Abstract
Background
Mycoplasma hominis, a well known cause of neonatal infection, has been reported as a pathogen in urogenital infections in adults; however, central nervous system (CNS) infections are rare. We report here the first case of M. hominis meningitis in China, post neurosurgical treatment for an intracerebral haemorrhage in a 71-year-old male.
Case presentation
We describe a 71-year-old man who developed M. hominis meningitis after neurosurgical treatment and was successfully treated with combined azithromycin and minocycline therapy of 2 weeks duration, despite delayed treatment because the Gram stain of cerebrospinal fluid (CSF) yielded no visible organisms. The diagnosis required 16S rDNA sequencing analysis of the cultured isolate from CSF. Literature review of M. hominis CNS infections yielded 19 cases (13 instances of brain abscess, 3 of meningitis, 1 spinal cord abscess and 1 subdural empyema each). Delay in diagnosis and initial treatment failure was evident in all cases. With appropriate microbiological testing, antibiotic therapy (ranging from 5 days to 12 weeks) and often, multiple surgical interventions, almost all the patients improved immediately.
Conclusions
Both our patient findings and the literature review, highlighted the pathogenic potential of M. hominis together with the challenges prompted by rare infectious diseases in particular for developing countries laboratories with limited diagnostic resources.
Electronic supplementary material
The online version of this article (doi:10.1186/s12879-016-1885-4) contains supplementary material, which is available to authorized users.
Keywords: Mycoplasma hominis, Post-operative infection, Meningitis, Hospital acquired pneumonia, Case report
Background
Mycoplasma hominis, initially described as pleuropneumonia-like organism, is a commensal of the human oral cavity, respiratory tract, and genitourinary tract [1–3]. However, its role in the pathogenesis of infections in adult patients, especially extragenital infections such as central nervous system (CNS) infection, post-operative wound infections, mediastinitis, and septic arthritis [2, 4–7], has been difficult to determine. M. hominis, which does not possess a cell wall and hence is not identifiable by Gram staining of clinical specimens, is difficult to detect [3, 8, 9]. Culturing M. hominis which is fastidious in nature, is both resource- and time-consuming because specialized media and incubation conditions are required. Direct 16S rDNA PCR amplification/sequencing on clinical specimens may be performed but sensitivity is moderate at best, and not all laboratories perform this test [2, 3, 8]. The true incidence of M. hominis infections is thus probably underestimated and delayed diagnosis leads to delayed treatment with suboptimal outcomes [10].
CNS infections due to M. hominis are rare in patients other than neonates. To the best of our knowledge, only 19 cases of such infections have been reported in the English literature (Table 1). We herein report a case of M. hominis meningitis in which the organism was detected in the cerebrospinal fluid (CSF) following neurosurgical intervention for cerebral haemorrhage. This is the first reported case of meningitis in an adult caused by M. hominis from China.
Table 1.
Literature reports of CNS infections caused by Mycoplasma hominis in non-neonatal patients (1950-2016.7)
| No. | Author & year | Pt age(years), sex | Pt Country | HU | Tr | SI | Clinical manifestation | Days to Dx after Ad | Dx basis | Antibiotics used prior to diagnosis | Final antibiotic regimen | Otc | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Paine et al. [14] | 20, M | USA | N | Y | Y | fever, headache, a stiff neck | 18 | C | S + P + St | Sm | CR | [14] |
| 2 | Payan et al. [15] | 29, M | USA | Y | Y | Y | fever, loss of consciousness | 23 | C | O + Cp + N + C | T + E | CR | [15] |
| 3 | Madoff S et al. [16] | 11, F | USA | N | N | Y | fever | 26 | GIT + IRT | V + E | Mc | De | [16] |
| 4 | McMahon et al. [24] | 76, M | USA | N | N | Y | fever, unresponsive | 18 | C | P + G | none | De | [24] |
