Abstract
Background
Serum C-reactive protein (CRP), an acute inflammatory response biomarker, has been recognized as an indicator of malignant disease progression. However, the prognostic significance of CRP levels collected before tumor removal in intrahepatic cholangiocarcinoma requires further investigation.
Methods
We sampled the CRP levels in 140 patients with intrahepatic cholangiocarcinoma who underwent hepatectomies with regional lymphadenectomies between 2006 and 2013. A retrospective analysis of the clinicopathological data was performed. We focused on the impact of serum CRP on the patients’ cancer-specific survival and recurrence-free survival rates.
Results
High levels of preoperative serum CRP were significantly associated with well-established clinicopathologic features, including gender, advanced tumor stage, and elevated carcinoembryonic antigen and carbohydrate antigen 19-9 levels (P < 0.05). Univariate analysis demonstrated a significant association between high levels of serum CRP and adverse cancer-specific survival (P = 0.001) and recurrence-free survival (P < 0.001). In patients with stage I/II intrahepatic cholangiocarcinoma, the serum CRP level was a prognostic indicator for cancer-specific survival. In patients with stage I/II or stage III/IV, the serum CRP level was a prognostic indicator for recurrence-free survival (P < 0.05). Additionally, multivariate analysis identified serum CRP level in intrahepatic cholangiocarcinoma as an independent prognostic factor (P < 0.05).
Conclusions
We confirmed a significant association of elevated pre-operative CRP levels with poor clinical outcomes for the tested patients with intrahepatic cholangiocarcinoma. Our results indicate that the serum CRP level may represent a useful factor for patient stratification in intrahepatic cholangiocarcinoma management.
Keywords: C-reactive protein, Intrahepatic cholangiocarcinoma, Prognosis
Background
Cholangiocarcinoma is a relatively rare neoplasm acquired by humans. Recently, high incidence rates have been reported in Eastern Asia, and especially in Thailand [1]. Based on the location in the body where it develops, cholangiocarcinoma is further classified into intrahepatic, perihilar extrahepatic, or distal extrahepatic. Intrahepatic cholangiocarcinoma (IHCC) originates from the second segment of the bile duct, and is the least common of the cholangiocarcinoma classifications that a person could acquire. It accounts for 8–10 % of total cholangiocarcinoma cases diagnosed [2]. Its etiology is unknown, although various risk factors, including primary sclerosing cholangitis [3], liver fluke infestation [4], hepatolithiasis [5], and hepatitis viruses [6, 7], have been identified. These risk factors all induce a chronic inflammation in the biliary epithelium and partially obstruct the bile duct [8]. These risk factors are considered to be favorable for potential cancer development [8]. IHCC is seemingly incurable, has a rapid progression, and is lethal in most cases, with the 5-years survival rate being less than 5 % for non-resectable cases [9]. Surgical resection offers only a chance to cure IHCC, but the outcomes vary widely across affected patients.
Several prognostic factors have been identified for the prediction of IHCC patient survival. These factors include staging [10], para-aortic lymph node status [11], positive node to the total node ratio [12], tumor size, and the presence of multiple tumors [13]. Other novel molecular biomarkers, such as hepatoma-derived growth factor [14], SOX4 [15], loss of FBXW7 expression [16], Homer1 [17], and inactivation of Smad4 [18], seem to be associated with poor IHCC patient prognosis. Despite these critical association findings, the majority of these histological predictors only apply to assessments conducted after surgical intervention. Consequently, there is an urgent need to identify pre-treatment prognostic markers that can be used for an improved risk stratified treatment approach for patients that have not undergone surgical intervention.
Serum C-reactive protein (CRP), an acute phase reactive protein, has been defined as an inflammatory biomarker produced in response to pro-inflammatory cytokine hepatocyte stimulation [19]. As a result, CRP is closely associated with the development and outcome of many diseases [19]. During the past decade, elevated serum CRP has been associated with poorer prognosis in patients with various malignant cancers, including gastric cancer, colorectal cancer, breast cancer, and urological cancer [20–25]. This linkage implies a close association between inflammation and malignancy. A recent study has also revealed that chronic inflammation and pro-inflammatory cytokines, like interleukin 6 (IL-6), play an important role in the development and progression of cholangiocarcinoma [26]. Based on these conclusions, it seems as though CRP may be a promising prognostic factor that could be incorporated into prognostic models to improve the predictive accuracy of the outcomes for IHCC patients. Already, a retrospective study has shown that the serum CRP was a prognostic factor in a patient with a small size perihilar cholangiocarcinoma [27]. In published literature, the relation between serum CRP and prognosis of IHCC has not been explored yet. In this study, we aimed to explore the prognostic significance of pre-treatment serum CRP levels on cancer-specific survival (CSS) and recurrence-free survival (RFS) in IHCC patients.
Methods
Patients
This retrospective study included 140 IHCC patients that were treated at Sun Yat-sen University Cancer Center between the years of 2006 and 2013. The patient cases were selected for inclusion in this study based on the following criteria: pathological diagnosis of IHCC, primary and curative tumor resection surgery without preoperative anticancer treatment, the availability of preoperative serum CRP levels, liver function and clinicopathological and follow-up data. The IHCC cohort included 93 (66 %) men and 47 (34 %) women with a mean age of 54.11 years. The average follow-up time after surgery was 20.9 months (median: 14.7 months; range: 0.55 to 87.9 months). Follow-up evaluations were performed every 3 months within the first year, every 6 months for the next 2 years, and annually 3 years after the surgery. The histopathological findings of this IHCC cohort were also reviewed, including tumor multiplicity, intraepithelial ductal spread and vascular invasion. Tumor differentiation was determined based on the criteria proposed in the WHO Classification of Tumours of the Digestive System (2010 version). At the T stage, the lymph node status and the tumor stage were defined according to the UICC/AJCC tumor-node-metastasis (TNM) Classification System (2010 version). Ultrasonography, computed tomography (CT), or magnetic resonance imaging (MRI) scanning were used to detect tumor recurrence, which included incidences of intrahepatic recurrence or metastasis. The time of detection was used as the time of recurrence. In our study, the RFS was defined as the time from surgery to IHCC recurrence or patient death from IHCC, whichever came first in each individual case. The CSS was the time from the date of surgery to the last follow-up visit or date of death from IHCC. The Institute Research Medical Ethics Committee of Sun Yat-sen University Cancer Center approved the methods used in this study.
