Abstract
Background
Pre-harvest sprouting (PHS) in wheat can cause substantial reduction in grain yield and end-use quality. Grain color (GC) together with other components affect PHS resistance. Several quantitative trait loci (QTL) have been reported for PHS resistance, and two of them on chromosome 3AS (TaPHS1) and 4A have been cloned.
Methods
To determine genetic architecture of PHS and GC and genetic relationships of the two traits, a genome-wide association study (GWAS) was conducted by evaluating a panel of 185 U.S. elite breeding lines and cultivars for sprouting rates of wheat spikes and GC in both greenhouse and field experiments. The panel was genotyped using the wheat 9K and 90K single nucleotide polymorphism (SNP) arrays.
Results
Four QTL for GC on four chromosomes and 12 QTL for PHS resistance on 10 chromosomes were identified in at least two experiments. QTL for PHS resistance showed varied effects under different environments, and those on chromosomes 3AS, 3AL, 3B, 4AL and 7A were the more frequently identified QTL. The common QTL for GC and PHS resistance were identified on the long arms of the chromosome 3A and 3D.
Conclusions
Wheat grain color is regulated by the three known genes on group 3 chromosomes and additional genes from other chromosomes. These grain color genes showed significant effects on PHS resistance in some environments. However, several other QTL that did not affect grain color also played a significant role on PHS resistance. Therefore, it is possible to breed PHS-resistant white wheat by pyramiding these non-color related QTL.
Electronic supplementary material
The online version of this article (doi:10.1186/s12864-016-3148-6) contains supplementary material, which is available to authorized users.
Keywords: Triticum aestivum, pre-harvest sprouting resistance, grain color, genome- wide association studies
Background
Pre-harvest sprouting (PHS) of wheat (Triticum aestivum L.) refers to the germination of wheat grains in matured spikes before harvest due to continuous wet weather during harvest seasons. PHS can result in a significant reduction in wheat grain yield and grain end-use quality, thus a reduction in grain sale price [1, 2]. Growing PHS-resistant cultivars is the most effective way to minimize PHS damage. PHS resistance QTL have been reported on almost all wheat chromosomes. One major QTL mapped on chromosome 3AS, designated as TaPHS1, has been cloned [3, 4]. Another major QTL on chromosome 4AL has been fine mapped with single nucleotide polymorphisms (SNPs) [5–7]. Recently, several candidate genes have been reported for the 4A QTL in different studies [6, 8]. In addition, several minor QTL have also been reported on chromosomes 2B [9–12], 3D [13], 4B, 4D [14] and many others [15].
Wheat grain color (GC) has long been associated with PHS, and red-grained wheats are usually more tolerant to PHS than the white-grained wheats [16–18]. The pigments, catechin and proanthocyanidins (PAs) synthesized through the flavonoid synthesis pathway, result in red GC [19, 20]. Early cytogenetic studies suggested that three genes, R-A1, R-B1 and R-D1, on homoeologous group 3 chromosomes control GC [21–23], and show a pleiotropic effect on wheat PHS resistance by accumulating catechin, a precursor of the red pigment, that inhibits grain germination [19, 24]. Flinthman [16] found that grain dormancy levels were increased in white-grained wheat NS-67 after adding a single GC (R) gene to one of group 3 chromosomes. Groos et al. [1] identified common QTL for GC and PHS resistance on chromosomes 3AL, 3BL, 3DL and 5A in a white × red wheat cross. The white-grained mutants of 'Chinese Spring' and 'AUS1490' showed increased sprouting, indicating that R genes enhanced PHS tolerance [17, 18]. Recently, Tamyb10 genes, the transcription factors of the flavonoid biosynthetic pathway, have been reported as candidate genes for the GC trait [25]. However, how much these R genes contribute to PHS resistance remains unknown. Therefore, simultaneous genome-wide association studies (GWAS) on both traits may reveal the relationship between R genes and PHS resistance.
Genome-wide association studies have been conducted in many plant species to discover and validate QTL and genes for various traits. By taking advantages of historical recombination events and linkage disequilibrium (LD) between causal genetic variants and nearby SNPs, GWAS detects statistical associations between genetic variations and phenotypic variations throughout the genome [26–29]. Therefore, GWAS can potentially increase mapping resolution by taking advantages of historical recombinations using highly diverse populations. To date, GWAS has not been reported for GC, and only several studies have been reported for wheat PHS resistance [11, 30–32]. In the current study, we analyzed a panel of elite breeding lines and cultivars from major U.S. winter wheat breeding programs using the wheat 9K and 90K arrays to (1) study the phenotypic variance of PHS resistance in U.S. winter wheat, (2) identify genome-wide QTL for GC and PHS resistance, and (3) determine the genetic relationship between GC and PHS resistance.
Methods
Plant materials
A set of 185 winter wheat accessions [33] was assembled to include 130 hard winter wheat and 55 soft winter wheat accessions as listed in Additional file 1: Table S1. A mapping population of 155 F6 recombinant inbred lines (RILs) derived from the cross of Tutoumai A x Siyang 936 [7, 34] was used to validate the SNPs that showed significant associations with the Qphs.hwwgr-4A.
Pre-harvest sprouting evaluation
In the greenhouse experiments, five plants per accession were grown in a 13 by 13 cm Dura-pot (Hummert Int. Topeka, KS) under the growth condition listed in Additional file 2: Table S2 after vernalization for 7 weeks at 4 °C in a cold chamber. The GWAS panel was evaluated for PHS in the greenhouse experiments of fall (August-December) 2011, spring (January-May) and fall 2012, and spring 2013. All experiments were conducted in a randomized complete block design with two replications of five plants.
The GWAS panel was also planted for PHS resistance evaluation in the Kansas State University Rocky Ford Wheat Research Farm, Manhattan, KS and the Agricultural Research Center-Hays, Hays, KS, respectively, in the summers of 2013 and 2014. About 30 seeds per accession were planted in a 1.22-m-long single-row plot, and each experiment had two replications.
When wheat plants reached physiological maturity, similar to Zadoks scale 91 [35], spikes that lost green color [36] were harvested from both greenhouse and field experiments, and evaluated for PHS in the lab. Five spikes per accession that were harvested from each replicate were air-dried for 5 d in a greenhouse, and then stored at -20 °C to maintain dormancy for PHS evaluation. After all accessions had been collected, the greenhouse- harvested spikes were air-dried 9 d and field-harvested spikes for 5 d at room temperature. The additional drying days were determined based on preliminary test results of randomly selected samples from field and greenhouse experiments that maximize phenotypic differences among genotypes. After the dried spikes had been immersed in de-ionized water for 12 h, they were enclosed in a moist chamber at 22 ± 1 °C with an attached humidifier that ran twice daily at 2 h each time to maintain high moisture in the chamber. After 7 d of incubation, the germinated and non-germinated kernels were hand-threshed and counted separately to calculate the percentage of germinated kernels from all five spikes of each replication.
Evaluation of grain color
Grain color was evaluated for grains harvested from one field experiment (2009-2010 Enid Oklahoma) and the fall 2011 greenhouse experiment at Manhattan KS. For each accession, ten seeds were soaked in 1 M sodium hydroxide (NaOH) for 1 h to increase the color contrast. Grain color intensity was determined visually using a scale of 1 to 4, where 1 represents white, 2 light red, 3 red and 4 dark red.
DNA isolation and genotyping
Leaf tissue was collected at the two-leaf stage, and genomic DNA was isolated using a modified cetyltrimethyl ammonium bromide (CTAB) method [33]. A total of 446 polymorphic SSR markers were selected to genotype the association panel based on PCR product quality, chromosome distribution in available genetic maps (http://wheat.pw.usda.gov/GG3/; verified 11 Aug. 2010), and previously reported associations with PHS resistance. One expression sequence tag (EST), ZXQ118 [12] and three gene markers of PM19A1 and PM19A2 [6] were used to determine the association between PHS resistance and Qphs.hwwgr-4A. Five sequence-tagged sites (STS) from three Tamyb10 genes [25] were analyzed to determine QTL for GC. Amplification, separation and scoring of polymorphic chain reaction (PCR) products followed Zhang et al. [33].
The GWAS panel was also genotyped with the Wheat 9K and 90K SNP arrays [37, 38] at USDA-ARS Cereal Crops Research Unit (Fargo, ND). SNPs with less than 5 % minor allele frequency (MAF) or with more than 15 % missing data were removed. A total of 5,921 and 21,600 SNPs were scored from the 9K and 90K SNP arrays, respectively. Association analysis was initially conducted using the 9K genotypic data, and 28 non-redundant SNPs with p < 0.001 were then selected and pooled together with the 90K data. Totally, 21,628 SNPs were used for the final analysis. Also, one SNP in the promoter region and two SNPs in the coding region of the TaPHS1 gene [3, 4] were analyzed using three Kompetitive Allele Specific PCR (KASP) assays. Sequences that harbored significant SNPs and SSR markers were searched against the W7984 reference sequence to estimate their putative chromosome positions.
Population structure and kinship
Population structure was characterized by a set of 1500 SNPs that are evenly distributed on all the 21 wheat chromosomes using the admixture model in STRUCTURE 2.3.4 [39]. K-values ran from 2 to 20 with 10 iterations set for each k-value. The burn-in time and replication number were set at 2 × 105 and 2 × 104, respectively. For each trait, Bayesian information criterion (BIC) [40] was applied to determine the optimum number of subpopulations. Marker-based kinship was estimated to approximate the probability of two individuals being identical by descent through adjusting the average probability of identical in the state between random individuals [41]. Kinship was calculated with the same set of 1,500 SNPs used for structure analysis using SPAGeDi package [42].