| 5 | Kersten RC et al. [17] | 20, M | USA | Y | Y | Y | fever, comatose | 19 | C | A + M + Su + V + Cf | M + Cf + Az + Am + Cl | Re | [17] |
| NM | 35 | C | Az + Am + Cl | M + Cf + D + Cd | CR | [17] | |||||||
| 6 | Cohen & Kubak. [25] | 18, F | USA | N | Y | Y | fever, altered mental status | 20 | C | E | D + Ci + C | CR | [25] |
| 7 | Zheng et al. [18] | 22, F | USA | N | N | Y | fever, left-sided weakness and numbness | 18 | IRT + IBA | Ct + N + Ca + M | none | CR | [19] |
| 8 | Douglas et al. [19] | 17, F | Australia | N | Y | Y | fever, headache, photophobia, nausea, vomiting | 13 | C + 16S | A + Ca + E | D + Cd | CR | [19] |
| 9 | House P et al. [20] | 40, F | Spain | N | N | Y | headache, left facial weakness, nausea, afebrile | 12 + “several” | C + 16S | V + Cf + M | Ci + M | CR | [20] |
| 10 | Kupila L et al. [21] | 40, M | Finland | N | Y | Y | haematuria and urine retention, confused | 14 | 16S | none | T | CR | [21] |
| 11 | McCarthy & Looke. [3] | 48, M | Australia | N | N | Y | fever | 36 | C + 16S | Cz + V | Ga + Cd | CR | [3] |
| 12 | McCarthy & Looke. [3] | 17, F | Australia | N | Y | Y | fever | 17 | C | V + Me | Ga + Mo | CR | [3] |
| 13 | Al Masalma et al. [22] | 41, F | Russia | N | N | Y | vertigo, coma headache, hemiparesis | 10 | 16S | V + Me | D | CR | [22] |
| 14 | Lee et al. [8] | 48, F | Netherlands | Y | Y | Y | fever | 15 | 16S | F + V | Mo | CR | [8] |
| 15 | Henao-Martínez et al. [10] | 40, M | Somalia | N | Y | Y | fever | 17 | C + 16S | V + PT + Ct + M | D | CR | [10] |
| 16 | Pailhorie ‘s et al. [23] | 43, M | France | N | Y | Y | fever, delirium tremens | 13 | Vitek MS + 16S | Me + V + Fo | L + D | CR | [23] |
| 17 | Whitson WJ [2] | 17, M | USA | Y | Y | Y | fever, bicep and deltoid weakness | 32 | C | PT + V + Ct + M | D + Mo | CR | [2] |
| 18 | Hos NJ [27] | 21, F | Germany | N | N | Y | fever, neck pain, nausea, vomiting, | 31 | C + 16S | A + Ct | Mo | CR | [27] |
| 19 | Reissier S [26] | 39, M | France | N | Y | Y | afbrile, loss of consciousness | 33 | C + 16S + RT-PCR | PT + Li + Ct + Me + V | Mo | De | [26] |
| 20 | Present study | 79, M | China | N | N | Y | fever, anepia and right-sided weakness | 17 | 16S | Me + V + CF | Az + D + Mi | CR |
Ad admitted, C culture, CR clinical recovery, De death, Dx diagnosis, GIT growth inhibition test, HU hormone use, IBA immunoblot assay, IRT immunofluo-rescence test, Otc outcome, Re recurrence, Ref reference, SI surgical intervention, 16S 16S rDNA sequencing, RT-PCR real-time PCR, Tr trauma, yrs: years
Ampicillin, (A) amoxicillin, (Am) azithromycin, (Az) chloramphenicol, (C) cefazolin, (Ca) clindamycin, (Cd) cefotaxime, (Cf) ciprofloxacin, (Ci) clavulanate potassium, (Cl) cephalothin (Cp), ceftriaxone (Ct), ceftazidime (Cz), cefoperazone/ sulbactam (CF), doxycycline (D), erythromycin (E),flucloxacillin (F), fosfomycin (Fo),gentamicin (G), gatifloxacin (Ga),levofloxacin (L), linezolid (Li), metronidazole (M), methacycline (Mc), meropenem (Me), minocyline (Mi), moxifloxacin (Mo),nafcillin (N), oxacillin (O),penicillin (P), piperacillin/tazobactam (PT), sulfadiazine (S), streptomycin (Sm), sulfathiazole (St), sulbactam (Su), tetracycline (T), vancomycin (V)
Case presentation
History and first admission
The patient was a 71-year-old man with a history of hypertension for 2 years who suddenly developed aphasia, and right-sided weakness and numbness while lifting water and was sent to the local hospital immediately on 21 September 2014. A cerebral computed tomography (CT) scan identified a cerebral hemorrhage rupturing into the ventricular system. He underwent craniotomy and evacuation of the hematoma. One week after the surgery, the patient developed hospital-acquired pneumonia, which was complicated by respiratory failure despite treatment with broad-spectrum antibiotics of imipenem.
MICU admission
The patient was soon transferred to the medical intensive care unit (MICU) of Peking Union Medical College Hospital (PUMCH) on October 14. On admission, he was febrile, comatose (Glasgow Coma Score of 9), dyspneic, and hypotensive. Mechanical ventilation was started after endotracheal intubation.