Detection of serum CRP
Serum CRP levels were detected by the biosensor. To be included in the study, the serum used for CRP detection needed to be collected within 5 days before the tumor resection surgery. The biosensor detection system was adjusted regularly with a calibration curve acquired from the four-parameter Logit log mode. All the reagents used in CRP detection were derived from the WHO standards.
Statistical analysis
A receiver operating characteristic (ROC) curve analysis was used to determine the preoperative serum CRP cutoff value that was acceptable for patient case inclusion in this study. The correlation between preoperative serum CRP level and the clinicopathologic features of the IHCC patients was evaluated by a χ 2 test. Using univariate analysis, survival curves were constructed using the Kaplan-Meier method. The differences between groups when considering survival were analyzed by the log-rank test. The effect of preoperative serum CRP and other clinicopathological variables on CSS and RFS were evaluated with the Cox proportional hazards regression model. For the variables with statistical significance in the univariate analysis, a further multivariate survival analysis was performed with the Cox regression model. The corresponding hazard ratio (HR) and 95 % confidence interval (CI) were extracted from the Cox regression models. All statistical analyses were performed using the SPSS statistical software package (SPSS Standard version 16.0, SPSS Inc.). A P < 0.05 in a two-sided analysis was considered to be statistically significant.
Results
Patient characteristics
All patients underwent curative resection for IHCC with the following intra-operative goals: complete tumor resection with lymphoadenectomy and leaving the cut surface free of tumor. Median tumor size was 5.5 cm (range from 0.5 to 15.0 cm). In this study, 140 IHCCs were located in right lobe (74, 53 %), left lobe (47, 34 %), both right and left lobes (17, 12 %), and quadrate lobe (2, 1 %), respectively. Most patients exhibited well-differentiated or moderately-differentiated tumors (n = 91, 65 %). Vascular invasion was observed in 54 (39 %) patients. Intraepithelial ductal spread was detected in 88 of 140 (63 %) patients with IHCC. The elevated levels of preoperative serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were observed in 53 (38 %) and 43 (31 %) patients, respectively. Cancer recurrence was observed in 87 patients, including 55 of intrahepatic relapse, 17 of multiple metastases, 12 of lymph node metastasis and 3 lung metastases. Patients received postoperative chemotherapy according to the status of lymph node metastasis and multiple tumor nodules. The characteristics and pathological features of IHCC patients are detailed in Table 1.
Table 1.
Correlation of preoperative serum C-reactive protein levels with patients’ clinico-pathological features in intra-hepatic cholangiocarcinoma
| Variable | All cases | Serum CRP mg/L | P valuea | |
|---|---|---|---|---|
| ≤1.8 | > 1.8 | |||
| Age (years) | 0.355 | |||
| ≤ 55b | 75 | 21 (28.0 %) | 54 (72.0 %) | |
| > 55 | 71 | 21 (32.3 %) | 44 (67.7 %) | |
| Gender | 0.018 | |||
| Male | 93 | 22 (23.7 %) | 71 (76.3 %) | |
| Female | 47 | 20 (42.6 %) | 27 (57.4 %) | |
| Location | 0.263 | |||
| Right lobe | 75 | 26 (34.7 %) | 49 (65.3 %) | |
| Left lobe | 46 | 13 (28.3 %) | 33 (71.7 %) | |
| Both lobes or quadrate lobe | 19 | 3 (15.8 %) | 16 (84.2 %) | |
| ALT | < 0.001 | |||
| Normal | 87 | 37 (42.5 %) | 50 (57.5 %) | |
| Elevated | 53 | 5 (9.4 %) | 48 (90.6 %) | |
| AST | 0.002 | |||
| Normal | 97 | 37 (38.1 %) | 60 (61.9 %) | |
| Elevated | 43 | 5 (11.6 %) | 38 (88.4 %) | |
| HbsAg | 0.417 | |||
| Positive | 55 | 15 (27.3 %) | 40 (72.7 %) | |
| Negative | 82 | 25 (30.5 %) | 57 (69.5 %) | |
| CEA (ng/ml) | 0.047 | |||
| ≤ 5 | 101 | 35 (34.7 %) | 66 (65.3 %) | |
| > 5 | 38 | 7 (16.7 %) | 31 (81.6 %) | |
| CA19-9 (U/ml) | 0.024 | |||
| ≤ 35 | 57 | 23 (40.4 %) | 34 (59.6 %) | |
| > 35 | 82 | 19 (23.2 %) | 63 (76.8 %) | |
| Tumor size (cm) | 0.119 | |||
| ≤ 5.5c | 71 | 25 (35.2 %) | 46 (64.8 %) | |
| > 5.5 | 69 | 17 (24.6 %) | 52 (75.4 %) | |
| Nodal metastasis | 0.158 | |||
| Yes | 29 | 6 (20.7 %) | 23 (79.3 %) | |
| No | 111 | 36 (32.4 %) | 75 (67.6 %) | |
| Tumor multiplicity | 0.830 | |||
| Single | 108 | 32 (29.6 %) | 76 (70.4 %) | |