Statistical analysis and genome-wide association analysis
Best linear unbiased predictions (BLUPs) were calculated for each accession evaluated in the greenhouse and field experiments using the 'lme4' package in R 3.2.2 [43] with year and location as random effects in the model. Genome-wide association analysis was conducted using two models: the generalized linear model (GLM) with the Q matrix as fixed effects, and the mixed linear model (MLM) with a Q matrix as fixed effects and a kinship matrix as random effects. These two models were applied to each experiment for GC and PHS resistance, and model fitness was determined based on the BIC values. Association analysis of SNP data was conducted using the genome association and prediction integrated tool (GAPIT) implemented in R [44], and association analysis of SSR data was conducted using PROC MIXED procedure in SAS 9.3 (SAS Institute Inc., Cary, NC). A threshold of p < 0.001 was set to claim significant associations between SSR markers and the traits (GC and PHS resistance), and p < 0.0001 was set to claim significant associations between SNPs and the traits. Linkage disequilibrium and haplotype analyses of the significant SNPs were performed with HAPLOVIEW v.4.2 (http://www.broadinstitute.org/scientific-community/science/programs/medical-and-population-genetics/haploview/haploview).
QTL analysis
A linkage map covering the 4A QTL region was constructed for the RIL population of Tutoumai A x Siyang 936 using KASP markers converted from significant SNPs from the association study, and previously mapped SSR markers in Liu et al. [45] and GBS-SNPs in Lin et al. [7] by JoinMap version 4.0 [46]. Recombination fractions were converted into centiMorgans (cM) using the Kosambi function [47]. Interval mapping (IM) using sprouting data from the 2005 and 2006 greenhouse experiments and their combined mean was performed using WinQTLCart 2.5 [48]. LOD thresholds to claim significant QTL for each dataset were determined from 1000 permutations [49].
Results
Phenotypic variations in grain color and pre-harvest sprouting
Twenty-nine accessions were scored as white wheats, and 156 accessions as red wheats. The GC scores were highly consistent between greenhouse and field grown seeds (Fig. 1) with a high correlation coefficient of 0.87 (P < 0.0001), indicating a low genotype-by-environment interaction for GC.
Fig. 1.
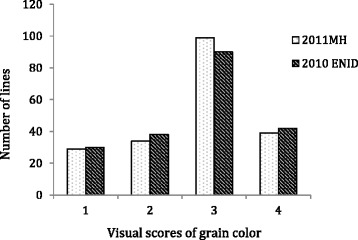
Frequency distribution of grain color (GC) scores evaluated using a 1 to 4 scale (white, light red, red and dark red) in the association mapping population. The seeds were harvested from the Manhattan 2011 greenhouse (2011MH) experiment and the Enid 2010 field (2010 ENID) experiment
Significant correlations for sprouting rates were observed among most of the eight experiments (Table 1). Cluster analysis showed high similarities in sprouting rates of accessions among all the field experiments, but significant differences between the field and the greenhouse experiments (Fig. 2a). The broad sense heritability across all eight experiments was high (0.83), with 0.62 in the greenhouse experiments and 0.92 in the field experiments. The population could be roughly divided into three subgroups (Fig. 2b), with average sprouting rates of 13.9 % in Group 1, 35.5 % in Group 2 and 60.3 % in Group 3, and average GC scores of 3.0 in Group 1, 2.6 in Group 2 and 1.7 in Group 3, indicating red wheats were more likely to have low sprouting rates. Most of the soft winter wheats were clustered to Group 1, as well as some hard white winter (HWW) wheat accessions from the Regional Germplasm Observation Nursery (RGON). The rest accessions from the RGON were mostly clustered to Group 2, whereas accessions from the Southern Regional Performance Nursery (SRPN) and the Northern Regional Performance Nursery (NRPN) were mainly clustered to Group 2 and Group 3.
Table 1.
Pairwise correlation coefficients among germination rates from all eight experiments and best linear unbiased predictions (BLUP) of the all greenhouse experiments and all field experiments
| Corr Coeff | 2011F | 2012S | 2012F | 2013S | 2013_MH | 2013_Hays | 2014_MH | 2014_Hays | GH_BLUP |
|---|---|---|---|---|---|---|---|---|---|
| 2012S | 0.238*** | ||||||||
| 2012F | 0.468*** | 0.313*** | |||||||
| 2013S | 0.167* | 0.543*** | 0.337*** | ||||||
| 2013_MH | 0.276*** | 0.171* | 0.212** | 0.153* | |||||
| 2013_Hays | 0.407*** | 0.227** | 0.402*** | 0.237*** | 0.741*** | ||||
| 2014_MH | 0.384*** | 0.312*** | 0.576*** | 0.242*** | 0.686*** | 0.721*** | |||
| 2014_Hays | 0.358*** | 0.228** | 0.431*** | 0.266*** | 0.747*** | 0.755*** | 0.821*** | ||
| GH_BLUP | 0.559*** | 0.718*** | 0.821*** | 0.710*** | 0.268*** | 0.437*** | 0.551*** | 0.448*** | |
| Field_BLUP | 0.399*** | 0.265*** | 0.463*** | 0.253*** | 0.869*** | 0.890*** | 0.909*** | 0.929*** | 0.484*** |
*p < 0.05; **p < 0.01, ***p < 0.001
Fig. 2.
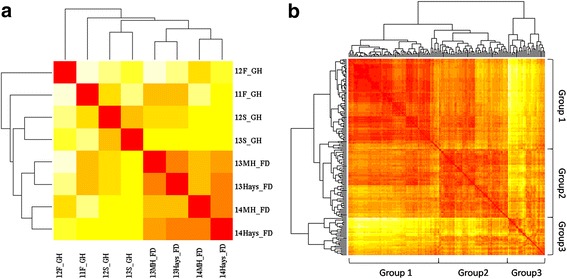
Heatmaps showing a the relationships of pre-harvest sprouting data among four greenhouse (GH) experiments conducted at Manhattan, KS in fall 2011(11F_GH), fall 2012(12F_GH), spring 2012(12S_GH), spring 2013(13S_GH) and four field experiments conducted at Manhattan in 2013 (13MH_FD) and 2014 (14MH_FD), and Hays in 2013 (14Hays_FD) and 2014 (14Hays_FD), and b the relationships and grouping of wheat accessions that were determined using the mean pre-harvest sprouting data collected from all four greenhouse and four field experiments. Similarity levels increase from light yellow (the lowest similarity) to dark red (the highest similarity)
Genome-wide association studies on grain color
According to the BIC values, the mixed model with population structure and kinship fit the best for the GC trait, thus was applied in the following analysis. GWAS detected four significant QTL on chromosomes 1B, 3A, 3B and 3D, which were represented by the gene markers for Tamyb10 genes (5 STS markers) and closely linked SSRs (6) and SNPs (12) (Table 2). Three major QTL for GC in the distal region of the long arms of group 3 chromosomes are significant for the data from both greenhouse and field experiments (Table 2). Among them, the QTL on 3DL, as indicated by significant markers Tamyb10-D1 and 3 SNPs, showed the largest effect and explained up to 23.0 % of the phenotypic variance for GC. The QTL on chromosome 3BL that was characterized by seven SNPs, two gene makers for Tamyb10-B1, and one SSR was significant in both greenhouse and field experiments, and explained up to 19.2 % of the phenotypic variance. A QTL on 3AL showed a moderate effect on GC, and explained about 11.1 % phenotypic variance. QTL on chromosomes 1B was identified in both field and greenhouse experiments and explained up to 11.7 % of the phenotypic variances. Also, three SSRs, Xwmc93, Xbarc145 and Xbarc148, were also significant for GC, but their positions cannot be determined because they were mapped to multiple chromosomes.
Table 2.
