Abstract
Background and aims
The internalization of aggregated low-density lipoproteins (agLDL) mediated by low-density lipoprotein receptor related protein (LRP1) may involve the actin cytoskeleton in ways that differ from the endocytosis of soluble LDL by the LDL receptor (LDLR). This study aims to define novel mechanisms of agLDL uptake through modulation of the actin cytoskeleton, to identify molecular targets involved in foam cell formation in vascular smooth muscle cells (VSMCs). The critical observation that formed the basis for these studies is that under pathophysiological conditions, nucleotide release from blood-derived and vascular cells activates SMC P2Y2 receptors (P2Y2Rs) leading to rearrangement of the actin cytoskeleton and cell motility. Therefore, we tested the hypothesis that P2Y2R activation mediates agLDL uptake by VSMCs.
Methods
Primary VSMCs were isolated from aortas of wild type (WT) C57BL/6 and.P2Y2R−/− mice to investigate whether P2Y2R activation modulates LRP1 expression. Cells were transiently transfected with cDNA encoding a hemagglutinin-tagged (HA-tagged) WT P2Y2R, or a mutant P2Y2R that unlike the WT P2Y2R does not bind the cytoskeletal actin-binding protein filamin-A (FLN-A).
Results
P2Y2R activation significantly increased agLDL uptake, and LRP1 mRNA expression decreased in P2Y2R−/− VSMCs versus WT. SMCs, expressing P2Y2R defective in FLN-A binding, exhibit 3-fold lower LDLR expression levels than SMCs expressing WT P2Y2R, while cells transfected with WT P2Y2R show greater agLDL uptake in both WT and P2Y2R−/− VSMCs versus cells transfected with the mutant P2Y2R.
Conclusions
Together, these results show that both LRP1 and LDLR expression and agLDL uptake are regulated by P2Y2R in VSMCs, and that agLDL uptake due to P2Y2R activation is dependent upon cytoskeletal reorganization mediated by P2Y2R binding to FLN-A.
Keywords: LRP1, P2Y2R
1. Introduction
The foremost cause of mortality in men and women in the United States is atherosclerosis [1]. A major event in its development is the accumulation of lipids, mainly cholesterol esters (CEs), in smooth muscle cells (SMCs) of blood vessels, which leads to foam cell formation [2,3]. Although dietary and life-style factors are implicated in the development of atherosclerosis, a number of molecular targets have been shown to play significant roles in the pathological progression of atherosclerosis [4,5].
There is little knowledge as to how vascular SMCs (VSMCs) are transformed into foam cells. Lipoproteins that are retained in the extracellular matrix are taken up by VSMCs and macrophages, which subsequently results in CE accumulation and foam cell formation [6]. Macrophages become foam cells through the uptake of diversely modified LDLs [7], whereas the aggregation of LDLs seems to be a key condition for lipid accumulation in VSMCs [6,8]. Scavenger receptors on macrophages mediate their transformation to foam cells following uptake of modified and oxidized LDLs (oxLDLs). Compared to their relative abundance in macrophages, scavenger receptors are not observed in VSMCs from human atherosclerotic lesions. Uptake of aggregated LDL (agLDL) is a pre-requisite condition for lipid accumulation in VSMCs, and the LDL receptor related protein 1 (LRP1) is an important mediator in this uptake [6,9]. However, little is known about how LRP1 expression is regulated.
P2 receptors for extracellular purine and pyrimidine nucleotides are ubiquitously expressed in human tissues, including the blood vessel wall [10] Kanapuli. Under pathological conditions, nucleotide release from blood-derived and vascular cells activates the P2Y2 receptor (P2Y2R) in SMCs, leading to rearrangement of the actin cytoskeleton and cell motility [11,12]. The P2Y2R mediates these effects through its interaction with FLN-A, an actin-binding 280 kDa protein [13].
Several studies have demonstrated that in human VSMCs in vitro, LRP1 mediates the internalization of aggregated LDL (agLDL) [14,15]. We hypothesized that the P2Y2R may regulate foam cell formation in VSMCs. The P2Y2R plays an important role in stress or injury and the development of inflammation in the vasculature and arterial wall disease [16,17]. Under pathological conditions, extracellular nucleotides, including the equipotent P2Y2R agonists ATP and UTP, are released from various cellular sources to activate P2 nucleotide receptors. P2Y2R activation by ATP or UTP [18,19] has been associated with various cellular processes, including cytoskeletal remodeling, cell proliferation and migration of VSMCs [11,13,16,20]. It has also been shown that reorganization of the cytoskeleton plays a significant role in the uptake of aggregated lipoproteins by macrophages that is different than receptor-mediated endocytosis [21]. Specifically, Sakr et al. have shown that actin polymerization occurs in SMCs with matrix-retained LDL, and the activities of myosin ATPase, Rho family GTPases, and other signaling molecules are needed for the internalization of matrix-retained LDL and agLDL [21].