| Multiple | 32 | 10 (31.2 %) | 22 (68.8 %) | |
| Intraepithelial ductal spread | 0.039 | |||
| Absent | 52 | 21 (40.4 %) | 31 (59.6 %) | |
| Present | 88 | 21 (23.9 %) | 67 (76.1 %) | |
| Vascular invasion | 0.940 | |||
| Absent | 86 | 26 (30.2 %) | 60 (69.8 %) | |
| Present | 54 | 16 (29.6 %) | 38 (70.4 %) | |
| Grade | 0.185 | |||
| I | 14 | 7 (50.0 %) | 7 (50.0 %) | |
| II | 77 | 23 (29.9 %) | 31 (70.1 %) | |
| III | 49 | 12 (24.5 %) | 37 (75.5 %) | |
| TNM | 0.011 | |||
| I-II | 78 | 30 (38.5 %) | 48 (61.5 %) | |
| III- IV | 62 | 12 (19.4 %) | 50 (80.6 %) | |
| Postoperative chemotherapy | 0.078 | |||
| Yes | 47 | 10 (21.3 %) | 37 (78.7 %) | |
| No | 93 | 32 (34.4 %) | 61 (65.6 %) | |
aChi-square test
bMedian age
cMedian size
CRP C-reactive protein, ALT alanine aminotransferase, AST aspartate aminotransferase, HbsAg hepatitis B surface antigen, CEA carcinoembryonic antigen, CA19-9 carbohydrate antigen 19-9, TNM tumor-node-metastasis
Preoperative serum CRP level cutoff selection
The mean pre-treatment plasma CRP level was 15.2 mg/L. To develop an optimal serum CRP cutoff value for further analysis, we subjected the serum CRP levels to an ROC curve analysis with respect to the survival and recurrence statuses (Fig. 1). The ROC curves showed the point on the curve closest to (0.0, 1.0), which maximizes both the sensitivity and specificity for the outcomes. Patients with cancer with CRP levels above the obtained cutoff value have a higher risk of tumor recurrence and cancer-related death than cases with levels below the value. Based on the gathered data, an optimal CRP level cutoff value of 1.8 mg/L was determined to differentiate between the opposing patient prognoses (area under the curve: 0.659; 95 % CI: 0.570–0.749) (Fig. 1a) as well as between the tumor recurrence and no further incidence of tumors (area under the curve: 0.659; 95 % CI: 0.566–0.752) (Fig. 1b).
Fig. 1.
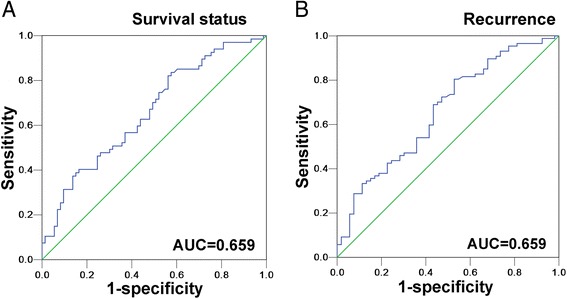
Receiver operating characteristic curve analysis determination of cutoff score for preoperative serum C-reactive protein levels. The sensitivity and specificity for each outcome were plotted: cancer-specific survival a, recurrence-free survival b
Relationship between preoperative serum CRP level and IHCC patient clinicopathological features
We separated patients into two groups according to low CRP levels (≤1.8 mg/L) or high CRP levels (>1.8 mg/L) according to the ROC curve analysis. We evaluated the associations between preoperative serum CRP levels and other clinicopathological factors gathered in the individual patient cases. An elevated serum CRP level was significantly correlated with gender, advanced tumor stage, elevated ALT, AST, carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9) levels and intraepithelial ductal spread (P < 0.05). No significant associations were found in between CRP level and age, tumor location, hepatitis B virus infection, tumor size, tumor grading, nodal metastasis, tumor multiplicity, vascular invasion and chemotherapy administration (Table 1).
Relationship between preoperative serum CRP level and IHCC patient survival
To investigate whether preoperative serum CRP level and other clinicopathological factors are associated with IHCC patient survival, we calculated univariate Cox proportional models for the CSS and RFS. Univariate analyses identified tumor size (≤5.5 vs >5.5 cm, P = 0.001), a high tumor stage (stage I/II vs III/IV, P = 0.007), nodal metastasis (absent vs presence, P = 0.011), vascular invasion (absent vs presence, P = 0.001), elevated CA19-9 (≤35 vs >35 U/ml, P = 0.016), and a high serum CRP level (≤1.8 vs >1.8 mg/L, P = 0.001) as poor prognostic factors for CSS. For a poor prognosis of RFS, having a larger tumor size (≤5.5 vs >5.5 cm, P = 0.001), a high tumor stage (stage I/II vs III/IV, P = 0.025), nodal metastasis (absent vs no presence, P = 0.016), vascular invasion (absent vs presence, P = 0.003), postoperative chemotherapy (chemotherapy vs on postoperative treatment, P < 0.001), and a high serum CRP level (≤1.8 vs >1.8 mg/L, P < 0.001) were identified as poor prognostic factors. Age, gender, tumor location, grading, elevated ALT, AST and CEA levels, and tumor multiplicity were not significantly associated with clinical outcomes for the set of patients (Table 2).
Table 2.