Quantitative trait loci (QTL) identified for wheat grain color (GC) evaluated for the seeds harvested from the field experiment of Enid, OK, in,2010 (Enid2010) and from the greenhouse (GH) experiment conducted in Manhattan KS, 2011 (GH2011)
| Chromosome | Marker name | Marker type | Chromosome Position (cM)a | Positive allele frequency | Enid2010 | GH2011 | Mean | |||
|---|---|---|---|---|---|---|---|---|---|---|
| p | R 2 (%)b | p | R 2 (%) | p | R 2 (%) | |||||
| 1B | Ra_c35710_395 | 90K | 58.08 | 0.92 | 7.31E-06 | 9.9 | 2.19E-05 | 9.3 | 3.16E-06 | 10.8 |
| 1B | RAC875_c1188_531 | 90K | 58.08 | 0.92 | 4.62E-06 | 10.4 | 8.06E-06 | 10.3 | 1.33E-06 | 11.7 |
| 3A | Xwmc559-1 | SSR | 107.20 | 0.94 | 1.25E-04 | 9.8 | 5.00E-04 | 10.6 | 1.57E-04 | 10.8 |
| 3A | Tamyb10-A1-66 | STS | 114.02 | 0.63 | 8.75E-06 | 7.5 | 1.86E-04 | 10.2 | 2.25E-05 | 9.4 |
| 3A | Tamyb10-A1-74 | STS | 114.02 | 0.61 | 3.12E-06 | 8.5 | 6.50E-05 | 11.1 | 7.32E-06 | 10.4 |
| 3B | BS00040742_51 | 90K | 68.26 | 0.36 | 9.42E-06 | 9.7 | - | - | 2.82E-05 | 8.6 |
| 3B | Tdurum_contig100004_204 | 90K | - | 0.38 | 4.81E-06 | 10.3 | 1.00E-05 | 10.1 | 7.54E-06 | 9.9 |
| 3B | BS00025679_51 | 90K | 76.22 | 0.58 | 2.45E-05 | 8.7 | 1.88E-06 | 11.8 | 6.30E-06 | 10.1 |
| 3B | Kukri_c60633_121 | 90K | 76.22 | 0.35 | 8.53E-06 | 9.8 | 2.35E-06 | 11.6 | 4.23E-06 | 10.5 |
| 3B | Kukri_c60633_257 | 90K | 76.22 | 0.33 | 3.63E-05 | 8.3 | 5.35E-06 | 10.7 | 9.98E-06 | 9.6 |
| 3B | Excalibur_rep_c97324_623 | 90K | 76.22 | 0.35 | 5.23E-06 | 10.3 | 1.64E-06 | 12.0 | 2.50E-06 | 11.0 |
| 3B | Tamyb10-B1-1 | STS | 77.36 | 0.26 | 2.26E-05 | 11.1 | 1.06E-05 | 11.0 | 7.46E-06 | 11.8 |
| 3B | Tamyb10-B1-2 | STS | 77.36 | 0.26 | 7.32E-07 | 11.1 | 8.21E-07 | 11.0 | 3.22E-07 | 11.8 |
| 3B | Xbarc84 | SSR | 80.77 | 0.31 | 1.89E-05 | 4.1 | - | - | 1.16E-04 | 6.4 |
| 3D | GENE-1785_118 | 90K | - | 0.42 | 4.74E-06 | 10.4 | 2.42E-09 | 19.2 | 6.49E-08 | 14.8 |
| 3D | D_GA8KES402JVT1Y_74 | 90K | 11.37 | 0.54 | 1.32E-07 | 14.0 | 3.31E-10 | 21.5 | 1.79E-09 | 18.7 |
| 3D | BS00067163_51 | 90K | 92.34 | 0.52 | 5.36E-08 | 15.0 | 8.39E-11 | 23.2 | 7.49E-10 | 19.7 |
| 3D | BS00063075_51 | 90K | - | 0.72 | 8.85E-05 | 7.5 | 5.03E-06 | 10.8 | 9.74E-06 | 9.7 |
| 3D | Tamyb10-D1-93 | STS | - | 0.56 | 4.31E-11 | 21.9 | 3.66E-13 | 17.5 | 6.51E-13 | 21.0 |
| 3D | Xbarc376 | SSR | - | 0.94 | - | - | 5.00E-04 | 24.6 | 7.00E-04 | 23.3 |
| 1A/1D | Xwmc93 | SSR | - | 0.37 | - | - | 6.10E-05 | 5.3 | 3.00E-04 | 7.2 |
| 1A/2D/3B | Xbarc145 | SSR | - | 0.11 | - | - | 6.62E-05 | 5.0 | 1.65E-04 | 6.7 |
| 1A/1D/3A/5B | Xbarc148 | SSR | - | 0.70 | 2.50E-07 | 16.6 | 1.03E-06 | 18.7 | 2.27E-07 | 18.4 |
aThe marker positions in the chromosome based on W7984 reference map
bPhenotypic variance explained by a significant marker significantly related to grain color
The association mapping population can be classified into eight genotypic groups based on the allele combinations of the gene markers of Tamyb10 genes on group 3 chromosomes. The average GC scores in each group tended to increase as the number of red color alleles increases. However, red wheat accessions T154, LA02-923, MO040192 and NC04-15533 do not contain the red alleles (abd) at any of the three loci, whereas white accessions KS05HW15-2 and OK06848W carry the red allele of Tamyb10-A1 (Abd), and white accessions KS05HW136-3 and CO03W139 carry the red allele of Tamyb10-D1 (abD) (Fig. 3), suggesting that other genes besides Tamyb10 may also contribute to GC, or the markers for Tamyb10 genes may not be diagnostic in some genetic backgrounds.
Fig. 3.
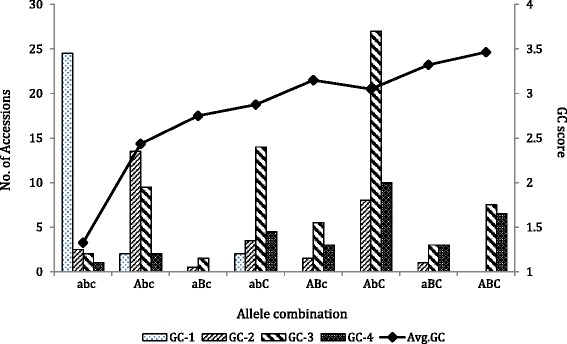
Distribution of grain color (GC) scores in the association mapping population predicted by Tamyb10 gene markers. Six allele combinations of three GC genes on chromosomes A, B and D separated 185 accessions into eight genotypes. Lower case represents a white grain allele and upper case represents a red grain allele in each locus. The three letters in each genotype represent three gene loci in the chromosomes A, B and D, respectively, e.g, Abc indicates red allele on 3A and white alleles on 3B and 3D. GC scores used a 1-4 scale with 1 for white grain and 4 for red grain
Genome-wide association studies on pre-harvest sprouting resistance
Generalized linear model with Q matrix of k = 3 was selected for GWAS on PHS resistance based on BIC values. Twelve QTL on ten chromosomes were significant for PHS resistance in at least two experiments (Table 3; Additional file 3: Table S3). Among them, QTL on chromosome 3AS, 3AL, 3B and 4AL were the most frequently identified QTL for PHS resistance. The 3AS QTL was detected in the fall 2011 greenhouse experiment and all the four field experiments, and explained 9.5 % to 15.8 % of the phenotypic variances for PHS resistance. Significant markers included one SSR, Xbarc57, five SNPs from the SNP chips and one SNP developed from the TaPHS1 gene sequence (Table 3; Additional file 3: Table S3), thus this QTL corresponds to TaPHS1. The 3AL QTL was identified by SNPs, SSR and the Tamyb10-a1 gene marker in spring 2012 and all the field experiments, and explained 6.8 % to 12.1 % of the phenotypic variances. Thus this QTL corresponds to the Tamyb10-a1 gene for GC on 3AL. The QTL on chromosome 4AL showed a wide range of effects among the experiments, and explained 9.9 % to 47.6 % phenotypic variance among the two greenhouse experiments (fall and spring 2012) and one field experiment (Manhattan, 2014). The most significant SNPs for Qphs.hwwgr-4A were Ex_c66324_1151, wsnp_Ex_c13031_20625900 and wsnp_Ex_rep_c66324_64493429. ZXQ118, an EST in the 4A QTL region (Zhang et al., 2008), and the gene markers for PM19A1 were also significant in the fall 2012 greenhouse experiment and the mean sprouting rates over all the greenhouse experiments, but explained much lower phenotypic variation than the previous three markers (Table 3; Additional file 3: Table S3). The QTL on chromosome 3B was significant in two greenhouse experiments (fall 2011 and spring 2012) and all field experiments, and explained 7.0 % to 12.3 % of the phenotypic variances for PHS resistance. Two QTL were identified on chromosome 3D with one at the distal end of the short arm (Qphs.hwwgr-3DS) and another at the distal end of the long arm (Qphs.hwwgr-3DL). Qphs.hwwgr-3DS was detected in the spring 2012 greenhouse, and two 2014 field experiments, and Qphs.hwwgr-3DL was significant in both field experiments in 2013. QTL identified on chromosome 7A was significant in the fall 2011 greenhouse and 2013 Manhattan field experiments and explained up to 13.5 % of the phenotypic variance.
Table 3.