Examination on MICU & anti-infectious therapy
Empiric antibiotic treatment consisting of meropenem (2.0 g intravenously q8h) and vancomycin (1.0 g intravenously q12h) was initiated for hospital-acquired pneumonia. A lumbar puncture showed an opening pressure > 33 cmH2O, 1201 x 106/L red cells, 201 x 106/L white cells (79 % polymorphonuclear forms), an undetectable glucose concentration, protein 2.32 g/L, and chloride 128 mmol/L. No organisms were seen on Gram stain of the CSF. A cerebral CT showed an extensive left temporal hematoma and moderate lateral ventricle enlargement (Fig. 1a).
Fig. 1.
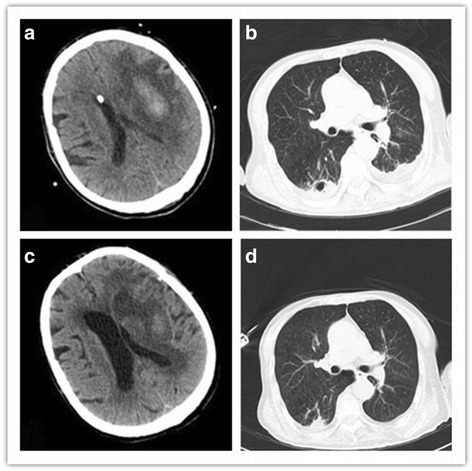
Computed tomography (CT) scans of the patient’s brain and lung during hospitalization. a. Cerebral CT scan revealed an extensive left hematoma in the temporal region of the hemispheres and moderate lateral ventricle enlargement with a drainage tube in it. The hematoma was surrounded by brain edema, narrowed gyri and the right–shifted cranial midline. b. Thoracic CT scan (lung windows) revealed a cavity with a wall of 7-mm thick surrounded by patchy shadowing in the right lower lobe. c. After 30 days of therapy, resolving brain swelling was seen in the CT scan with decreased edema. d. After 30 days of therapy, thoracic CT scan (lung window) revealed that the area of cavitation had decreased substantially
During his stay in ICU, repeated cultures of tracheal aspirates grew multidrug resistant (MDR) Acinetobacter baumannii, which was only susceptible to cefoperazone/sulbactam, and intermediately susceptible to tigecycline and minocycline. A follow-up chest CT scan revealed a thick-walled cavity in the right lower lobe (Fig. 1b). As a result, meropenem was changed to cefoperazone/sulbactam (3.0 g q8h), while the dose of vancomycin was increased to 1.5 g q12h to optimize the serum trough level (Fig. 2).
Fig. 2.
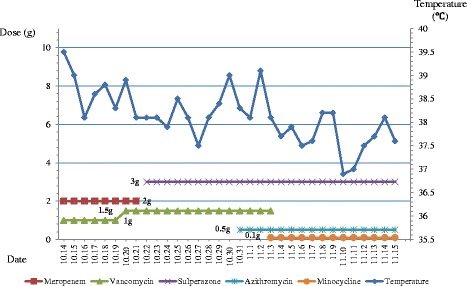
The correlation between the change of body temperature and the use of antibiotics during hospitalization
Etiological examination
Microbiology laboratory examination
CSF specimens were plated onto 5 % sheep blood agar and chocolate agar and were incubated at 37 °C under aerobic and anaerobic conditions, and in air with 5 % CO2. On October 30, cultures revealed non-hemolytic, semi-translucent pinpoint colonies on sheep blood agar plate after 4 days of incubation under anaerobic conditions (Fig. 3). Gram-stain smears of the CSF sample showed no evidence of bacteria.
Fig. 3.
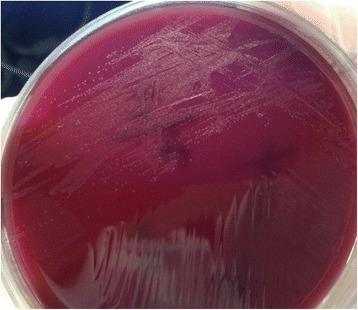
Non-hemolytic, semi-translucent pinpoint colonies of M. hominis were shown on 5 % blood sheep agar after 4 days of incubation
MALDI-TOF analysis of the cultured isolate
Two systems of the matrix-assisted laser desorption/ionization–time of flight mass spectrometry (MALDI-TOF MS) were used, i.e. the MALDI Biotyper (Bruker) and VITEK MS IVD (bioMérieux) as instructed by the manufacturer. The spectra were analyzed by using the MALDI Biotyper database library V.3.3.1.2 and the VITEK® MS IVD v2.0 database respectively. Both MALDI Biotyper and VITEK MS IVD consistently yielded no identifying profile and assigned “no identification” to this isolate.