Univariate analyses of serum C-reactive protein (CRP) levels and clinicopathologic variables in 140 patients with intrahepatic cholangiocarcinoma (Cox proportional-hazards regression)
| Variables | All cases | Cancer-specific survival | Recurrence-free survival | ||
|---|---|---|---|---|---|
| Hazard Ratio (95 % CI) | P value | Hazard Ratio (95 % CI) | P value | ||
| Age (years) | |||||
| ≤ 55a | 75 | 1 | 1 | ||
| > 55 | 65 | 0.930 (0.572–1.513) | 0.772 | 0.862 (0.562–1.321) | 0.496 |
| Gender | |||||
| Male | 93 | 1 | 1 | ||
| Female | 47 | 0.770 (0.455–1.301) | 0.329 | 0.770 (0.488–1.214) | 0.216 |
| Location | |||||
| Right lobe | 75 | 1 | 1 | ||
| Left lobe | 46 | 1.329 (0.772–2.289) | 0.304 | 1.224 (0.760–1.972) | 0.407 |
| Both lobes or quadrate lobe | 19 | 1.192 (0.855–1.662) | 0.300 | 1.217 (0.900–1.646) | 0.202 |
| ALT | |||||
| Normal | 87 | 1 | 1 | ||
| Elevated | 53 | 1.295 (0.794–2.110) | 0.300 | 1.218 (0.791–1.874) | 0.371 |
| AST | |||||
| Normal | 97 | 1 | 1 | ||
| Elevated | 43 | 1.305 (0.783–2.175) | 0.308 | 1.149 (0.729–1.812) | 0.550 |
| HbsAg | |||||
| Positive | 55 | 1.027 (0.629–1.675) | 0.916 | 1.212 (0.785–1.871) | 0.385 |
| Negative | 82 | 1 | 1 | ||
| Tumor size (cm) | |||||
| ≤ 5.5b | 71 | 1 | 1 | ||
| > 5.5 | 69 | 2.315 (1.407–3.810) | 0.001 | 2.072 (1.347–3.188) | 0.001 |
| Grade | |||||
| I | 14 | 1 | 1 | ||
| II | 77 | 0.490 (0.169–1.418) | 0.188 | 0.363 (0.141–0.934) | 0.036 |
| III | 49 | 0.910 (0.544–1.521) | 0.719 | 0.794 (0.507–1.244) | 0.314 |
| Nodal metastasis | |||||
| No | 111 | 1 | 1 | ||
| Yes | 29 | 2.048 (1.169–3.589) | 0. 011 | 1.847 (1.123–3.038) | 0. 016 |
| Tumor multiplicity | |||||
| Single | 108 | 1 | 1 | ||
| Multiple | 32 | 1.266 (0.729–2.198) | 0.403 | 1.214 (0.742–1.985) | 0.440 |
| Intraepithelial ductal spread | |||||
| Absent | 52 | 0.821 (0.507–1.329) | 0.422 | 0.897 (0.585–1.375) | 0.618 |
| Present | 88 | 1 | 1 | ||
| Vascular invasion | |||||
| Absent | 86 | 1 | 1 | ||
| Present | 54 | 2.298 (1.416–3.731) | 0.001 | 1.901 (1.238–2.918) | 0.003 |
| TNM | |||||
| I-II | 78 | 1 | 1 | ||
| III-IV | 62 | 1.977 (1.203–3.249) | 0.007 | 1.644 (1.065–2.536) | 0.025 |
| CEA (ng/ml) | |||||
| ≤ 5 | 101 | 1 | 1 | ||
| > 5 | 38 | 1.276 (0.734–2.219) | 0.388 | 1.317 (0.819–2.119) | 0.255 |
| CA19-9 (U/ml) | |||||
| ≤ 35 | 57 | 1 | 1 | ||
| > 35 | 82 | 1.889 (1.125–3.169) | 0.016 | 1.441 (0.930–2.234) | 0.102 |
| Postoperative chemotherapy | |||||
| No | 93 | 1 | 1 | ||
| Yes | 47 | 1.520 (0.925–2.498) | 0.098 | 2.515 (1.619–3.908) | <0.001 |
| Serum CRP (mg/L) | |||||
| ≤ 1.8 | 42 | 1 | 1 | ||
| > 1.8 | 98 | 2.965 (1.551–5.667) | 0.001 | 2.751 | <0.001 |
ALT alanine aminotransferase, AST aspartate aminotransferase, HbsAg hepatitis B surface antigen, CEA carcinoembryonic antigen, CA19-9 carbohydrate antigen 19-9, TNM tumor-node-metastasis
aMedian age
bMedian size
Among the 140 IHCC patients, death occurred in 11 of 42 (26 %) patients with a low serum CRP level and in 56 of 98 (57 %) patients with a high serum CRP level (P = 0.001). In the Kaplan–Meier survival analysis, there was highly significant association between a high serum CRP level and shortened patient survival time (P = 0.001, Kaplan-Meier Method) (Fig. 2a). A stratified survival analysis was also performed to evaluate the serum CRP levels in subsets of the IHCC patients that were at different clinical stages. Our results demonstrated that a high serum CRP level was a prognostic factor in IHCC patients with stage I/II cancer (P = 0.006, Kaplan-Meier Method) (Fig. 2c) but not stage III/IV cancer (P = 0.126, Kaplan-Meier Method) (Fig. 2d).
Fig. 2.
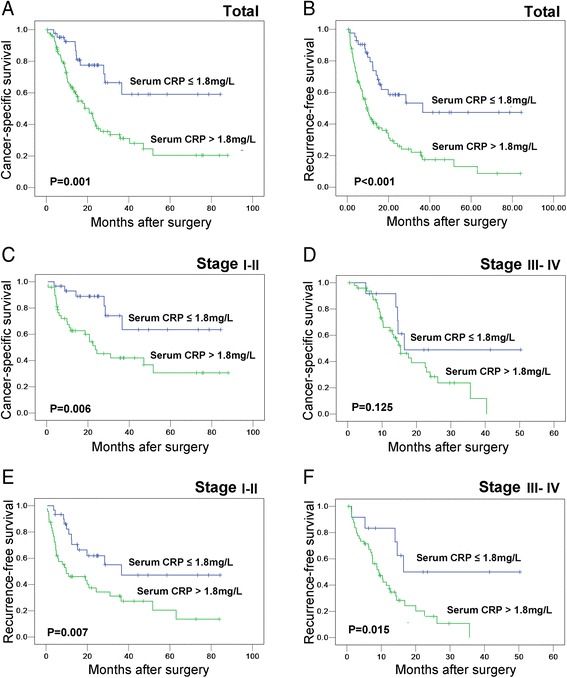
Kaplan-Meier survival analysis of preoperative serum C-reactive protein (CRP) levels in patients with intrahepatic cholangiocarcinoma (IHCC) (log-rank test). The preoperative serum CRP levels and the probability of cancer-specific survival (CSS, a) and recurrence-free survival (RFS) of IHCC patients b The preoperative serum CRP levels and the probability of cancer-specific survival in IHCC patients at stage I-II c and stage III-IV d The preoperative serum CRP levels and the probability of RFS in IHCC patients at stage I-II e and stage III-IV f
Results in the RFS analysis were similar to that in CSS analysis. Patients with high serum CRP level showed a significant trend toward worse RFS compared to the RFS of patients with low serum CRP levels (P < 0.001, Kaplan-Meier Method) (Fig. 2b). Additionally, a stratified survival analysis showed that a high serum CRP level was a predictor for RFS in both stage I/II cancers (P = 0.007, Kaplan-Meier Method) (Fig. 2e) and stage III/IV cancers (P = 0. 015, Kaplan-Meier Method) (Fig. 2f).