Representative markers for quantitative trait loci (QTL) of wheat pre-harvest sprouting resistance identified in at least two of the experiments using sprouting rates (%) evaluated in the fall 2011 (2011F), spring 2012 (2012S), fall 2012 (2012F) and spring 2013 (2013S) greenhouse experiments, the 2013 and 2014 Manhattan (2013MH and 2014MH) and 2013 and 2014 Hays (2013Hays and 2014Hays) field experiments, and using the best linear unbiased predictions (BLUP) of each accession from all the greenhouse (GH_BLUP) and field (Field_BLUP) experiments
| Chromosome | Marker name | Marker type | Chromosome position (cM)a | Positive allele frequency | p | R 2 (%)b | Experiment |
|---|---|---|---|---|---|---|---|
| 1A | BS00011787_51 | 90K | 34.28 | 0.49 | 3.30E-05 | 9.2 | 2013MH |
| 1A | Kukri_c60564_136 | 90K | 50.20 | 0.93 | 6.54E-05 | 9.1 | 2011F |
| 1D | Ex_c6765_2118 | 90K | 48.90 | 0.83 | 5.74E-04 | 6.7 | 2013S |
| 1D | wsnp_Ku_c19622_29138795 | 90K | - | 0.67 | 3.38E-04 | 7.3 | 2013S |
| 1D | Xgwm337 | SSR | - | 0.18 | 4.98E-04 | 17.8 | 2012S |
| 1.30E-04 | 13.3 | 2013S | |||||
| 4.70E-04 | 13.5 | GH_BLUP | |||||
| 2D | BobWhite_c1477_315 | 90K | - | 0.33 | 9.48E-04 | 6.2 | 2013S |
| 2D | Xgwm539 | SSR | - | 0.11 | 5.00E-04 | 11.7 | 2013MH |
| 6.00E-04 | 15.1 | 2014Hays | |||||
| 3.38E-04 | 16.7 | Field_BLUP | |||||
| 3AS | wsnp_Ex_rep_c67702_66370241 | 9K | 9.12 | 0.78 | 4.42E-05 | 9.5 | 2011F |
| 3AS | wsnp_Ra_c2339_4506620 | 9K | 9.12 | 0.38 | 5.28E-05 | 8.7 | 2013MH |
| 5.66E-05 | 8.5 | Field_BLUP | |||||
| 3AS | KASP-TaPHS1.1 | STS | - | 0.78 | 5.55E-05 | 9.3 | 2011F |
| 3AS | Xbarc57.2 | SSR | - | 0.79 | 6.74E-06 | 14.0 | 2013MH |
| 6.56E-06 | 14.2 | 2013Hays | |||||
| 9.46E-05 | 15.3 | 2014MH | |||||
| 2.00E-04 | 13.7 | 2014Hays | |||||
| 3.78E-06 | 15.9 | Field_BLUP | |||||
| 3AL | wsnp_Ku_c5359_9531713 | 9K | L201.88 | 0.89 | 2.50E-04 | 7.6 | 2012S |
| 3AL | Tamyb10-A1-66 | STS | 114.02 | 0.61 | 4.67E-07 | 12.1 | 2013MH |
| 1.82E-05 | 9.2 | 2013Hays | |||||
| 3.00E-04 | 6.8 | 2014MH | |||||
| 2.83E-05 | 8.6 | 2014Hays | |||||
| 2.01E-06 | 10.9 | Field_BLUP | |||||
| 3AL | Xwmc559-1 | SSR | 107.20 | 0.89 | 9.00E-04 | 10.4 | 2013Hays |
| 3B | wsnp_BE446087B_Ta_2_1 | 9K | 46.66 | 0.91 | 3.22E-05 | 9.3 | 2013Hays |
| 3BL | Xbarc77 | SSR | - | 0.94 | 5.33E-04 | 12.3 | 2012S |
| 4.00E-04 | 7.6 | 2013MH | |||||
| 2.00E-04 | 9.5 | 2014Hays | |||||
| 4.82E-04 | 8.1 | Field_BLUP | |||||
| 3BL | Xgwm108 | SSR | - | 0.81 | 9.00E-04 | 7.0 | 2013MH |
| 2.00E-04 | 7.5 | 2014MH | |||||
| 5.68E-04 | 7.3 | Field_BLUP | |||||
| 3BL | Xgwm181 | SSR | - | 0.92 | 3.00E-04 | 12.2 | 2011F |
| 3DS | BS00067117_51 | 90K | - | 0.47 | 8.26E-06 | 11.1 | 2014MH |
| 8.56E-05 | 7.4 | 2014Hays | |||||
| 3DS | Kukri_c50527_241 | 90K | - | 0.35 | 8.46E-04 | 6.3 | 2012S |
| 2.97E-05 | 9.7 | 2014MH | |||||
| 3DS | BobWhite_c3111_636 | 90K | - | 0.32 | 1.15E-05 | 10.8 | 2014MH |
| 8.88E-05 | 7.4 | 2014Hays | |||||
| 7.09E-05 | 8.3 | Field_BLUP | |||||
| 3DL | BS00067163_51 | 90K | 92.34 | 0.52 | 7.79E-05 | 8.3 | 2013MH |
| 3DL | Tamyb10-D1-93 | STS | - | 0.56 | 6.00E-04 | 5.7 | 2013MH |
| 3.00E-04 | 6.7 | 2013Hays | |||||
| 4A | Ex_c66324_1151 | 90K | 76.97 | 0.42 | 3.67E-17 | 47.6 | 2012F |
| 2.37E-05 | 9.9 | 2014MH | |||||
| 1.34E-12 | 31.5 | GH_BLUP | |||||
| 4A | wsnp_Ex_rep_c66324_64493429 | 90K | 76.97 | 0.43 | 1.51E-16 | 45.3 | 2012F |
| 2.60E-05 | 9.8 | 2014MH | |||||
| 2.99E-12 | 30.4 | GH_BLUP | |||||
| 4A | Xbarc236 | SSR | 92.92 | 0.23 | 7.41E-05 | 14.1 | 2012F |
| 4A | Xwmc757 | SSR | - | 0.11 | 9.84E-04 | 11.9 | 2012S |
| 5A | BobWhite_c4004_61 | 90K | 35.36 | 0.83 | 2.62E-04 | 7.6 | 2013S |
| 5A | Excalibur_c54774_408 | 90K | 47.99 | 0.77 | 5.60E-04 | 6.7 | 2012S |
| 6B.2 | wsnp_Ex_c19525_28494827 | 90K | 94.46 | 0.91 | 1.21E-05 | 10.2 | 2013MH |
| 8.19E-06 | 10.8 | 2013Hays | |||||
| 2.81E-05 | 9.3 | Field_BLUP | |||||
| 6B.2 | Excalibur_c15109_942 | 90K | 95.60 | 0.64 | 4.29E-04 | 7.0 | 2013S |
| 6B | Xgwm88 | SSR | - | 0.91 | 9.55E-04 | 8.9 | 2012S |
| 7A | wsnp_Ex_c26509_35755018 | 9K | 69.63 | 0.95 | 1.41E-06 | 13.5 | 2011F |
| 7A | Xwmc603 | SSR | - | 0.92 | 1.00E-04 | 25.4 | 2011F |
| 7A | Xgwm130 | SSR | - | 0.94 | 5.00E-04 | 10.6 | 2013MH |
| 1A/1D/3A/5B | Xbarc148 | SSR | - | 0.83 | 1.38E-08 | 22.8 | 2013MH |
| 3.38E-05 | 11.9 | 2013Hays | |||||
| 2.22E-05 | 14.5 | 2014Hays | |||||
| 3.43E-06 | 14.7 | Field_BLUP | |||||
| 5ABD | Xbarc232 | SSR | - | 0.92 | 6.00E-04 | 10.3 | 2013MH |
| 4.40E-05 | 12.6 | 2014MH | |||||
| 3.97E-05 | 13.6 | 2014Hays | |||||
| 3.80E-04 | 10.9 | Field_BLUP |
aThe positions of markers on W7984 reference sequence
bPhenotypic variance explained by a significant marker significantly related to pre-harvest sprouting resistance
Some QTL were only significant in a single environment. For example, the two QTL identified on chromosomes 2B, Qphs.hwwgr-2B.1 and Qphs.hwwgr-2B.2, were associated with PHS resistance each in one greenhouse experiment (spring 2013 and 2012, respectively), and the Qgc.hwwgr-6B.1 was identified only in one field experiment (Manhattan, 2013) (Table 4). Therefore, these QTL may be more sensitive to environmental conditions.
Table 4.
Quantitative trait loci (QTL) for pre-harvest sprouting resistance identified in only one of the experiments conducted in the spring 2012 (2012S) and spring 2013 (2013S) greenhouse experiments and the 2013 Manhattan field experiment (2013MH)
| Chromosome | Marker name | Marker type | Chromosome position (cM)a | Positive allele frequency | p | R 2 (%)b | Experiment |
|---|---|---|---|---|---|---|---|
| 2B.1 | Excalibur_c1787_1199 | 90K | 7.97 | 0.81 | 2.97E-04 | 7.4 | 2013S |
| 2B.1 | BS00044806_51 | 90K | 10.24 | 0.72 | 4.98E-04 | 6.9 | 2013S |
| 2B.1 | Tdurum_contig51145_476 | 90K | 10.24 | 0.76 | 3.65E-04 | 7.2 | 2013S |
| 2B.1 | BS00022203_51 | 90K | - | 0.17 | 7.08E-04 | 6.5 | 2013S |
| 2B.1 | Excalibur_c3524_318 | 90K | - | 0.81 | 7.05E-04 | 6.5 | 2013S |
| 2B.1 | Kukri_c16758_443 | 90K | 10.24 | 0.73 | 1.31E-04 | 8.3 | 2013S |
| 2B.1 | wsnp_JD_c3288_4296662 | 9K | 10.24 | 0.77 | 7.57E-04 | 6.4 | 2013S |
| 2B.1 | BS00065556_51 | 90K | - | 0.77 | 2.56E-04 | 7.6 | 2013S |
| 2B.2 | wsnp_Ex_c13865_21720466 | 9K | 83.07 | 0.42 | 6.42E-04 | 6.6 | 2012S |
| 2B.2 | wsnp_RFL_Contig3273_3319580 | 90K | 83.07 | 0.41 | 7.30E-04 | 6.4 | 2012S |
| 2B.2 | RAC875_c26697_589 | 90K | 83.07 | 0.35 | 4.27E-04 | 7.0 | 2012S |
| 2B.2 | Tdurum_contig28795_322 | 90K | - | 0.41 | 7.63E-04 | 6.4 | 2012S |
| 6B.1 | RAC875_c23251_624 | 90K | 43.28 | 0.89 | 2.59E-05 | 9.4 | 2013MH |
| 6B.1 | BS00066799_51 | 90K | 43.28 | 0.92 | 7.13E-05 | 8.3 | 2013MH |
| 6B.1 | CAP8_c1361_367 | 90K | 43.28 | 0.89 | 2.59E-05 | 9.4 | 2013MH |
aThe positions of markers on W7984 reference sequence
bPhenotypic variance explained by a marker that was significantly associated with pre-harvest sprouting resistance
Relationships between grain color and pre-harvest sprouting resistance
Analysis of variance (ANOVA) was conducted by taking CG as the explanatory variable and PHS resistance as the response variable, and it showed that GC had significant effects on PHS resistance in all the field experiments (P < 0.0001), but not in any of the greenhouse experiments (Table 5). White wheat had significantly higher sprouting rates than red wheats (P < 0.0001) in the field experiments, but the difference was not significant between red-grained accessions with different color scores (data not shown).
Table 5.