16S rDNA gene identification
Bacterial DNA was obtained from isolates by using QIAamp DNA minikit (Qiagen, Hilden, Germany) following the manufacturer’s instructions starting from 200 μL of bacterial pellet suspension about 2-3 McFarland followed by a series of extracting steps with different reagents. Amplification of the 16S rDNA gene was performed by broad-range bacterial polymerase chain reaction (PCR) assay using the universal primers: 27 F (5’-AGAGTTTGATCCTGGCTCAG-3’) and 1522R (5’-AAGGAGGTGATCCAGCCGCA-3’) as described before [11, 12]. Purified PCR products and sequencing primers (the same as for amplification) were mixed and sent to Ruibiotech (Beijing, China) for sequencing. Species identification was performed by comparing the obtained sequences against those in the GenBank database using the BLASTn software (http://www.ncbi.nlm.nih.gov/blast). By querying 16S rDNA sequences against those in the GenBank database, the isolate best matched with 3 M. hominis reference strains (GenBank accession numbers: CP011538.1, NR_041881.1 and CP009652.1) with an identity of 100 % (1385/1385), followed by Mycoplasma equirhinis, with an identity of 97 % (1314/1353). All M. hominis 16S rDNA nucleotide sequences available in GenBank till 2015 (n = 10) are summarized in Additional file 1.
Post-treatment course
On November 2, the microbiology laboratory reported isolation from the CSF sample of M. hominis, which was susceptible to doxycycline, and intermediately suscepible to azithromycin by the bioMérieux® SA Mycoplasma IST2 kit (Biomerieux, France). Combination therapy with azithromycin (0.5 g qd) and minocycline (100 mg q12h) was then started (Fig. 2). On November 16, 14 days after appropriate antimicrobial therapy was started, the patient was transferred back to the local hospital. Repeated brain and chest CT scans before discharge showed marked improvement of the cerebral edema and size of the brain swelling (Fig. 1c), and almost complete resolution of the pulmonary cavity and pleural effusion (Fig. 1d).
Discussion
A PubMed search was performed using the following key words: “Mycoplasma hominis” AND “encephalitis” OR “cerebritis” OR “cephalitis” OR “neuraxitis” OR “phrenitis” OR “meningitis” OR “brain abscess” OR “cranial infection” OR "myelitis" OR " polyradiculoneuritis " OR "spinal cord infection". A total of 58 manuscripts were found, most reporting M. hominis infection in neonates, which most likely arising from contact with maternal genital flora [13]. By carefully reading all the papers, we found that there were only 19 reported non-neonatal-associated cases of CNS infection caused by M. hominis; none was reported from China (Table 1). Of these 20 cases, brain abscess was the most common CNS infections (n = 13) [3, 10, 14–23], followed by meningitis (n = 4) [8, 24–26], spinal cord abscess (n = 1) [2], subdural empyema (n = 1) [27], (Table 1).
Current microbiological analysis, mostly based on direct examination and culture of pus specimens, underestimate the role of fastidious microorganisms, such as M. hominis [22], in CNS infections. Predisposing host factors such as immunosuppression, malignancy, trauma, and manipulation or surgery of the genitourinary tract are considered to be risk factors for extra-genital infections caused by this microorganism [8, 15, 17, 18, 28]. In most M. hominis brain infections thus far described, patients usually presented with prior head trauma or had undergone neurosurgical procedures [8, 10, 14, 15, 17, 19, 21, 23–27]. This was also the case in our patient who had an intracererbal haematoma evacuated 7 days prior to developing meningitis.
Three routes of intracranial infection are typically considered: direct contamination during trauma, direct contamination during surgery, or seeding of the cerebral site secondary to bacteraemia due to genitourinary manipulation [21, 23]. In traumatized brain tissue where CNS capillaries are damaged, M. hominis can easily reach the ischemic brain tissue through blood circulation. In our case, the patient was exposed to the aforementioned last 2 hypothetical sources. Cerebral hemorrhage was cured by a neurosurgical intervention, but he also underwent urinary catheterization after the coma during hospitalization. Therefore, we were unable to definitively identify the source of infection.
