Abstract
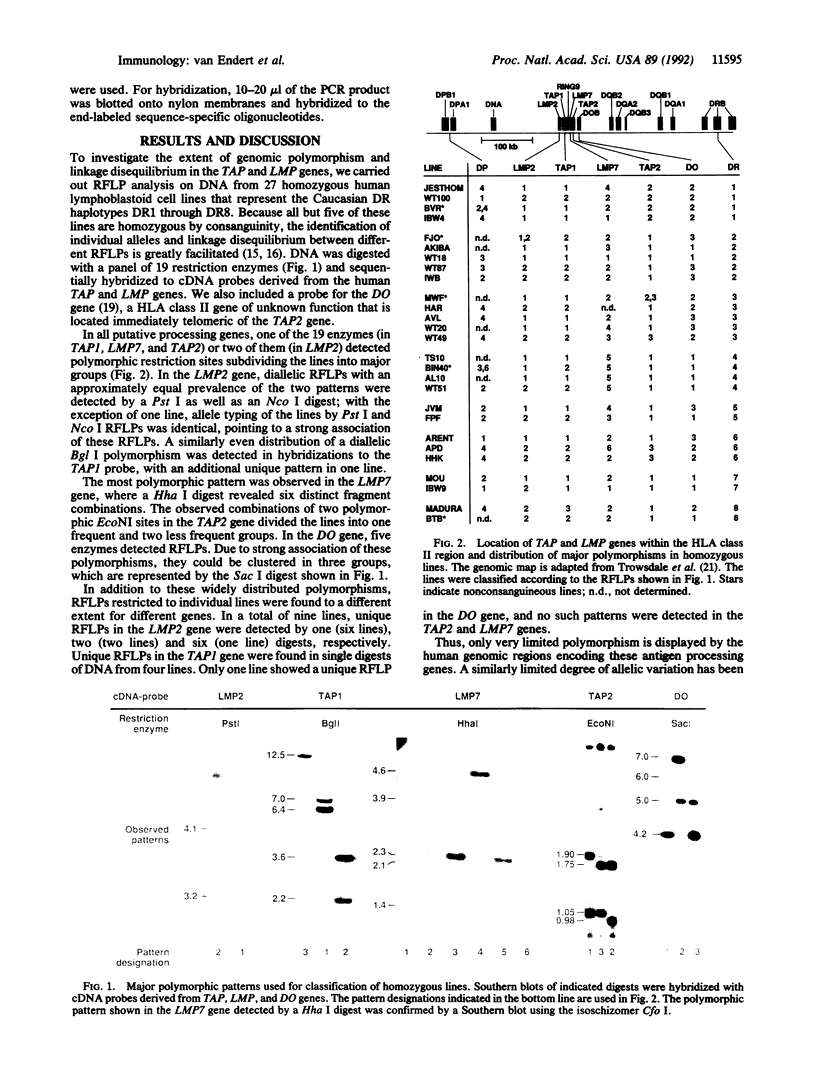
Recently, two subunits of a large cytosolic protease and two putative peptide transporter proteins were found to be encoded by genes within the class II region of the major histocompatibility complex (MHC). These genes have been suggested to be involved in the processing of antigenic proteins for presentation by MHC class I molecules. Because of the high degree of polymorphism in MHC genes, and previous evidence for both functional and polypeptide sequence polymorphism in the proteins encoded by the antigen-processing genes, we tested DNA from 27 consanguineous human cell lines for genomic polymorphism by restriction fragment length polymorphism (RFLP) analysis. These studies demonstrate a strong linkage disequilibrium between TAP1 and LMP2 RFLPs. Moreover, RFLPs, as well as a polymorphic stop codon in the telomeric TAP2 gene, appear to be in linkage disequilibrium with HLA-DR alleles and RFLPs in the HLA-DO gene. A high rate of recombination, however, seems to occur in the center of the complex, between the TAP1 and TAP2 genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attaya M., Jameson S., Martinez C. K., Hermel E., Aldrich C., Forman J., Lindahl K. F., Bevan M. J., Monaco J. J. Ham-2 corrects the class I antigen-processing defect in RMA-S cells. Nature. 1992 Feb 13;355(6361):647–649. doi: 10.1038/355647a0. [DOI] [PubMed] [Google Scholar]
- Bahram S., Arnold D., Bresnahan M., Strominger J. L., Spies T. Two putative subunits of a peptide pump encoded in the human major histocompatibility complex class II region. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10094–10098. doi: 10.1073/pnas.88.22.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. I., Denney D., Jr, Foster L., Belt T., Todd J. A., McDevitt H. O. Allelic variation in the DR subregion of the human major histocompatibility complex. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6234–6238. doi: 10.1073/pnas.84.17.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. I., Denney D., Jr, MacMurray A., Foster L., Watling D., McDevitt H. O. Molecular mapping of class II polymorphisms in the human major histocompatibility complex. I. DR beta. J Immunol. 1987 Jul 15;139(2):562–573. [PubMed] [Google Scholar]
- Bodmer J. G., Marsh S. G., Albert E. D., Bodmer W. F., Dupont B., Erlich H. A., Mach B., Mayr W. R., Parham P., Sasazuki T. Nomenclature for factors of the HLA system, 1991. WHO Nomenclature Committee for factors of the HLA system. Tissue Antigens. 1992 Apr;39(4):161–173. doi: 10.1111/j.1399-0039.1992.tb01932.x. [DOI] [PubMed] [Google Scholar]
- Brown M. G., Driscoll J., Monaco J. J. Structural and serological similarity of MHC-linked LMP and proteasome (multicatalytic proteinase) complexes. Nature. 1991 Sep 26;353(6342):355–357. doi: 10.1038/353355a0. [DOI] [PubMed] [Google Scholar]
- Collins F. S. Cystic fibrosis: molecular biology and therapeutic implications. Science. 1992 May 8;256(5058):774–779. doi: 10.1126/science.1375392. [DOI] [PubMed] [Google Scholar]
- Colonna M., Bresnahan M., Bahram S., Strominger J. L., Spies T. Allelic variants of the human putative peptide transporter involved in antigen processing. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3932–3936. doi: 10.1073/pnas.89.9.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynne R., Powis S. H., Beck S., Kelly A., Kerr L. A., Trowsdale J. A proteasome-related gene between the two ABC transporter loci in the class II region of the human MHC. Nature. 1991 Sep 26;353(6342):357–360. doi: 10.1038/353357a0. [DOI] [PubMed] [Google Scholar]