Effect of grain color (GC) that was evaluated in the field at Enid, OK in 2010 (2010Enid) and the greenhouse at Manhattan KS 2011 (2011F_GH) on pre-harvest sprouting (PHS) resistance evaluated in four greenhouse experiments (GH_experiments) conducted in Manhattan, KS, and four field experiments conducted in Manhattan (MH) and Hays, KS in 2013 and 2014, respectively
| Experiments | 2010Enid (GC) | 2011F_GH (GC) | ||
|---|---|---|---|---|
| p | R 2 (%)a | p | R 2 (%)a | |
| GH_experiments (PHS) | NS | - | NS | - |
| 2013MH (PHS) | <2e-16 | 43.7 | <2e-16 | 44.5 |
| 2013Hays (PHS) | <2e-16 | 42.9 | <2e-16 | 43.6 |
| 2014MH (PHS) | 1.27E-10 | 26.3 | 3.13E-11 | 27.5 |
| 2014Hays (PHS) | 1.13E-15 | 35.6 | 3.41E-15 | 34.8 |
| Field_BLUP b(PHS) | <2e-16 | 43.9 | <2e-16 | 44.1 |
aPhenotypic variance explained by grain color in each PHS experiment, which is derived from the analysis of variance (ANOVA) where grain color (GC) was used as the explanatory variable and pre-harvest sprouting (PHS) resistance as the response variable
bField_BLUP = Best Linear Unbiased Predictions calculated from all four field experiment
Common QTL for GC and PHS resistance were identified on the long arms of chromosomes 3A and 3D (Table 6), but not on 3BL. The QTL on chromosome 3AL, identified by Tamyb10-A1, was significant for GC in both field and greenhouse experiments and for PHS resistance in all the field experiments. For the QTL on chromosome 3D as represented by Tamyb10-D1, one SNP was significant for GC in both experiments and also for PHS resistance in the 2013 field experiments at both Manhattan and Hays. Unlike the 3A and 3D QTL, QTL on chromosome 3B, represented by the Tamyb10-B1 as well as seven linked SNPs and one SSR, was significant for only GC, not PHS resistance in any experiments. Therefore, Tamyb10-A1 and Tamyb10-D1, but not Tamyb10-B1, were very likely to have pleiotropic effects on PHS resistance under the field conditions.
Table 6.
Common Quantitative trait loci (QTL) identified for grain color evaluated in 2010 field (2010Enid) and 2011 greenhouse (GH) experiments and pre-harvest sprouting resistance evaluated in Manhattan (MH) and Hays in 2013 and 2014 experiments, respectively
| Grain color | PHS resistance | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chromosome | Marker name | Chromosome position (cM)a | 2010Enid | 2011GH | Mean | 2013MH | 2013Hays | 2014MH | 2014Hays | Field_BLUP | ||||||||
| p | R 2 (%)b | p | R 2 (%)b | p | R 2 (%)b | p | R 2 (%)c | p | R 2 (%)c | p | R 2 (%)c | p | R 2 (%)c | p | R 2 (%)c | |||
| 3AL | Xwmc559-1 | 107.20 | 1.25E-04 | 9.8 | 5.00E-04 | 10.6 | 1.57E-04 | 10.8 | - | - | 9.00E-04 | 10.4 | - | - | - | - | - | - |
| 3AL | Tamyb10-A1-66 | 114.02 | 8.75E-06 | 7.5 | 1.86E-04 | 10.2 | 2.25E-05 | 9.4 | 4.67E-07 | 12.1 | 1.82E-05 | 9.2 | 3.00E-04 | 6.8 | 2.83E-05 | 8.6 | 2.01E-06 | 10.9 |
| 3AL | Tamyb10-A1-74 | 114.02 | 3.12E-06 | 8.5 | 6.50E-05 | 11.1 | 7.32E-06 | 10.4 | 8.07E-07 | 11.6 | 3.47E-05 | 8.6 | - | - | 1.16E-04 | 7.3 | 1.12E-05 | 9.4 |
| 3DL | BS00067163_51 | 92.34 | 5.36E-08 | 15.0 | 8.39E-11 | 23.2 | 7.49E-10 | 19.7 | 7.79E-05 | 8.3 | - | - | - | - | - | - | - | - |
| 3DL | Tamyb10-D1-93 | - | 4.31E-11 | 21.9 | 3.66E-13 | 17.5 | 6.51E-13 | 21.0 | 6.00E-04 | 5.7 | 3.00E-04 | 6.7 | - | - | - | - | - | - |
| 1A/1D/3A/5B | Xbarc148 | - | 2.50E-07 | 16.6 | 1.03E-06 | 18.7 | 2.27E-07 | 18.4 | 1.38E-08 | 22.8 | 3.38E-05 | 11.9 | - | - | 2.22E-05 | 14.5 | 3.43E-06 | 14.7 |
aThe marker positions in a chromosome based on W7984 reference map
bPhenotypic variance explained by a marker that is significantly associated with grain color
cPhenotypic variance explained by a marker that is significantly associated with pre-harvest sprouting resistance
Validation of the significant SNPs for the 4A QTL in a bi-parental population
Seventeen KASP assays were designed based on the sequences of the significant SNPs identified in the 4A QTL region for PHS resistance. Four of the KASP markers (Additional file 4: Table S4) showed co-segregation among the F6 RILs of 'Tutoumai A' × 'Siyang 936', and were mapped between the two previously reported flanking GBS SNPs (GBS212432, GBS109947) for the QTL [7] at 1.02 cM to GBS212432 and 2.10 cM to GBS109947 (Fig. 4). These four SNPs showed the highest LOD scores in all experiments, and explained up to 31.76 % of the phenotypic variance in the population.
Fig. 4.
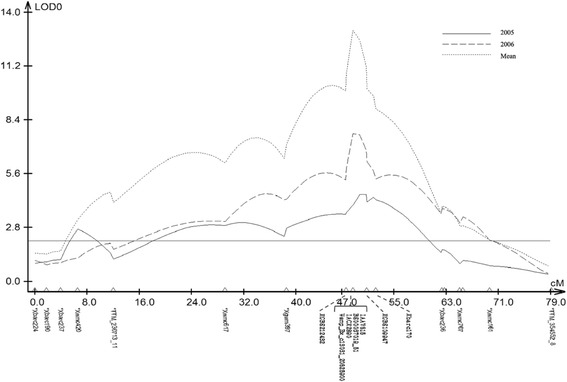
Interval mapping (IM) of a quantitative trait locus (QTL) for pre-harvest sprouting (PHS) resistance on chromosome 4A using SSRs, GBS-SNPs and SNPs identified from genome-wide association study (GWAS). The line parallel to the X-axis is the threshold line for the significant LOD value of 2.42 (P < 0.05). Genetic distances are in centiMorgans (cM)
Linkage disequilibrium
Linkage disequilibrium (LD) parameter r 2 was calculated to determine the linkage relationship between SNPs from different QTL and link the markers with unknown positions to known QTL. LD was calculated for the 125 SNPs that were significantly linked to nine PHS resistance QTL in at least two experiments. Strong LD was detected for SNPs within each PHS resistance QTL region, but not between different QTL (Fig. 5b), indicating that those QTL for PHS resistance were independent. Pair-wise r 2 values were also estimated for the 17 SNPs that were tightly linked to the five GC QTL. Similarly, strong LD was not detected among the SNPs linked to GC QTL in group 3 chromosomes (Fig. 5a).
Fig. 5.
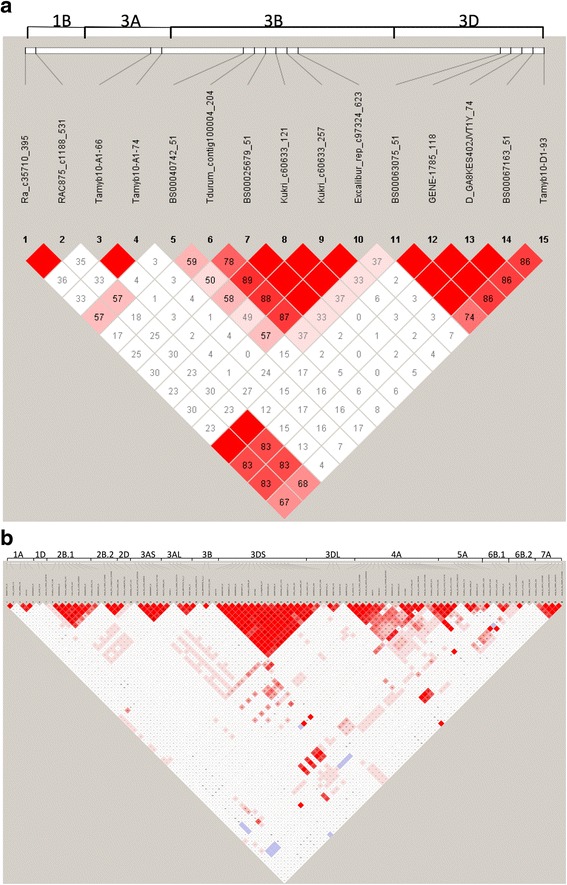
LD plots of SNP markers that showed significantly association with GC (a) and pre-harvest sprouting (PHS) resistance (b). The chromosome numbers are labeled above the chromosome maps (the long white bar) and marker names are labeled between the LD plot and chromosome maps
Genetic positions of most significant SNPs for GC on chromosome 3D and Tamyb10-D1 could not be determined using the W7984 reference sequence, and SNPs significantly related to GC on chromosome 3D, D_GA8KES402JVT1Y_74 and BS00067163_51, were far apart from each other on the chromosome 3D. However, LD analysis suggested that these SNPs were tightly linked to Tamyb10-D1, and thus they linked to the same 3D QTL for GC (Fig. 5a).