Identification of M. hominis infections by culture is challenging due to the slow growth of the colonies and the absence of cell wall, which contributes to a negative result on Gram staining [10], as in our patient. Moreover, even when culture is successful, it is difficult to rule out the possibility of contamination. Although M. hominis, being less fastidious than other mycoplasmas, is able to grow on conventional blood agar medium, specific laboratory methods are required for its identification. In most reports of cerebral infections, 16S rDNA sequencing is required for definitive identification as was the case here [8]. It has been recently highlighted that MALDI-TOF MS could be useful for the rapid identification of M. hominis [23, 29]. However, we were unable to identify M. hominis by MALDI TOF MS even though the spectra of this species is represented in the VITEK® MS IVD v2.0 database. In this regard, similar results were also found for the MALDI Biotyper by Nulens E and his colleges [30]. Thus, further improvement of M. hominis spectra database seems necessary. Nevertheless, neither the Bruker nor Vitek database misidentified the strain as another species.
Since cases of intracranial infections caused by M. hominis are rare, clinicians usually fail to consider the diagnosis in the absence of microbiological evidence. In addition, it is difficult to distinguish brain abscesses or meningitis caused by mycoplasmas from those caused by bacteria or viruses, due to the lack of specific clinical manifestations. This may lead to delayed initiation of antimicrobial therapy with serious clinical consequence [31], as M. hominis is not susceptible to most first-line antibiotics used to treat brain abscesses [21]. The possibility of a M. hominis infection should be suspected when Gram stain reveals abundant neutrophils but no bacteria, and empirical treatment shows poor efficacy during this period.
In all previously published case studies of M. hominis brain abscesses, treatment involved abscess drainage, debridement, and specific antimicrobial therapy. The importance of surgical treatment is evident from a case report of a patient who responded to surgical therapy alone [32]. Infections caused by Mycoplasma spp. (i.e. a microorganism lacking both cell wall and folic acid synthesis) require ad-hoc antibiotic treatment [33, 34]. There are no CLSI (Clinical and Laboratory Standards Institute) and EUCAST (European Committee on Antimicrobial Susceptibility Testing) breakpoints for M. hominis at present. Nevertheless, the organism is generally considered to be susceptible to tetracyclines, lincosamides, streptogramins and quinolones, but not to the macrolides although tetracyclines-resistant M. hominis has been reported [10, 35–37]. However, poor passage through the blood brain barrier could lead to low antibiotic concentrations in the brain, with the possible major exception of fourth generation quinolones that, besides exhibiting low MICs for M. hominis, do possess reasonable CSF penetration [35–43]. Because of the tendency for chronic and often latent infection, long-term antimicrobial treatment against M. hominis is warranted. However, surgical drainage and debridement remain the key to recovery, since patients may respond to surgical treatment alone [29].
In our case, initial treatment with meropenem and vancomycin showed little efficiency since a low-grade fever still persisted (as shown in Fig. 2). MDR A. baumannii was also isolated from the lower respiratory tract possibly due to nosocomial infection. Persistent fever despite various antibiotic treatment with meropenem, vancomycin, and cefoperazone/sulbactam, as well as the satisfactory response to azithromycin and minocycline, strongly suggested that M. hominis, rather than A. baumannii was the primary pathogen in our patient.
Conclusions
The prevalence of brain infections caused by M. hominis may be increasing, presenting a diagnostic and therapeutic challenge to clinicians. We reported here the first case of meningitis caused by M. hominis in an adult in China, who was successfully treated with azithromycin and minocycline. The pathogenic potential of M. hominis, the need for early diagnosis, and the importance of initial appropriate chemotherapy must be highlighted also in developing countries, where the challenges in diagnostic capacity for clinical laboratories are greater.
Acknowledgements
We thank Dr. Zhihong Guan from the Department of neurology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China where the patient is in care.
Availability of data and materials
The Near Complete length GenBank Mycoplasma hominis16S rRNA sequences supporting the conclusions of this article are included within the article (Additional file 1).
Authors’ contributions
MZ, PW, FK and YCX conceived and designed the experiments. MZ, PW, JD, MX and FW collected the information about the case, contributed to the acquisition, analysis and interpretation of data. MZ, SC, BD and FK wrote and revised the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Consent for publication
Written informed consent was obtained from the patient for publication of this Case report. A copy of the written consent is available for review by the Editor of this journal.
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board of Peking Union Medical College Hospital (No. S-263). Written informed consent was obtained from the patient.
Abbreviations
- BBB
Blood brain barrier
- CLSI
Clinical and laboratory standards institute
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- CT
Computed tomography
- EUCAST
European committee on antimicrobial susceptibility testing
- MALDI-TOF MS
Matrix-assisted laser desorption/ionization–time of flight mass spectrometry
- MDR
Multidrug resistant
- MICU
Medical intensive care unit
- PCR
Polymerase chain reaction
- PUMCH
Peking union medical college hospital
Additional file
Near complete length GenBank Mycoplasma hominis 16S rRNA sequences analysis. A summary of all the Mycoplasma hominis16S rRNA sequences deposited in GeneBank. (DOCX 16 kb)
Contributor Information
Menglan Zhou, Email: mumuxi529@139.com, Email: 18811387499@163.com.