Independent prognostic value of preoperative serum CRP levels in IHCC patients
To determine the independent prognostic value of the serum CRP levels for CSS and RFS, we performed multivariate analyses using a Cox proportional hazard model. The clinicopathologic variables, specifically tumor stage, tumor size, nodal metastasis, vascular invasion, CA19-9 level, administration of postoperative chemotherapy, and serum CRP levels, were tested in the multivariate analyses. The clinicopathological variables were found to be of statistical significance in the univariate analyses. In the multivariate analyses, we found that high serum CRP level was a prognostic factor for poor CSS (P = 0.004) and RFS (P < 0.001) of IHCC patients. Additionally, it appeared that independent of tumor stage, nodal metastasis and CA19-9 level were not prognostic factors for poor CSS and RFS (Table 3). Despite this finding, tumor size and vascular invasion were found to be independent prognostic predictors for poor CSS and RFS, as well as administration of postoperative chemotherapy for poor RFS (P < 0.05) (Table 3).
Table 3.
Cox multivariate analyses of prognostic factors on patients’ survival
| Variables | Hazards ratio | 95 % CI | P value |
|---|---|---|---|
| Cancer-specific survivala | |||
| CA19-9, U/ml (≤35 v >35) | 1.553 | 0.904–2.668 | 0.111 |
| Tumor size, cm (≤5.5 v >5.5) | 2.143 | 1.239–3.706 | 0.006 |
| Nodal metastasis (absent v present) | 1.390 | 0.703–2.749 | 0.343 |
| Vascular invasion (absent v present) | 1.954 | 1.181–3.233 | 0.009 |
| TNM (I-II v III-IV) | 0.884 | 0.459–1.702 | 0.711 |
| Serum CRP, mg/L (≤1.8 v >1.8) | 2.646 | 1.356–5.164 | 0.004 |
| Recurrence- free survivalb | |||
| Postoperative chemotherapy (yes v no) | 1.788 | 1.042–3.067 | 0.035 |
| Tumor size, cm (≤5.5 v >5.5) | 1.920 | 1.153–3.197 | 0.012 |
| Nodal metastasis (absent v present) | 1.277 | 0.689–2.367 | 0.437 |
| Vascular invasion (absent v present) | 1.733 | 1.107–2.715 | 0.016 |
| TNM (I-II v III-IV) | 0.663 | 0.369–1.191 | 0.169 |
| Serum CRP, mg/L (≤1.8 v >1.8) | 2.827 | 1.636–4.886 | < 0.001 |
CI confidence interval, CA19-9 carbohydrate antigen 19-9, TNM tumor-node-metastasis, CRP C-reactive protein
aThe total number of patients and total number of events in this model were 140 and 67, respectively
bThe total number of patients and total number of events in this model were 140 and 87, respectively
Discussion
CRP is an acute-phase reactant, and plays a role in microbial infection, trauma, infarction, autoimmune diseases, and malignant cancers [28]. Recently, high levels of serum CRP have been associated with metastatic disease and a poor prognosis in various malignant cancers, including perihilar cholangiocarcinoma. However, the role of CRP status in IHCC, and its utility to clinicians, remains unknown. In our study, we retrospectively assessed IHCC patient cases in order to explore the prognostic value of preoperative CRP. We sought to determine the relative survival rates for IHCC patients who underwent curative operations to remove cancerous tumors.
Our study demonstrated that preoperative serum CRP levels were strongly associated with adverse CSS and RFS in IHCC patients. Furthermore, the preoperative serum CRP level appeared independent from tumor characteristics and treatment allocation. Other groups have reported similar results [23, 29–32], in which preoperative serum CRP level was significantly correlated with other pathologic parameters found in solid cancers, such as decreased survival times, increased loco-regional recurrence rates, more severe post-operative complications, and a shorter relapse time after surgery. A more recent study has found that C-reactive protein, at the time of diagnosis, predicts poorer outcomes in hepatocellular carcinoma patients that are unable to have tumor removal surgeries [33]. More importantly, high CRP level was significantly correlated with poor survival and found to be independently predictive of survival in patients with perihilar cholangiocarcinoma as evidenced by the in the univariate and multivariate analyses [27]. In summation, it appears the pre-operative CRP level may be useful prognostic predictor in many cancer patients who have undergone a radical treatment to remove the cancer. Despite this finding, the prognostic significance in IHCC patients that have not undergone tumor removal surgery still needs further study.
In our study, subgroup analyses, with respect to the TNM stage, supported the prognostic relevance of serum CRP, except for the CSS rate in the subgroup of TNM stage III–IV. This finding is inconsistent with the previous studies, which imply a strengthened association between serum CRP concentration and prognosis in patients with advanced cancers, included colorectal cancer, gastric cancer, ovarian cancer, and renal cell cancer [20, 34–37]. Notably, there is also a report [38] which shows no significant prognostic value when considering serum CRP level for predicting cancer recurrence rate in stage II and III colorectal cancer. Taken together, differences in the geographic backgrounds, biological characteristics of different tumors, selection of the CRP cutoff valve, patient heterogeneity, small sample sizes, and different definitions of end points (disease-free, cancer specific survival, or overall survival) may contribute to these discrepancies.