Discussion
QTL for grain color
Wheat GC has been a classic example for dissection of a quantitative trait [50] and three genes on wheat chromosomes group 3 have long been proposed as the genes controlling wheat GC. Several previous studies have mapped the three genes as major QTL as well as some minor QTL on chromosomes 2B, 2D, 5A and 6B for GC [1, 25, 51]. Being the first association study for wheat GC, we not only validated the effects of these three GC genes, Tamyb10-A1, Tamyb10-B1 and Tamyb10-D1, on the long arms of chromosomes group 3, but also identified a new QTL on the chromosome 1B for GC, suggesting that QTL on other chromosomes than these well-known QTL on chromosomes group 3 may also play a role in regulating GC in some wheat germplasm lines.
Groos et al. [1] mapped all group 3 QTL in a bi-parental population, but they did not discuss their effects of each QTL. In this study, a diverse association panel makes it possible to compare the effects of all the three QTL. Among the three genes on the chromosome group 3, Tamyb10-D1 had the largest effect on GC (R2 = 0.24) and Tamyb10-A1 the smallest (R2 = 0.11) in the association mapping panel whereas their minor allele frequencies (MAF) were similar (Table 2), indicating that the large effect of Tamyb10-D1 was not due to a higher MAF than other two genes. On the other hand, one single gene changed GC from white to red, and adding one or two additional GC genes only slightly increased redness (Fig. 3). Besides, QTL on chromosome 1B also contribute to GC, which was not reported previously, thus it is likely a new QTL for GC. That the red allele of the 1B QTL presents in the four red wheat accessions that do not carry the red alleles (abd) at any of the three Tamyb10 genes supports this assumption. Therefore, when breeding for white wheat cultivars, breeders not only need to remove the three Tamyb10 genes, but also should watch for other genes that may contribute to GC.
In this study, wheat GC was visually scored after increasing color intensities using sodium hydroxide solution. High repeatability in GC between the greenhouse and field experiments (Fig. 1) indicates that the GC scoring method used in the experiments is highly repeatable. All of the four QTL identified for GC were detected in both experiments, which provided genetic evidence that QTL for GC are relatively stable across environments.
QTL for pre-harvest sprouting resistance
QTL for PHS resistance have been mapped on almost all wheat chromosomes in previous bi-parental mapping studies. Although association studies on PHS resistance have been conducted using several types of markers [11, 30], the current study is the first report to use high density SNPs for GWAS on PHS resistance. We identified 12 QTL that were significance in at least two experiments.
For the QTL on 3AS, the causal gene (TaPHS1) has been cloned [3, 4]. One of the reported functional SNPs in the coding region [4] was significant in one greenhouse (fall 2011), whereas the functional SNP in the promoter region [3] was not significant in any of the experiments (Table 3; Additional file 3: Table S3). However, the most significant markers linked to the 3AS PHS resistance QTL were not the functional SNPs (Additional file 3: Table S3), which was probably due to environmental effects on phenotyping [3]. We had similar result for markers in the 4A QTL region. Only one of the candidate gene markers of PM19A1 was significantly associated with PHS resistance in the fall 2012 experiment, although the 4A QTL showed an extremely large effect on PHS in that experiment (Table 3; Additional file 3: Table S3). This was probably due to the fact that the gene expression was affected by environments or the gene markers are not diagnostic.
The QTL identified at the distal end of chromosome 3DS was not reported previously. LD analysis indicated that Qgc.hwwgr-3DS is different but could be a homeologue of Qgc.hwwgr-3AS (Fig. 5b). For the QTL on chromosome 3B, the sequences of the linked SSR markers are not found in the W7984 reference sequence, thus we cannot determine whether or not the significant SSR markers and SNPs on 3B linked to the same QTL. Similarly, we cannot determine the QTL positions on chromosome 7A.
QTL identified on chromosome 1A could be the same QTL reported by Knox et al. [52] in durum because Xwmc183 was located near the QTL region mapped in our study based on the W7984 reference sequence. The QTL on chromosome 2D is the same QTL as QPhs.ccsu-2D.4 [53] because of the common SSR Xgwm539. However, we cannot determine whether the QTL that were identified on chromosomes 1D, 5A, 5B, 6A and 6B were the same QTL reported in previous studies [1, 9, 30, 51, 54] due to the lack of common markers.
Variation of PHS resistance across environments
PHS is a complicated trait affected by many factors, including seed dormancy (SD) [15, 55–57], GC [1, 58], spike morphology, as well as environmental factors such as temperature, moisture and photoperiod after flowering [59, 60]. In the current study, PHS resistance of the tested accessions and QTL effects varied across environments with more variation observed among the greenhouse experiments than that among the field experiments (Fig. 2a). A total of four greenhouse experiments were conducted in the fall greenhouse cycles of 2011 and 2012 with the harvest time in winter, and the spring cycles of 2012 and 2013 with the harvest time in summer. The two seasons were highly different in growing and post-harvesting temperatures, which has been shown to influence PHS resistance [3, 6]. Meanwhile, in the field experiments at Manhattan and Hays, dry hot winds shortened maturity period, which greatly reduced environment effects on wheat PHS resistance. Therefore, PHS resistance was similar in the four field experiments.
Qphs.hwwgr-3AS and Qphs.hwwgr-4A were the major QTL for PHS resistance, and most frequently identified in all experiments. However, Qphs.hwwgr-3AS was detected more frequently in the field experiments, while Qphs.hwwgr-4A was detected more frequently in the greenhouse conditions (Table 3; Additional file 3: Table S3), which might be due to high temperatures in field conditions during late grain maturation that suppressed the expression of Qphs.hwwgr-4A [6].
According to the heat map derived from individual PHS ratings across all the experiments, the population can be roughly divided into three clusters (Fig. 2b). Most of the soft winter wheats had low germination rates, and were clustered to Group 1. Wheat cultivars from RGON were mostly clustered to Group 1 and Group 2, whereas accessions in SRPN and NRPN showed higher germination rates, and were mainly clustered to Group 2 and Group 3. These results indicated that the soft winter wheat accessions grown in the humid climate during harvest season had a higher selective pressure on PHS resistance than the hard winter wheat accessions from the Great Plains that grown under relatively drier climate.
Validation of the markers for the QTL on 4A
In this study, a RIL population from ‘Tutoumai A’ x ‘Siyang 936’ was used to validate the position of significant SNPs for 4A PHS resistance QTL. Four polymorphic SNPs from GWAS were successfully mapped to the QTL region, and they are more closely linked to PHS resistance than previously reported flanking markers, GBS212432 and GBS109947, for this QTL [7]. This result indicates that GWAS provides more power to increase marker density and mapping resolution, whereas bi-parental populations can further validate the positions of new markers. Barrero et al. [6] proposed PM19A1 and PM19A2 as the candidate genes for the 4A QTL and identified causal deletions in PM19A1 and PM19A2. We analyzed the markers developed based on the causal variation in Tutoumai A and Siyang 936, but did not find any polymorphism between the two parents. Therefore, a different gene or different causal SNP in the gene may control the PHS resistance of 4A QTL in this population, which was also supported by the results from the GWAS that the candidate gene markers contributed much lower phenotypic variation for PHS resistance than three other SNP markers (Ex_c66324_1151, wsnp_Ex_c13031_20625900, wsnp_Ex_rep_c66324_64493429) (Table 3; Additional file 3: Table S3).
Effect of grain color QTL on pre-harvest sprouting resistance
GC has been considered as an important factor for PHS resistance, and previous studies showed that seed dormancy level of a white-grained wheat line was improved by the introgression of an R gene [16]. In the current study, GC explained 26 % to 44 % of the phenotypic variance for PHS resistance, and Tamyb10-A1 and Tamyb10-D1 showed significant effects on both GC and PHS resistance, which agree with a previous study [1]. Tamyb10 genes encode R2R3-type MBY transcription factors, which regulate the accumulation of PA in the biosynthesis pathways [25]. Therefore, it is possible that these transcription factors showed pleiotropic effects by regulating more than one metabolism pathway, and had effects on improving wheat PHS resistance. However, the GC gene on 3BL, Tamyb10-B1, did not show any effect on PHS resistance in this study (Table 2; Table 6).
In this study, GC was significantly related to PHS resistance in field experiments, but had barely any effect in the greenhouse experiments (Table 5). Also, the Tamyb10-A1 gene affected PHS resistance in all of the four field experiments, and the Tamyb10-D1 gene only affected PHS resistance in the 2013 experiments. Such results suggested that environmental factors could be important triggers of pleiotropic effects of the GC genes on PHS resistance. That Tamyb10-B1 did not show any effect on PHS resistance might be due to the field environments of this study that could not trigger the expression of pleiotropic effect of the gene.
Although some GC genes contributed to wheat PHS resistance, many QTL for PHS resistance did not affect GC. Therefore, some red wheats can be highly susceptible to PHS, while some white wheats can be highly resistant [61, 62]. Breeding for PHS resistance, attention should be paid to these QTL with a major effect on PHS in most environments without a pleiotropic effect on GC, such as these on 3AS and 4AL. Pyramiding several of these genes in one cultivar should be able to avoid PHS damage in U.S. HWW.
Conclusions
Using genome-wide association mapping, we identified four QTL for GC and 12 QTL for PHS resistance using 9K and 90K wheat SNP arrays. Besides three Tamyb10 genes cloned from group 3 chromosomes showed significant effects on GC, a new QTL on chromosome 1B also contributed to GC. Among them, Tamby10-A1 and Tamyb10-D1 showed a pleiotropic effect on PHS resistance under field conditions. Several other QTL that did not affect GC trait, especially the QTL on chromosomes 3AS and 4AL, showed significant effects on PHS resistance, thus they can be pyramided to improve PHS resistance in white wheat cultivars.
Acknowledgements
This is contribution number 17-001-J from the Kansas Agricultural Experiment Station. Authors thank Dr. William Bockus in Plant Pathology Department, Kansas State University for help with Manhattan field experiments. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. USDA is an equal opportunity provider and employer.