Peng Wang, Email: pumch2005@126.com.
Sharon Chen, Email: sharon.chen@health.nsw.gov.au.
Bin Du, Email: dubin98@gmail.com.
Jinlong Du, Email: dujinlong2009123@sina.com.
Fengdan Wang, Email: wfdpumc@126.com.
Meng Xiao, Email: cjtcxiaomeng@aliyun.com.
Fanrong Kong, Email: kongfr_nk@yahoo.com.au.
Yingchun Xu, Phone: 86-13911303028, Phone: 86-10-6915-9766, Email: xycpumch@139.com.
References
- 1.Dienes L, Edsall G. Observations on the L-organism of Klieneberger. Proc Soc Exp Biol Med. 1937;36:740–744. doi: 10.3181/00379727-36-9380. [DOI] [Google Scholar]
- 2.Whitson WJ, Ball PA, Lollis SS, Balkman JD, Bauer DF. Post-operative Mycoplasma hominis infections after neurosurgical intervention. J Neurosurg Pediatr. 2014;14:212–218. doi: 10.3171/2014.4.PEDS13547. [DOI] [PubMed] [Google Scholar]
- 3.McCarthy KL, Looke DF. Successful treatment of post-neurosurgical intracranial Mycoplasma hominis infection using gatifloxacin. J Infect. 2008;57:344–346. doi: 10.1016/j.jinf.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Pastural M, Audard V, Bralet MP, Rémy P, Salomon L, Tankovic J, et al. Mycoplasma hominis infection in renal transplantation. Nephrol Dial Transplant. 2002;17:495–496. doi: 10.1093/ndt/17.3.495. [DOI] [PubMed] [Google Scholar]
- 5.Mattila PS, Carlson P, Sivonen A, Savola J, Luosto R, Salo J, et al. Life-threatening Mycoplasma hominis mediastinitis. Clin Infect Dis. 1999;29:1529–1537. doi: 10.1086/313529. [DOI] [PubMed] [Google Scholar]
- 6.Flouzat-Lachaniette CH, Guidon J, Allain J, Poignard A. An uncommon case of Mycoplasma hominis infection after total disc replacement. Eur Spine J. 2013;22(Suppl 3):S394–S398. doi: 10.1007/s00586-012-2511-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor-Robinson D. Infections due to species of Mycoplasma and Ureaplasma: an update. Clin Infect Dis. 1996;23:671–682. doi: 10.1093/clinids/23.4.671. [DOI] [PubMed] [Google Scholar]
- 8.Lee EH, Winter HL, van Dijl JM, Metzemaekers JD, Arends JP. Diagnosis and antimicrobial therapy of Mycoplasma hominis meningitis in adults. Int J Med Microbiol. 2012;302:289–292. doi: 10.1016/j.ijmm.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Waites KB, Schelonka RL, Xiao L, Grigsby PL, Novy MJ. Congenital and opportunistic infections: Ureaplasma species and Mycoplasma hominis. Semin Fetal Neonatal Med. 2009;14:190–199. doi: 10.1016/j.siny.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Henao-Martinez AF, Young H, Nardi-Korver JJ, Burman W. Mycoplasma hominis brain abscess presenting after a head trauma: a case report. J Med Case Rep. 2012;6:253. doi: 10.1186/1752-1947-6-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kupila L, Rantakokko-Jalava K, Jalava J, Nikkari S, Peltonen R, Meurman O, et al. Aetiological diagnosis of brain abscesses and spinal infections: application of broad range bacterial polymerase chain reaction analysis. J Neurol Neurosurg Psychiatry. 2003;74:728–733. doi: 10.1136/jnnp.74.6.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drancourt M, Berger P, Raoult D. Systematic 16S rRNA gene sequencing of atypical clinical isolates identified 27 new bacterial species associated with humans. J Clin Microbiol. 2004;42:2197–2202. doi: 10.1128/JCM.42.5.2197-2202.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hata A, Honda Y, Asada K, Sasaki Y, Kenri T, Hata D. Mycoplasma hominis meningitis in a neonate: case report and review. J Infect. 2008;57:338–343. doi: 10.1016/j.jinf.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Paine TF, Murray R, Perlmutter I, Finland M. Brain abscess and meningitis associated with a pleuropneumonia-like organism: clinical and bacteriological observations in a case with recovery. Ann Intern Med. 1950;32:554–562. doi: 10.7326/0003-4819-32-3-554. [DOI] [Google Scholar]