- Kelly A., Powis S. H., Glynne R., Radley E., Beck S., Trowsdale J. Second proteasome-related gene in the human MHC class II region. Nature. 1991 Oct 17;353(6345):667–668. doi: 10.1038/353667a0. [DOI] [PubMed] [Google Scholar]
- Lindahl K. F. His and hers recombinational hotspots. Trends Genet. 1991 Sep;7(9):273–276. doi: 10.1016/0168-9525(91)90306-B. [DOI] [PubMed] [Google Scholar]
- Livingstone A. M., Powis S. J., Diamond A. G., Butcher G. W., Howard J. C. A trans-acting major histocompatibility complex-linked gene whose alleles determine gain and loss changes in the antigenic structure of a classical class I molecule. J Exp Med. 1989 Sep 1;170(3):777–795. doi: 10.1084/jem.170.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMurray A. J., Bell J. I., Denney D., Jr, Watling D., Foster L. S., McDevitt H. O. Molecular mapping class II polymorphisms in the human major histocompatibility complex. II. DQ beta. J Immunol. 1987 Jul 15;139(2):574–586. [PubMed] [Google Scholar]
- Martinez C. K., Monaco J. J. Homology of proteasome subunits to a major histocompatibility complex-linked LMP gene. Nature. 1991 Oct 17;353(6345):664–667. doi: 10.1038/353664a0. [DOI] [PubMed] [Google Scholar]
- Monaco J. J., McDevitt H. O. Identification of a fourth class of proteins linked to the murine major histocompatibility complex. Proc Natl Acad Sci U S A. 1982 May;79(9):3001–3005. doi: 10.1073/pnas.79.9.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Navarrete V., Seelig A., Gernold M., Frentzel S., Kloetzel P. M., Hämmerling G. J. Subunit of the '20S' proteasome (multicatalytic proteinase) encoded by the major histocompatibility complex. Nature. 1991 Oct 17;353(6345):662–664. doi: 10.1038/353662a0. [DOI] [PubMed] [Google Scholar]
- Powis S. J., Deverson E. V., Coadwell W. J., Ciruela A., Huskisson N. S., Smith H., Butcher G. W., Howard J. C. Effect of polymorphism of an MHC-linked transporter on the peptides assembled in a class I molecule. Nature. 1992 May 21;357(6375):211–215. doi: 10.1038/357211a0. [DOI] [PubMed] [Google Scholar]
- Powis S. J., Townsend A. R., Deverson E. V., Bastin J., Butcher G. W., Howard J. C. Restoration of antigen presentation to the mutant cell line RMA-S by an MHC-linked transporter. Nature. 1991 Dec 19;354(6354):528–531. doi: 10.1038/354528a0. [DOI] [PubMed] [Google Scholar]
- Spies T., Bresnahan M., Bahram S., Arnold D., Blanck G., Mellins E., Pious D., DeMars R. A gene in the human major histocompatibility complex class II region controlling the class I antigen presentation pathway. Nature. 1990 Dec 20;348(6303):744–747. doi: 10.1038/348744a0. [DOI] [PubMed] [Google Scholar]
- Spies T., DeMars R. Restored expression of major histocompatibility class I molecules by gene transfer of a putative peptide transporter. Nature. 1991 May 23;351(6324):323–324. doi: 10.1038/351323a0. [DOI] [PubMed] [Google Scholar]
- Tonnelle C., DeMars R., Long E. O. DO beta: a new beta chain gene in HLA-D with a distinct regulation of expression. EMBO J. 1985 Nov;4(11):2839–2847. doi: 10.1002/j.1460-2075.1985.tb04012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowsdale J., Hanson I., Mockridge I., Beck S., Townsend A., Kelly A. Sequences encoded in the class II region of the MHC related to the 'ABC' superfamily of transporters. Nature. 1990 Dec 20;348(6303):741–744. doi: 10.1038/348741a0. [DOI] [PubMed] [Google Scholar]
- Trowsdale J., Ragoussis J., Campbell R. D. Map of the human MHC. Immunol Today. 1991 Dec;12(12):443–446. doi: 10.1016/0167-5699(91)90017-n. [DOI] [PubMed] [Google Scholar]