Funding
This study was partially funded by the National Research Initiative Competitive Grants CAP Project (2011-68002-30029) from the USDA National Institute of Food and Agriculture.
Availability of supporting data
All supporting data were included as additional files.
Authors’ contributions
GB conceived and designed this study. ML, DZ, SL, GZ carried out the experiments, ML, DZ, JY analyzed the data, and ML, GB, AF wrote the manuscript. All authors revised the manuscript and approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Abbreviations
- PHS
Pre-harvest sprouting
- GC
Grain color
- QTL
Quantitative trait loci
- GWAS
Genome-wide association study
- SNP
Single nucleotide polymorphism
- LD
Linkage disequilibrium
- RIL
Recombinant inbred line
- STS
Sequence-tagged site
- PCR
Polymorphic chain reaction
- MAF
Minor allele frequency
- KASP
Kompetitive Allele Specific PCR
- BIC
Bayesian information criterion
- BLUP
Best linear unbiased prediction
- GAPIT
Genome association and prediction integrated tool
- IM
Interval mapping
- GBS
Genotyping-by-sequencing
- RGON
Regional Germplasm Observation Nursery
- SRPN
Southern Regional Performance Nursery
- NRPN
Northern Regional Performance Nursery
- SD
Seed dormancy
Additional files
Table S1. Pedigree, sources, origins and preharvest sprouting phenotypic data of 185 accessions in the genome-wide association study panel. (XLSX 121 kb)
Table S2. Environmental statistics of greenhouse and field conditions in Manhattan and Hays (XLSX 44 kb)
Table S3. Quantitative trait loci identified at least twice among eight experiments using the association mapping population and the Best Linear Unbiased Prediction (BLUP) of greenhouse and field data of wheat sprouting rates in a spike. (XLSX 59 kb)
Table S4. Kompetitive Allele Specific PCR assays developed from significant SNPs for the 4A pre-harvest sprouting resistance quantitative trait locus. (XLSX 33 kb)
References
- 1.Groos C, Gay G, Perretant MR, Gervais L, Bernard M, Dedryver F, Charmet G. Study of the relationship between pre-harvest sprouting and grain color by quantitative trait loci analysis in a white × red grain bread-wheat cross. Theor Appl Genet. 2002;104:39–47. doi: 10.1007/s001220200004. [DOI] [PubMed] [Google Scholar]
- 2.Mares DJ, Mrva K, Cheong J, Williams K, Watson B, Storlie E, Sutherland M, Zou Y. A QTL located on chromosome 4A associated with dormancy in white- and red-grained wheats of diverse origin. Theor Appl Genet. 2005;111:1357–1364. doi: 10.1007/s00122-005-0065-5. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura S, Abe F, Kawahigashi H, Nakazono K, Tagiri A, Matsumoto T, Utsugi S, Ogawa T, Handa H, Ishida H, Mori M, Kawaura K, Ogihara Y, Miura H. A wheat homolog of MOTHER OF FT AND TFL1 acts in the regulation of germination. Plant Cell. 2011;23:3215–3229. doi: 10.1105/tpc.111.088492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S, Sehgal SK, Li J, Lin M, Trick HN, Yu J, Gill BS, Bai G. Cloning and characterization of a critical regulator for preharvest sprouting in wheat. Genetics. 2013;195:263–273. doi: 10.1534/genetics.113.152330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabral AL, Jordan MC, McCartney CA, You FM, Humphreys DG, MacLachlan R, Pozniak CJ. Identification of candidate genes, regions and markers for pre-harvest sprouting resistance in wheat (Triticum aestivum L.) BMC Plant Biol. 2014;14:340. doi: 10.1186/s12870-014-0340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrero JM, Cavanagh C, Verbyla KL, Tibbits JF, Verbyla AP, Huang BE, Rosewarne MG, Stephen S, Wang P, Whan A, Rigault P, Hayden JM, Gubler F. Transcriptomic analysis of wheat near-isogenic lines identifies PM19-A1 and A2 as candidates for a major dormancy QTL. Genome Biol. 2015;16:93. doi: 10.1186/s13059-015-0665-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin M, Cai S, Wang S, Liu S, Zhang G, Bai G. Genotyping-by-sequencing (GBS) identified SNP tightly linked to QTL for pre-harvest sprouting resistance. Theor Appl Genet. 2015;128:1385–1395. [DOI] [PubMed]
- 8.Torada A, Koike M, Ogawa T, Takenouchi Y, Tadamura K, Wu J, Matsumoto T, Kawaura K, Ogihara Y. A Causal Gene for Seed Dormancy on Wheat Chromosome 4A Encodes a MAP Kinase Kinase. Curr Biol. 2016;http://dx.doi.org/10.1016/j.cub.2016.01.063. [DOI] [PubMed]
- 9.Kulwal PL, Singh R, Balyan HS, Gupta PK. Genetic basis of pre-harvest sprouting tolerance using single-locus and two-locus QTL analyses in bread wheat. Funct Integr Genomics. 2004;4:94–101. doi: 10.1007/s10142-004-0105-2. [DOI] [PubMed] [Google Scholar]
- 10.Munkvold JD, Tanaka J, Benscher D, Sorrells ME. Mapping quantitative trait loci for preharvest sprouting resistance in white wheat. Theor Appl Genet. 2009;119:1223–1235. doi: 10.1007/s00122-009-1123-1. [DOI] [PubMed] [Google Scholar]
- 11.Kulwal P, Ishikawa G, Benscher D, Feng Z, Yu LX, Jadhav A, Mehetre S, Sorrells ME. Association mapping for pre-harvest sprouting resistance in white winter wheat. Theor Appl Genet. 2012;125:793–805. doi: 10.1007/s00122-012-1872-0. [DOI] [PubMed] [Google Scholar]
- 12.Zhang XQ, Li C, Tay A, Lance R, Mares D, Cheong J, Cakir M, Ma J, Appels R. A new PCR-based marker on chromosome 4AL for resistance to pre-harvest sprouting in wheat (Triticum aestivum L.) Mol Breed. 2008;22:227–236. doi: 10.1007/s11032-008-9169-3. [DOI] [Google Scholar]
- 13.Imtiaz M, Ogbonnaya FC, Oman J, Ginkel MV. Characterization of quantitative trait loci controlling genetic variation for preharvest sprouting in synthetic backcross derived wheat lines. Genetics. 2008;178:1725–1736. doi: 10.1534/genetics.107.084939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato K, Nakamura W, Tabiki T, Miura H, Sawada S. Detection of loci controlling seed dormancy on group 4 chromosomes of wheat and comparative mapping with rice and barley genomes. Theor Appl Genet. 2001;102:980–985. doi: 10.1007/s001220000494. [DOI] [Google Scholar]
- 15.Anderson JA, Sorrells ME, Tanksley SD. RFLP analysis of genomic regions associated with resistance to preharvest sprouting in wheat. Crop Sci. 1993;33:453–459. doi: 10.2135/cropsci1993.0011183X003300030008x. [DOI] [Google Scholar]
- 16.Flintham JE. Different genetic components control coatimposed and embryo-imposed dormancy in wheat. Seed Sci Res. 2000;10:43–50. doi: 10.1017/S0960258500000052. [DOI] [Google Scholar]
- 17.Warner RL, Kudrna DA, Spaeth SC, Jones SS. Dormancy in white-grain mutants of Chinese Spring wheat (Triticum aestivum L.) Seed Sci Res. 2000;10:51–60. [Google Scholar]
- 18.Himi E, Mares DJ, Yanagisawa A, Noda K. Effect of grain colour gene (R) on grain dormancy and sensitivity of the embryo to abscisic acid (ABA) in wheat. J Exp Bot. 2002;53(374):1569–1574. doi: 10.1093/jxb/erf005. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto T, Everson EH. Biochemical and physiological studies of wheat seed pigmentation. Agron J. 1958;50:733–734. doi: 10.2134/agronj1958.00021962005000120005x. [DOI] [Google Scholar]
- 20.McCallum JA, Walker JRL. Proanthocyanidins in wheat bran. Cereal Chem. 1990;67(3):282–285. [Google Scholar]
- 21.Sears ER. Cytogenetic studies with polyploid species of wheat. II. Additional chromosomal aberrations in Triticum vulgare. Genetics. 1944;29:232–246. doi: 10.1093/genetics/29.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allan RE, Vogel OA. Monosomic analysis of red seed color in wheat. Crop Sci. 1965;5:474–475. doi: 10.2135/cropsci1965.0011183X000500050030x. [DOI] [Google Scholar]
- 23.Metzger RJ, Silbaugh BA. Location of genes for seed coat color in hexaploid wheat, Triticum aestivum L. Crop Sci. 1970;10:495–496. doi: 10.2135/cropsci1970.0011183X001000050012x. [DOI] [Google Scholar]
- 24.Stoy V, Sundin K. Effects of growth regulating substances in cereal grain germination. Cereal Res Commun. 1976;4:157–163. [Google Scholar]
- 25.Himi E, Maekawa M, Miura H, Noda K. Development of PCR markers for Tamyb10 related to R-1, red grain color gene in wheat. Theor Appl Genet. 2011;122:1561–1576. doi: 10.1007/s00122-011-1555-2. [DOI] [PubMed] [Google Scholar]
