Wong C et al. Bcl-2, Bcl-x(L), and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood 2012; 119: 5807–5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Gaizo Moore V, Schlis KD, Sallan SE, Armstrong SA, Letai A. BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia. Blood 2008; 111: 2300–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005; 435: 677–681. [DOI] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 2006; 10: 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 2008; 68: 3421–3428. [DOI] [PubMed] [Google Scholar]
- Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 2013; 19: 202–208. [DOI] [PubMed] [Google Scholar]
- Leverson JD, Phillips DC, Mitten MJ, Boghaert ER, Diaz D, Tahir SK et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med 2015; 7: 279ra40. [DOI] [PubMed] [Google Scholar]
- Vandenberg CJ, Cory S. ABT-199, a new Bcl-2-specific BH3 mimetic, has in vivo efficacy against aggressive Myc-driven mouse lymphomas without provoking thrombocytopenia. Blood 2013; 121: 2285–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids MS, Roberts AW, Anderson MA, Pagel JM, Kahl BS, Gerecitano JF et al. The BCL-2-specific BH3-mimetic ABT-199 (GDC-0199) is active and well-tolerated in patients with relapsed non-hodgkin lymphoma: interim results of a phase I study. Blood 2012; 120: 2.22767573 [Google Scholar]
- Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol 2012; 30: 488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AW, Advani RH, Kahl BS, Persky D, Sweetenham JW, Carney DA et al. Phase 1 study of the safety, pharmacokinetics, and antitumour activity of the BCL2 inhibitor navitoclax in combination with rituximab in patients with relapsed or refractory CD20+ lymphoid malignancies. Br J Haematol 2015; 170: 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med 2016; 374: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooswinkel RW, van de Kooij B, Verheij M, Borst J. Bcl-2 is a better ABT-737 target than Bcl-xL or Bcl-w and only Noxa overcomes resistance mediated by Mcl-1, Bfl-1, or Bcl-B. Cell Death Dis 2012; 3: e366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somervaille TC, Cleary ML. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell 2006; 10: 257–268. [DOI] [PubMed] [Google Scholar]
- Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature 2006; 442: 818–822. [DOI] [PubMed] [Google Scholar]
- Glaser S, Lee EF, Trounson E, Bouillet P, Wei A, Fairlie WD et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev 2012; 26: 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard DS, Liesveld J, Phillips GL2nd, Hayslip J, Weiss H, Jordan CT et al. A phase I study using bortezomib with weekly idarubicin for treatment of elderly patients with acute myeloid leukemia. Leuk Res 2013; 37: 1502–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton TM, Perentesis JP, Gamis AS, Alonzo TA, Gerbing RB, Ballard J et al. A Phase 2 study of bortezomib combined with either idarubicin/cytarabine or cytarabine/etoposide in children with relapsed, refractory or secondary acute myeloid leukemia: a report from the Children's Oncology Group. Pediatr Blood Cancer 2014; 61: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ. Tumor surveillance via the ARF-p53 pathway. Genes Dev 1998; 12: 2984–2991. [DOI] [PubMed] [Google Scholar]
- Ramakers-van Woerden NL, Beverloo HB, Veerman AJ, Camitta BM, Loonen AH, van Wering ER et al. In vitro drug-resistance profile in infant acute lymphoblastic leukemia in relation to age, MLL rearrangements and immunophenotype. Leukemia 2004; 18: 521–529. [DOI] [PubMed] [Google Scholar]
- Jayanthan A, Incoronato A, Singh A, Blackmore C, Bernoux D, Lewis V et al. Cytotoxicity, drug combinability, and biological correlates of ABT-737 against acute lymphoblastic leukemia cells with MLL rearrangement. Pediatr Blood Cancer 2011; 56: 353–360. [DOI] [PubMed] [Google Scholar]
- Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci USA 2004; 101: 6164–6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak EM, Jansen ES, Happo L, Cragg MS, Tai L, Smyth GK et al. Puma and to a lesser extent Noxa are suppressors of Myc-induced lymphomagenesis. Cell Death Differ 2009; 16: 684–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenzel A, Labi V, Chmelewskij W, Ploner C, Geley S, Fiegl H et al. Suppression of B-cell lymphomagenesis by the BH3-only proteins Bmf and Bad. Blood 2010; 115: 995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujtaba T, Dou QP. Advances in the understanding of mechanisms and therapeutic use of bortezomib. Discov Med 2011; 12: 471–480. [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Mora-Jensen H, Weniger MA, Perez-Galan P, Wolford C, Hai T et al. ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc Natl Acad Sci USA 2009; 106: 2200–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforov MA, Riblett M, Tang WH, Gratchouck V, Zhuang D, Fernandez Y et al. Tumor cell-selective regulation of NOXA by c-MYC in response to proteasome inhibition. Proc Natl Acad Sci USA 2007; 104: 19488–19493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Bougie P, Menoret E, Juin P, Dousset C, Pellat-Deceunynck C, Amiot M. Noxa controls Mule-dependent Mcl-1 ubiquitination through the regulation of the Mcl-1/USP9X interaction. Biochem Biophys Res Commun 2011; 413: 460–464. [DOI] [PubMed] [Google Scholar]
- Happo L, Cragg MS, Phipson B, Haga JM, Jansen ES, Herold MJ et al. Maximal killing of lymphoma cells by DNA-damage inducing therapy requires not only the p53 targets Puma and Noxa but also Bim. Blood 2010; 116: 5256–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 2003; 302: 1036–1038. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Metcalf D, Huang DCS, Tarlinton DM, Kay TWH, Köntgen F et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 1999; 286: 1735–1738. [DOI] [PubMed] [Google Scholar]
- Labi V, Erlacher M, Kiessling S, Manzl C, Frenzel A, O'Reilly L et al. Loss of the BH3-only protein Bmf impairs B cell homeostasis and accelerates gamma irradiation-induced thymic lymphoma development. J Exp Med 2008; 205: 641–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavau C, Luo RT, Du C, Thirman MJ. Retrovirus-mediated gene transfer of MLL-ELL transforms primary myeloid progenitors and causes acute myeloid leukemias in mice. Proc Natl Acad Sci USA 2000; 97: 10984–10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift S, Lorens J, Achacoso P, Nolan GP. Rapid production of retroviruses for efficient gene delivery to mammalian cells using 293 T cell-based systems. Curr Protoc Immunol 2001; 31:VI:10.17C:10.17.14–10.17.29. [DOI] [PubMed]
- Mason KD, Vandenberg CJ, Scott CL, Wei AH, Cory S, Huang DC et al. In vivo efficacy of the Bcl-2 antagonist ABT-737 against aggressive Myc-driven lymphomas. Proc Natl Acad Sci USA 2008; 105: 17961–17966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.