Extracellular nucleotides induce rapid dynamic reorganization of the actin network. As such, UTP-stimulated P2Y receptor activation was shown to induce reorganization of the actin cytoskeleton in rat VSMCs expressing P2Y2 or P2Y4 receptors [11], which, was coupled to activation of RhoA, a small GTPase that regulates actin cytoskeleton reorganization. UTP, an agonist of the P2Y2R, was shown to significantly increase the spreading and migration of SMCs isolated from mouse aortas [13]. P2Y2R/FLN-A interaction is necessary for P2Y2R-mediated actin cytoskeleton reorganization, since UTP-induced spreading and migration was not observed when SMCs from P2Y2R knockout mice were transfected with mutant P2Y2R cDNA encoding a P2Y2R that does not bind to FLN-A [13]. In the present study, we investigated the role of P2Y2R in the internalization of matrix-bound agLDL in VSMCs, and we found that an interaction between FLN-A and P2Y2 R was a necessary factor in the uptake of agLDL.
2. Materials and methods
2.1. Materials
Mouse aortas were surgically removed from 6 to 8 week old male P2Y2R knockout mice and C57BL/6 control mice and enzymatically digested with 0.1% (w/v) collagenase II (Worthington Biochemical Corporation, Lakewood, NJ) in Medium Nutrient Mix 199 (M199) and suspended in Dulbecco's Modified Eagle's Medium Nutrient Mix F12 in a 1:1 ratio (DMEM F12), 100 μg/ml normocin, 5% (v/v) fetal bovine serum (FBS), 5% (v/v) 20X Smooth Muscle Growth Supplement (SMGS), 1% (v/v) 100X penicillin-streptomycin solution containing 10,000 IU units/ml penicillin and 10,000 μg/ml streptomycin and penicillin-streptomycin glutamine (Life Technologies, Carlsbad, CA). Uridine 5’-triphosphate trisodium salt dehydrate, pyridoxal phosphate-6-azo (benzene-2,4-disulfonic acid) tetrasodium salt hydrate (PPADS) and ribonucleic acid (RNA) synthesis inhibitor Actinomycin D were purchased from Sigma-Aldrich (St. Louis, MO). Lipofectamine® 2000 Reagent, SOC medium and Electromax DH 10B™ competent cells used for cloning and transfection protocols were obtained through Life Technologies (Carlsbad, CA). Lipoproteins (unlabeled LDL or oxLDL labeled with 1,1’-dioctadecyl-3,3,3′,3’-tetramethyl-indocarbocyanine perchlorate (DiI-oxLDL) for LDL uptake studies) were purchased from Biomedical Technologies (Stoughton, MA). Alexa Fluor® 546 Monoclonal Antibody Labeling kit (Molecular Probes, Eugene OR) was used for labeling LDL. Primer sequences for LRP1, GAPDH, P2Y1R, P2Y2R and P2Y4R were designed using the National Center for Biotechnology Information Local Alignment Search Tool (BLAST). The mRNA relative abundance was calculated using the delta-delta (ΔΔ) Ct method. GAPDH was used as a loading control gene.
2.2. Cell preparation and culture
Primary VSMCs isolated from mouse aortas were grown in cell culture. The cells were obtained from enzymatically-digested aortas surgically removed from P2Y2R−/− and wild type (WT) C57BL/6 mice. The aortas were placed in a 0.5% (v/v) penicillinstreptomycin phosphate buffer solution (PBS) for 30 min to destroy surface contaminants. The aortas were then removed from the PBS and placed in enough M199 media to cover them. They were next cut open longitudinally and connective tissue and fat were removed. Aortas were placed in a culture dish with fresh PBS and 0.1% (w/v) collagenase, chopped into small pieces (2e4 mm) and transferred into a 50 ml tube. The tubes were incubated for 30 min in a water bath with gentle shaking. Following incubation, the tissue suspension was filtered through a 100 μm strainer and centrifuged for 5 min at 210g. The cell pellet was collected and resuspended in M199 medium supplemented with 20% (v/v) FBS and 1% (v/v) penicillin-streptomycin with 100 μg/ml normocin, 5% (v/v) SMGS. The cells were grown in M199 supplemented with 20% (v/v) FBS and 1% (v/v) penicillin-streptomycin, plus normocin. Cells were seeded in 6-well plates and incubated at 37 °C for 24 h in a humidified atmosphere with 5% CO2. After 24 h, the media were aspirated, the cells rinsed with sterile PBS and fresh media were added. Thereafter, the cells were replenished with fresh media every 3 days. The cells were identified as VSMCs by their characteristic “hill-and-valley” growth pattern. Following the first passage at 70% confluence, cells were incubated in DMEM F12 culture medium supplemented with 15% (v/v) FBS, 5% (v/v) SMGS, 1% (v/v) penicillin-streptomycin with 5% (w/v) L-glutamine. Human 1321N1 astrocytoma cells were cultured in DMEM with 10% (v/v) fetal calf serum, penicillin (100 IU/ml), streptomycin (100 μg/ml) and L-glutamine (2 mM).