- 15.Payan DG, Seigal N, Madoff S. Infection of a brain abscess of Mycoplasma hominis. J Clin Microbiol. 1981;14:571–573. doi: 10.1128/jcm.14.5.571-573.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madoff S, Hooper DC. Nongenitourinary infections caused by Mycoplasma hominis in adults. Rev Infect Dis. 1988;10:602–613. doi: 10.1093/clinids/10.3.602. [DOI] [PubMed] [Google Scholar]
- 17.Kersten RC, Haglund L, Kulwin DR, Ma'luf R, DeConciliis C. Mycoplasma hominis orbital abscess. Arch Ophthalmol. 1995;113:1096–1097. doi: 10.1001/archopht.1995.01100090018009. [DOI] [PubMed] [Google Scholar]
- 18.Zheng X, Olson DA, Tully JG, Watson HL, Cassell GH, Gustafson DR, et al. Isolation of Mycoplasma hominis from a brain abscess. J Clin Microbiol. 1997;35:992–994. doi: 10.1128/jcm.35.4.992-994.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Douglas MW, Fisher DA, Lum GD, Roy J. Mycoplasma hominis infection of a subdural haematoma in the peripartum period. Pathology. 2003;35:452–454. doi: 10.1080/00313020310001602684. [DOI] [PubMed] [Google Scholar]
- 20.House P. Dunn J, Carroll K, MacDonald J. Seeding of a cavernous angioma with Mycoplasma hominis: case report. Neurosurgery. 2003;53:749–752. doi: 10.1227/01.NEU.0000080064.21806.28. [DOI] [PubMed] [Google Scholar]
- 21.Kupila L, Rantakokko-Jalava K, Jalava J, Peltonen R, Marttila RJ, Kotilainen E, et al. Brain abscess caused by Mycoplasma hominis: a clinically recognizable entity? Eur J Neurol. 2006;13:550–551. doi: 10.1111/j.1468-1331.2006.01209.x. [DOI] [PubMed] [Google Scholar]
- 22.Al Masalma M, Drancourt M, Dufour H, Raoult D, Fournier PE. Mycoplasma hominis brain abscess following uterus curettage: a case report. J Med Case Rep. 2011;5:278. doi: 10.1186/1752-1947-5-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pailhories H, Rabier V, Eveillard M, Mahaza C, Joly-Guillou ML, Chennebault JM, et al. A case report of Mycoplasma hominis brain abscess identified by MALDI-TOF mass spectrometry. Int J Infect Dis. 2014;29:166–168. doi: 10.1016/j.ijid.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 24.McMahon DK, Dummer JS, Pasculle AW, Cassell G. Extragenital Mycoplasma hominis infections in adults. Am J Med. 1990;89:275–281. doi: 10.1016/0002-9343(90)90338-E. [DOI] [PubMed] [Google Scholar]
- 25.Cohen M, Kubak B. Mycoplasma hominis meningitis complicating head trauma: case report and review. Clin Infect Dis. 1997;24:272–273. doi: 10.1093/clinids/24.2.272. [DOI] [PubMed] [Google Scholar]
- 26.Reissier S, Masson R, Guérin F, Viquesnel G, Petitjean-Lecherbonnier J, Pereyre SInt J, et al. Fatal nosocomial meningitis caused by Mycoplasma hominis in an adult patient: case reportand review of the literature. Infect Dis. 2016;48:81–83. doi: 10.1016/j.ijid.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Hos NJ, Bauer C, Liebig T, Plum G, Seifert H, Hampl J. Autoinfection as a cause of postpartum subdural empyema due to Mycoplasma hominis. Infection. 2015;43(2):241–244. doi: 10.1007/s15010-014-0713-2. [DOI] [PubMed] [Google Scholar]
- 28.Simberkoff MS, Toharsky B. Mycoplasmemia in adult male patients. JAMA. 1976;236:2522–2524. doi: 10.1001/jama.1976.03270230044029. [DOI] [PubMed] [Google Scholar]
- 29.Pereyre S, Tardy F, Renaudin H, Cauvin E, Del Prá Netto Machado L, Tricot A, et al. Identification and subtyping of clinically relevant human and ruminant mycoplasmas by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J ClinMicrobiol. 2013;51:3314–3323. doi: 10.1128/JCM.01573-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nulens E, Van Praet J, Selleslag D, Van Landschoot T, Dekeyzer D, Descheemaecker P, et al. A disseminated Mycoplasma hominis infection in a patient with an underlying defect in humoral immunity. Infection. 2016;44(3):379–381. doi: 10.1007/s15010-015-0859-6. [DOI] [PubMed] [Google Scholar]