- 26.Flint-Garcia SA, Thornsberry JM, Buckler ES., IV Structure of linkage disequilibrium in plants. Annu Rev Plant Biol. 2003;54:357–374. doi: 10.1146/annurev.arplant.54.031902.134907. [DOI] [PubMed] [Google Scholar]
- 27.Nordborg M, Weigel D. Next-generation genetics in plants. Nature. 2008;456:720–723. doi: 10.1038/nature07629. [DOI] [PubMed] [Google Scholar]
- 28.Myles S, Peiffer J, Brown PJ, Ersoz ES, Zhang Z, Costich DE, Buckler ES. Association mapping: critical considerations shift from genotyping to experimental design. Plant Cell. 2009;21:2194–2202. doi: 10.1105/tpc.109.068437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipka AE, Kandianis CB, Hudson ME, Yu J, Drnevich J, Bradbury PJ, Gore MA. From association to prediction: statistical methods for the dissection and selection of complex traits in plants. Curr Opin Plant Biol. 2015;24:110–118. doi: 10.1016/j.pbi.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Rehman Arif MA, Neumann K, Nagel M, Kobiljski B, Lohwasser U, Börner A. An association mapping analysis of dormancy and pre-harvest sprouting in wheat. Euphytica. 2012;188:409–417. doi: 10.1007/s10681-012-0705-1. [DOI] [Google Scholar]
- 31.Jaiswal V, Mir RR, Mohan A, Balyan HS, Gupta PK. Association mapping for pre-harvest sprouting tolerance in common wheat (Triticum aestivum L.) Euphytica. 2012;188:89–102. doi: 10.1007/s10681-012-0713-1. [DOI] [Google Scholar]
- 32.Albrecht T, Oberforster M, Kempf H, Ramgraber L, Schacht J, Kazman E, Zechner E, Neumayer A, Hartl L, Mohler V. Genome-wide association mapping of preharvest sprouting resistance in a diversity panel of European winter wheats. J Appl Genet. 2015;56(3):277–85. doi: 10.1007/s13353-015-0286-5. [DOI] [PubMed] [Google Scholar]
- 33.Zhang D, Bai G, Zhu C, Yu J, Carver BF. Genetic diversity, population structure, and linkage disequilibrium in U.S. elite winter wheat. Plant Genome. 2010;3:117–127. doi: 10.3835/plantgenome2010.03.0004. [DOI] [Google Scholar]
- 34.Liu S, Cai S, Graybosch R, Chen C, Bai G. Quantitative trait loci for resistance to pre-harvest sprouting in US hard white winter wheat Rio Blanco. Theor Appl Genet. 2008;117:691–699. doi: 10.1007/s00122-008-0810-7. [DOI] [PubMed] [Google Scholar]
- 35.Zadoks JC, Chang TT, Konzak CF. A decimal code for the growth stages of cereals. Weed Res. 1974;14(6):415–421. doi: 10.1111/j.1365-3180.1974.tb01084.x. [DOI] [Google Scholar]
- 36.Trethowan RM. Evaluation and selection of bread wheat (Triticum aestivum L) for preharvest sprouting tolerance. Aust J Agric Res. 1995;46:463–474. doi: 10.1071/AR9950463. [DOI] [Google Scholar]
- 37.Cavanagh CR, Chao S, Wang S, Huang BE, Stephen S, Kiani S, Forrest K, Saintenac C, Brown-Guedira GL, Akhunova A, et al. Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. Proc Natl Acad Sci U S A. 2013;110:8057–8062. doi: 10.1073/pnas.1217133110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang S, Wong D, Forrest K, Allen A, Chao S, Huang BE, Maccaferri M, Salvi S, Milner SG, Cattivelli L, Mastrangelo AM, Whan A, Stephen S, Barker G, Wieseke R, Plieske J, International Wheat Genome Sequencing Consortium. Lillemo M, Mather D, Appels R, Dolferus R, Brown-Guedira G, Korol A, Akhunova AR, Feuillet C, Salse J, Morgante M, Pozniak C, Luo M-C, Dvorak J, et al. Characterization of polyploid wheat genomic diversity using a high-density 90 000 single nucleotide polymorphism array. Plant Biotech J. 2014;12:787–796. doi: 10.1111/pbi.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. doi: 10.1214/aos/1176344136. [DOI] [Google Scholar]
- 41.Yu J, Pressoir G, Briggs WH, Bi IV, Yamasak M, Doeble JF, McMullen MD, Gaut BS, Nielson DM, Holland JB, Kresovich S, Kresovich S. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat Genet. 2006;38(2):203–208. doi: 10.1038/ng1702. [DOI] [PubMed] [Google Scholar]
- 42.Hardy OJ, Vekemans X. spagedi: a versatile computer program to analyze spatial genetic structure at the individual or population levels. Mol Ecol Notes. 2002;2:618–620. doi: 10.1046/j.1471-8286.2002.00305.x. [DOI] [Google Scholar]
- 43.Bates D, Maechler M, Bolker B, Walker S. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-7. 2014;http://CRAN.R-project.org/package=lme4.
- 44.Lipka AE, Tian F, Wang Q, Peiffer J, Li M, Bradbury PJ, Gore MA, Buckler ES, Zhang Z. GAPIT: genome association and prediction integrated tool. Bioinformatics. 2012;28(18):2397–2399. doi: 10.1093/bioinformatics/bts444. [DOI] [PubMed] [Google Scholar]
- 45.Liu S, Bai G, Cai S, Chen C. Dissection of genetic components of preharvest sprouting resistance in white wheat. Mol Breed. 2011;27:511–523. doi: 10.1007/s11032-010-9448-7. [DOI] [Google Scholar]
- 46.Van Ooijen JW. JoinMap® 4, Software for the calculation of genetic linkage maps in experimental populations. Wageningen, Netherlands: Kyazma BV; 2006. [Google Scholar]
- 47.Kosambi DD. The estimation of map distances from recombination values. Ann Eugen. 1944;12:172–175. doi: 10.1111/j.1469-1809.1943.tb02321.x. [DOI] [Google Scholar]
- 48.Wang S, Basten CJ, Zeng ZB. Windows QTL Cartographer 2.5. Raliegh: Departmentof Statistics, North Carolina State University; 2005. [Google Scholar]
- 49.Doerge RW, Churchill GA. Permutation tests for multiple loci affecting a quantitative character. Genetics. 1996;142:285–294. doi: 10.1093/genetics/142.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nilsson-Ehle H. Kreuzungsuntersuchungen an hafer und weizen. Lunds Universitets Arsskrift, N.F. Afd 2, Bd 5, Nr 2. 1909;pp 122.
- 51.Kumar A, Kumar J, Singh R, Garg T, Chhuneja P, Balyan HS, Gupta PK. QTL analysis for grain colour and pre-harvest sprouting in bread wheat. Plant Sci. 2009;177:114–122. doi: 10.1016/j.plantsci.2009.04.004. [DOI] [Google Scholar]
- 52.Knox RE, Clarke FR, Clarke JM, Fox SL. Genetic analysis of pre-harvest sprouting in a durum wheat cross. Euphytica. 2005;143:261–264. doi: 10.1007/s10681-005-7874-0. [DOI] [Google Scholar]
- 53.Mohan A, Kulwal P, Singh R, Kumar V, Mir RR, Kumar J, Prasad M, Balyan HS, Gupta PK. Genome-wide QTL analysis for pre-harvest sprouting tolerance in bread wheat. Euphytica. 2009;168:319–329. doi: 10.1007/s10681-009-9935-2. [DOI] [Google Scholar]
- 54.Roy JK, Prasad M, Varshney RK, Balyan HS, Blake TK. Identification of a microsatellite on chromosomes 6B and a STS on 7D of bread wheat showing an association with preharvest sprouting tolerance. Theor Appl Genet. 1999;99:336–340. doi: 10.1007/s001220051241. [DOI] [Google Scholar]
- 55.Bewley JD, Black M. Physiological and biochemistry of seeds in relation to germination. Heidelberg: Springer; 1982. pp. 61–81. [Google Scholar]
- 56.Mares DJ, Mrva K. Mapping quantitative trait loci associated with variation in grain dormancy in Australian wheat. Crop Pasture Sci. 2001;52:1257–1265. doi: 10.1071/AR01049. [DOI] [Google Scholar]
- 57.Ogbonnaya FC, Imtiaz M, Ye G, Hearnden PR, Hernandez E, Eastwood RF, Ginkel MV, Shorter SC, Winchester JM. Genetic and QTL analyses of seed dormancy and preharvest sprouting resistance in the wheat germplasm CN10955. Theor Appl Genet. 2008;116:891–902. doi: 10.1007/s00122-008-0712-8. [DOI] [PubMed] [Google Scholar]
- 58.Gfeller F, Svejda F. Inheritance of post-harvest seed dormancy and kernel color in spring wheat lines. Can J Plant Sci. 1960;40:1–6. doi: 10.4141/cjps60-001. [DOI] [Google Scholar]
- 59.Argel PJ, Humphreys LR. Environmental effects on seed development and hardseededness in Stylosanthes hamata cv. Verano. I. Temperature. Crop Pasture Sci. 1983;34:261–270. doi: 10.1071/AR9830261. [DOI] [Google Scholar]
- 60.Ceccato DV, Daniel Bertero H, Batlla D. Environmental control of dormancy in quinoa (Chenopodium quinoa) seeds: two potential genetic resources for preharvest sprouting tolerance. Seed Sci Res. 2011;21:133–141. doi: 10.1017/S096025851100002X. [DOI] [Google Scholar]
- 61.Torada A, Amano Y. Effect of seed coat color on seed dormancy in different environments. Euphytica. 2002;126(1):99–105. doi: 10.1023/A:1019603201883. [DOI] [Google Scholar]
- 62.Bi HH, Sun YW, Xiao YG, Xia LQ. Characterization of DFR allelic variations and their associations with pre-harvest sprouting resistance in a set of red-grained Chinese wheat germplasm. Euphytica. 2014;195(2):197–207. doi: 10.1007/s10681-013-0986-z. [DOI] [Google Scholar]