The underling mechanism regarding the elevated CRP level indicating a potential poorer outcome for cancer patients is unknown. There are three possible explanations. First, this phenomenon could be explained by causality, or that an elevated CRP level promotes tumor progression. Second, this could be explained by reverse causality, or that tumor progression increases the CRP level. Lastly, there could be a confounding explanation. A third factor, for example inflammation, could increase both CRP level and tumor malignancy. Notably, there are evidence [26] that the inflammatory field effect, reflected by elevated CRP, may be directly involved in tumor progression. This could explain the CRP level’s prognostic significance in IHCC, which has a development and progression that are closely related with inflammation [39, 40]. For example, IL-6, one of the main inducers of CRP production, has been shown to be associated with development and progression of cholangiocarcinoma and other cancers [28, 41]. Moreover, CRP was shown to directly enhance tumor cell proliferation under stressed conditions in a recent study on myeloma [42]. Clearly, the causal mechanisms of CRP regarding the progression of IHCC and other cancers need to be clarified in further investigations.
The findings presented in this report may have impacts for the design of future clinical trials. Most studies in advanced IHCC only stratify prognoses according to variables like tumor staging and grading, presence or absence of vascular invasion/extra-hepatic spread, and CEA or CA19-9 levels. The strong and independent prognostic significance of serum CRP levels found in this study provides evidence to collect serum CRP levels in future clinical trials for the potential determination of an IHCC patient’s prognosis.
There are a few limitations in the design of this study. CRP is known to be a non-specific marker of inflammation. It is possible that an undocumented super infection could influence CRP concentrations in the serum. Furthermore, as IHCC is mainly located within the liver, the synthetic function of the hepatocytes may be affected. This change in functioning may act as a confounding factor that correlates directly with both the development of IHCC and the serum concentration of CRP. Moreover, our study is limited by its retrospective nature and a rather heterogeneous group of patients.
Based on the results of our study, it is possible that a higher preoperative CRP level in IHCC patients was associated with a higher risk for recurrence and earlier death because of the disease. Whether patients can be selected for resection, or increase the chances of curative resection, can only be evaluated in a controlled prospective clinical trial. However, to the best of our knowledge, our study represents the first one validates the prognostic value of serum CRP levels in IHCC patient prognoses.
Conclusions
In conclusion, the preoperative CRP level was a strong and independent predictor of a poor prognostic outcome, as indicated by the univariate and multivariate analyses. Adding the serum CRP into TNM stage factor could improve the ability to discriminate between IHCC patients’ outcomes. Our data seem to indicate that CRP could function as an independent prognostic factor of outcomes in IHCC. Additionally, the data support the consideration of the preoperative CRP level for therapy stratification. The causal role of CRP in tumor progression merits further investigation in preclinical studies.
Acknowledgements
The authors would like to thank the Guangdong Natural Science who funded this work under the funds for distinguished young scholar (No. 2015A030306001). Funders and study sponsors had no role in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Funding
Guangdong Natural Science funded this work under the funds for distinguished young scholar (No. 2015A030306001).
Availability of data and materials
The dataset supporting the conclusions of this article is available on request from e-mail: caimuyan@hotmail.com.
Authors’ contributions
MYC is responsible for the study design. ZYL performed the experiments and drafted the manuscript. ZYL and MYC carried out the data analysis and interpretation. PLZ, JWC, YC, LXY, JPY and DX participated in the data collection. ZXL provided the patients’ clinical data. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was approved by the Institute Research Medical Ethics Committee of Sun Yat-sen University Cancer Center. No informed consent (written or verbal) was obtained for use of retrospective data from the patients within this study, most of whom were deceased, since this was not deemed necessary by the Ethics Committee, who waived the need for consent. All samples were anonymised.
Abbreviations
- CRP
C-reactive protein
- CSS
Cancer-specific survival
- IHCC
Intrahepatic cholangiocarcinoma
- RFS
Recurrence-free survival
- ROC
Receiver operating characteristic
Contributor Information
Zi-Ying Lin, Email: 1532230295@qq.com.
Zhen-Xing Liang, Email: 1051322781@qq.com.
Pei-Lin Zhuang, Email: pelion@163.com.
Jie-Wei Chen, Email: chenjiew@sysucc.org.cn.
Yun Cao, Email: caoyun@sysucc.org.cn.
Li-Xu Yan, Email: ylxyss@163.com.
Jing-Ping Yun, Email: yunjp@sysucc.org.cn.
Dan Xie, Email: xied@mail.sysu.edu.cn.
Mu-Yan Cai, Phone: 86-20-87342269, Email: caimuyan@hotmail.com.