- 31.Brouwer MC, Tunkel AR, McKhann GM, van de Beek D. Brain abscess. N Engl J Med. 2014;371:447–456. doi: 10.1056/NEJMra1301635. [DOI] [PubMed] [Google Scholar]
- 32.Mossad SB, Rehm SJ, Tomford JW, Isada CM, Taylor PC, Rutherford I, et al. Sternotomy infection with Mycoplasma hominis: a cause of "culture negative" wound infection. J Cardiovasc Surg. 1996;37:505–509. [PubMed] [Google Scholar]
- 33.McCormack WM. Susceptibility of mycoplasmas to antimicrobial agents: clinical implications. Clin Infect Dis. 1993;17(Suppl 1):S200–S201. doi: 10.1093/clinids/17.Supplement_1.S200. [DOI] [PubMed] [Google Scholar]
- 34.Krausse R, Schubert S. In-vitro activities of tetracyclines, macrolides, fluoroquinolones and clindamycin against Mycoplasma hominis and Ureaplasma spp. isolated in Germany over 20 years. Clin Microbiol Infect. 2010;16:1649–1655. doi: 10.1111/j.1469-0691.2010.03155.x. [DOI] [PubMed] [Google Scholar]
- 35.Kenny GE, Cartwright FD. Susceptibilities of Mycoplasma hominis, M. pneumoniae, and Ureaplasma urealyticum to GAR-936, dalfopristin, dirithromycin, evernimicin, gatifloxacin, linezolid, moxifloxacin, quinupristin-dalfopristin, and telithromycin compared to their susceptibilities to reference macrolides, tetracyclines, and quinolones. Antimicrob Agents Chemother. 2001;45:2604–2608. doi: 10.1128/AAC.45.9.2604-2608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redelinghuys MJ, Ehlers MM, Dreyer AW, Lombaard HA, Kock MM. Antimicrobial susceptibility patterns of Ureaplasma species and Mycoplasma hominis in pregnant women. BMC Infect Dis. 2014;14:171. doi: 10.1186/1471-2334-14-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mardassi BB, Aissani N, Moalla I, Dhahri D, Dridi A, Mlik B. Evidence for the predominance of a single tet(M) gene sequence type in tetracycline-resistant Ureaplasma parvum and Mycoplasma hominis isolates from Tunisian patients. J Med Microbiol. 2012;61(Pt9):1254–1261. doi: 10.1099/jmm.0.044016-0. [DOI] [PubMed] [Google Scholar]
- 38.Cottagnoud P, Tauber MG. Fluoroquinolones in the treatment of meningitis. CurrInfect Dis Rep. 2003;5:329–336. doi: 10.1007/s11908-003-0011-0. [DOI] [PubMed] [Google Scholar]
- 39.Lutsar I, Friedland IR, Wubbel L, McCoig CC, Jafri HS, Ng W, et al. Pharmacodynamics of gatifloxacin in cerebrospinal fluid in experimental cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother. 1998;42:2650–2655. doi: 10.1128/aac.42.10.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scotton PG, Pea F, Giobbia M, Baraldo M, Vaglia A, Furlanut M. Cerebrospinal fluid penetration of levofloxacin in patients with spontaneous acute bacterial meningitis. Clin Infect Dis. 2001;33:e109–e111. doi: 10.1086/323406. [DOI] [PubMed] [Google Scholar]
- 41.Shimada J, Nogita T, Ishibashi Y. Clinical pharmacokinetics of sparfloxacin. Clin Pharmacokinet. 1993;25:358–369. doi: 10.2165/00003088-199325050-00002. [DOI] [PubMed] [Google Scholar]
- 42.Lutsar I, McCracken GH, Jr, Friedland IR. Antibiotic pharmacodynamics in cerebrospinal fluid. Clin Infect Dis. 1998;27:1117–1127. doi: 10.1086/515003. [DOI] [PubMed] [Google Scholar]
- 43.Kanellakopoulou K, Pagoulatou A, Stroumpoulis K, Vafiadou M, Kranidioti H, Giamarellou H, et al. Pharmacokinetics of moxifloxacin in non-inflamed cerebrospinal fluid of humans: implication for a bactericidal effect. J Antimicrob Chemother. 2008;61:1328–1331. doi: 10.1093/jac/dkn110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Near Complete length GenBank Mycoplasma hominis16S rRNA sequences supporting the conclusions of this article are included within the article (Additional file 1).