References
- 1.Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang YY, Wiangnon S, Sripa B, Hong ST. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci. 2010;101(3):579–585. doi: 10.1111/j.1349-7006.2009.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baheti AD, Tirumani SH, Rosenthal MH, Shinagare AB, Ramaiya NH. Diagnosis and management of intrahepatic cholangiocarcinoma: a comprehensive update for the radiologist. Clin Radiol. 2014;69(12):463–470. doi: 10.1016/j.crad.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Tsaitas C, Semertzidou A, Sinakos E. Update on inflammatory bowel disease in patients with primary sclerosing cholangitis. World J Hepatol. 2014;6(4):178–187. doi: 10.4254/wjh.v6.i4.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim MK, Ju YH, Franceschi S, Oh JK, Kong HJ, Hwang SS, Park SK, Cho SI, Sohn WM, Kim DI, et al. Clonorchis sinensis infection and increasing risk of cholangiocarcinoma in the Republic of Korea. AmJTrop Med Hyg. 2006;75(1):93–96. [PubMed] [Google Scholar]
- 5.Ohta T, Nagakawa T, Konishi I, Ueno K, Kanno M, Akiyama T, Kayahara M, Izumi R, Konishi K, Miyazaki I, et al. Clinical experience of intrahepatic cholangiocarcinoma associated with hepatolithiasis. Jpn J Surg. 1988;18(1):47–53. doi: 10.1007/BF02470846. [DOI] [PubMed] [Google Scholar]
- 6.Ariizumi SI, Yamamoto M. Intrahepatic cholangiocarcinoma and cholangiolocellular carcinoma in cirrhosis and chronic viral hepatitis. Surg Today. 2015;45(6):682–687. doi: 10.1007/s00595-014-1031-0. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Wang H, Li D, Hu J, Wang H, Zhou D, Li Q, Jiang X, Zhou H, Hu H. Occult Hepatitis B Virus Infection in Chinese Cryptogenic Intrahepatic Cholangiocarcinoma Patient Population. J Clin Gastroenterol. 2014;48(10):878-82. [DOI] [PubMed]
- 8.Gatto M, Alvaro D. New insights on cholangiocarcinoma. World J Gastrointest Oncol. 2010;2(3):136–145. doi: 10.4251/wjgo.v2.i3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farley DR, Weaver AL, Nagorney DM. “Natural history” of unresected cholangiocarcinoma: patient outcome after noncurative intervention. Mayo Clin Proc. 1995;70(5):425–429. doi: 10.4065/70.5.425. [DOI] [PubMed] [Google Scholar]
- 10.Li T, Qin LX, Zhou J, Sun HC, Qiu SJ, Ye QH, Wang L, Tang ZY, Fan J. Staging, prognostic factors and adjuvant therapy of intrahepatic cholangiocarcinoma after curative resection. Liver Int. 2014;34(6):953–960. doi: 10.1111/liv.12364. [DOI] [PubMed] [Google Scholar]
- 11.Nakayama T, Tsuchikawa T, Shichinohe T, Nakamura T, Ebihara Y, Hirano S. Pathological confirmation of para-aortic lymph node status as a potential criterion for the selection of intrahepatic cholangiocarcinoma patients for radical resection with regional lymph node dissection. World J Surg. 2014;38(7):1763–1768. doi: 10.1007/s00268-013-2433-7. [DOI] [PubMed] [Google Scholar]
- 12.Nari GA, Palacios OG, Lopez-Ben S, Albiol M, Falgueras L, Castro-Gutierrez E, Figueras J. Hilar cholangiocarcinoma: The number of positive nodes and positive node/total node ratio is a significant prognostic factor for survival. Cirugia Espanola. 2014;92(4):247–253. doi: 10.1016/j.ciresp.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Popescu I, Dumitrascu T. Curative-intent surgery for hilar cholangiocarcinoma: prognostic factors for clinical decision making. Langenbeck’s Arch Surg. 2014;399(6):693–705. doi: 10.1007/s00423-014-1210-x. [DOI] [PubMed] [Google Scholar]
- 14.Guo S, Liu HD, Liu YF, Liu L, Sun Q, Cui XJ. Hepatoma-derived growth factor: a novel prognostic biomarker in intrahepatic cholangiocarcinoma. Tumour Biol. 2015;36(1):353–364. doi: 10.1007/s13277-014-2651-0. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Zhang J, Zhan X, Lin T, Yang M, Hu J, Han B, Hu S. SOX4 is associated with poor prognosis in cholangiocarcinoma. Biochem Biophys Res Commun. 2014;452(3):614–621. doi: 10.1016/j.bbrc.2014.08.124. [DOI] [PubMed] [Google Scholar]
- 16.Enkhbold C, Utsunomiya T, Morine Y, Imura S, Ikemoto T, Arakawa Y, Kanamoto M, Iwahashi S, Saito Y, Ishikawa D, et al. Loss of FBXW7 expression is associated with poor prognosis in intrahepatic cholangiocarcinoma. Hepatol Res. 2014;44(14):346–352. doi: 10.1111/hepr.12314. [DOI] [PubMed] [Google Scholar]
- 17.Wu SY, Yu MX, Li XG, Xu SF, Shen J, Sun Z, Zhou X, Chen XZ, Tu JC. Identification of Homer1 as a potential prognostic marker for intrahepatic cholangiocarcinoma. Asian Pac J Cancer Prev. 2014;15(7):3299–3304. doi: 10.7314/APJCP.2014.15.7.3299. [DOI] [PubMed] [Google Scholar]
- 18.Yan XQ, Zhang W, Zhang BX, Liang HF, Zhang WG, Chen XP. Inactivation of Smad4 is a prognostic factor in intrahepatic cholangiocarcinoma. Chin Med J. 2013;126(16):3039–3043. [PubMed] [Google Scholar]
- 19.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805–1812. doi: 10.1172/JCI200318921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baba H, Kuwabara K, Ishiguro T, Hatano S, Matsuzawa T, Fukuchi M, Kumagai Y, Ishibashi K, Mochiki E, Ishida H. C-reactive protein as a significant prognostic factor for stage IV gastric cancer patients. Anticancer Res. 2013;33(12):5591–5595. [PubMed] [Google Scholar]
- 21.Pathak S, Nunes QM, Daniels IR, Smart NJ. Is C-reactive protein useful in prognostication for colorectal cancer? A systematic review. Color Dis. 2014;16(10):769–776. doi: 10.1111/codi.12700. [DOI] [PubMed] [Google Scholar]
- 22.Han Y, Mao F, Wu Y, Fu X, Zhu X, Zhou S, Zhang W, Sun Q, Zhao Y. Prognostic role of C-reactive protein in breast cancer: a systematic review and meta-analysis. Int J Biol Markers. 2011;26(4):209–215. doi: 10.5301/JBM.2011.8872. [DOI] [PubMed] [Google Scholar]
- 23.Eggers H, Seidel C, Schrader AJ, Lehmann R, Wegener G, Kuczyk MA, Steffens S. Serum C-reactive protein: a prognostic factor in metastatic urothelial cancer of the bladder. Med Oncol. 2013;30(4):705. doi: 10.1007/s12032-013-0705-6. [DOI] [PubMed] [Google Scholar]
- 24.Ishino Y, Saigusa S, Ohi M, Yasuda H, Tanaka K, Toiyama Y, Mohri Y, Kusunoki M. Preoperative C-reactive protein and operative blood loss predict poor prognosis in patients with gastric cancer after laparoscopy-assisted gastrectomy. Asian J Endosc Surg. 2014;7(4):287–294. doi: 10.1111/ases.12126. [DOI] [PubMed] [Google Scholar]
- 25.Liu ZQ, Chu L, Fang JM, Zhang X, Zhao HX, Chen YJ, Xu Q. Prognostic role of C-reactive protein in prostate cancer: a systematic review and meta-analysis. Asian J Androl. 2014;16(3):467–471. doi: 10.4103/1008-682X.123686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerhardt T, Milz S, Schepke M, Feldmann G, Wolff M, Sauerbruch T, Dumoulin FL. C-reactive protein is a prognostic indicator in patients with perihilar cholangiocarcinoma. World J Gastroenterol. 2006;12(34):5495–5500. doi: 10.3748/wjg.v12.i34.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao X, Huang J, Zhong H, Shen N, Faggioni R, Fung M, Yao Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. 2014;141(2):125–139. doi: 10.1016/j.pharmthera.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Al Ghazal A, Steffens S, Steinestel J, Lehmann R, Schnoeller TJ, Schulte-Hostede A, Wegener G, Jentzmik F, Schrader M, Kuczyk MA, et al. Elevated C-reactive protein values predict nodal metastasis in patients with penile cancer. BMC Urol. 2013;13:53. doi: 10.1186/1471-2490-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci. 2011;48(4):155–170. doi: 10.3109/10408363.2011.599831. [DOI] [PubMed] [Google Scholar]
- 31.Aziz A, Rink M, Gakis G, Kluth LA, Dechet C, Miller F, Otto W, Gierth M, Denzinger S, Schwentner C, et al. Preoperative C-Reactive Protein in the Serum: A Prognostic Biomarker for Upper Urinary Tract Urothelial Carcinoma Treated with Radical Nephroureterectomy. Urol Int. 2014;93(3):352–360. doi: 10.1159/000362248. [DOI] [PubMed] [Google Scholar]
- 32.Selby J, Prabhudesai A. Can C-reactive protein predict the severity of a post-operative complication after elective resection of colorectal cancer? Int J Color Dis. 2014;29(10):1211–1215. doi: 10.1007/s00384-014-1977-9. [DOI] [PubMed] [Google Scholar]
- 33.Sieghart W, Pinter M, Hucke F, Graziadei I, Schoniger-Hekele M, Muller C, Vogel W, Trauner M, Peck-Radosavljevic M. Single determination of C-reactive protein at the time of diagnosis predicts long-term outcome of patients with hepatocellular carcinoma. Hepatology. 2013;57(6):2224–2234. doi: 10.1002/hep.26057. [DOI] [PubMed] [Google Scholar]
- 34.Kersten C, Louhimo J, Algars A, Lahdesmaki A, Cvancerova M, Stenstedt K, Haglund C, Gunnarsson U. Increased C-reactive protein implies a poorer stage-specific prognosis in colon cancer. Acta Oncol. 2013;52(8):1691–1698. doi: 10.3109/0284186X.2013.835494. [DOI] [PubMed] [Google Scholar]
- 35.Fukuchi M, Kuwabara K, Tsuji Y, Baba H, Ishibashi K, Chika N, Hatano S, Matsuzawa T, Kumamoto K, Kumagai Y, et al. C-reactive protein is a negative independent factor in patients with stage IV colorectal cancer undergoing oxaliplatin-based chemotherapy. Anticancer Res. 2013;33(11):5051–5055. [PubMed] [Google Scholar]
- 36.Mahmoud FA, Rivera NI. The role of C-reactive protein as a prognostic indicator in advanced cancer. Curr Oncol Rep. 2002;4(3):250–255. doi: 10.1007/s11912-002-0023-1. [DOI] [PubMed] [Google Scholar]
- 37.Shibutani M, Maeda K, Nagahara H, Noda E, Ohtani H, Nishiguchi Y, Hirakawa K. Prognostic significance of the preoperative serum C-reactive protein level in patients with stage IV colorectal cancer. Surg Today. 2015;45(3):315–321. doi: 10.1007/s00595-014-0909-1. [DOI] [PubMed] [Google Scholar]
- 38.Fujii T, Yajima R, Tabe Y, Yamaguchi S, Tsutsumi S, Asao T, Kuwano H. Elevated C-reactive protein is associated with the tumor depth of invasion but not with disease recurrence in stage II and III colorectal cancer. Hepato-Gastroenterology. 2013;60(126):1343–1347. doi: 10.5754/hge11214. [DOI] [PubMed] [Google Scholar]
- 39.Thanan R, Oikawa S, Yongvanit P, Hiraku Y, Ma N, Pinlaor S, Pairojkul C, Wongkham C, Sripa B, Khuntikeo N, et al. Inflammation-induced protein carbonylation contributes to poor prognosis for cholangiocarcinoma. Free Radic Biol Med. 2012;52(8):1465–1472. doi: 10.1016/j.freeradbiomed.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 40.Thanan R, Pairojkul C, Pinlaor S, Khuntikeo N, Wongkham C, Sripa B, Ma N, Vaeteewoottacharn K, Furukawa A, Kobayashi H, et al. Inflammation-related DNA damage and expression of CD133 and Oct3/4 in cholangiocarcinoma patients with poor prognosis. Free Radic Biol Med. 2013;65:1464–1472. doi: 10.1016/j.freeradbiomed.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 41.Braconi C, Patel T. Cholangiocarcinoma: new insights into disease pathogenesis and biology. Infect Dis Clin N Am. 2010;24(4):871–884. doi: 10.1016/j.idc.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang J, Wezeman M, Zhang X, Lin P, Wang M, Qian J, Wan B, Kwak LW, Yu L, Yi Q. Human C-reactive protein binds activating Fcgamma receptors and protects myeloma tumor cells from apoptosis. Cancer Cell. 2007;12(3):252–265. doi: 10.1016/j.ccr.2007.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusions of this article is available on request from e-mail: caimuyan@hotmail.com.