2.3. P2Y2 receptor cDNA constructs
The open reading frame of wild type human P2Y2 receptor cDNA was modified using PCR to incorporate the HA epitope (YPYDVP-DYA) from influenza virus at the N-terminus of the expressed protein, as described previously [22]. Three primers synthesized in the DNA Core Facility (University of Missouri, Columbia, MO) were used in PCR to generate cDNA encoding HA-tagged deleted (del) (using primer 1 and 3) or 4A (using primer 1 and 2) mutant P2Y2 receptors; Primer 1: 5′ -AGGCTCGTACGCTTTGCCCGAGATGCCAAGGCTCGCCGCAGGCTGGGCCTGCGCAGATC-3’; Primer 2: 5′–ATCATGGATCCTTACT TGGCATCTCGGGC-3’; Primer 3: 5′-CACACCCTAACTGACAC -3’. The PCR products were resolved by agarose gel electrophoresis and the products were purified using the PCR Wizard kit from Amersham Biosciences (Piscataway, NY), digested with BsiWI and BamHI, and inserted into pLXSN. The mutant cDNAs were sequenced to verify that the mutations were incorporated correctly.
2.4. DH5α transformation, culture preparation and cell transfection
Plasmid DNA (100 ng/200 μl cells) encoding HA-tagged WT P2Y2R or a mutant P2Y2R that does not bind FLN-A, were added to DH5 competent cells and cells were heat-shocked at 42 °C for 2 min with gentle shaking, followed by addition of 4 vol of Lauria-Bertani (LB) media (400 μl) and incubation at 37 °C with gentle shaking for 50–60 min. The cells were pelleted at 5,000c for 10 min. Approximately 400 μl of the supernatant was removed and the pellet was re-suspended in the remaining 50 μl of supernatant. Then, cultures were prepared by inoculating cells in 1.5% (w/v) agar on petri dishes and incubating at 37 °C for 24 h. The colonies were collected and incubated with LB media with 1 μg/ml ampicillin in culture tubes at 37 °C with gentle shaking, until evidence of cloudy suspension appeared. The cultures were then centrifuged for 10 min at 10,000g and the pellet was collected followed by DNA isolation and purification using the Qiagen DNA Isolation kit with the manufacturer's protocol. VSMCs were transiently transfected with the plasmid DNA constructs using Lipofectamine® 2000 reagent from Life Technologies (Carlsbad, CA) following the manufacturer's instructions. The retroviral vector pLXSN was used to stably express the HA-tagged human P2Y2 receptor in P2 receptor-null human 1321N1 astrocytoma cells, as previously described [23]. In brief, the recombinant P2Y2R-pLXSN construct or pLXSN (control) was used to transfect PA317 amphotropic packaging cells for production of the viral vectors. Then, 1321N1 cells were infected with the viral vectors and cultured in DMEM plus 5% (v/v) FBS, 100 units/ml penicillin, and selected for neomycin resistance with 1 mg/ml G418 from Life Technologies (Carlsbad, CA).
2.5. Protein abundance of LRP1 in mouse VSMCs
Serum-starved WT C57BL/6 cell cultures were incubated in the absence or presence of UTP (1, 10, 25, 50 or 100 μM). Cell lysates were prepared in 2X Laemmli sample buffer. Equivalent amounts of protein (100 μg) were subjected to 7.5% (w/v) SDS-PAGE and transferred to nitrocellulose membranes for immunoblotting. Membranes were blocked for 1 h with 5% (w/v) non-fat dry milk in Tris-buffered saline (0.137 M NaCl, 0.025 M Tris, pH 7.4) containing 0.1% (v/v) Tween-20 (TBST) and immunoblotted overnight at 4 °C in TBST containing 1% (w/v) BSA and anti-LRP1 antibody (1:3000 dilution; Abcam, Cambridge, MA), followed by horseradish peroxidase-conjugated donkey anti-mouse polyclonal antibody (1:10,000 dilution; Abcam, Cambridge, MA). For signal normalization, membranes were probed with rabbit polyclonal anti-mouse β-actin antibody (1:2000 dilution; Abcam, Cambridge, MA).
2.6. LRP1, LDLR, P2Y1R, P2Y2R and P2Y4R mRNA expression in VSMCs
P2Y2R−/− and C57BL/6 control VSMCs were grown to 90% confluence in a monolayer in 12-well plates in DMEM F12 media supplemented with 10% (v/v) FBS, 1% (v/v) penicillin/streptomycin. Cells were serum starved by incubating for 24 h with DMEM F12 at 37 °C in a humidified atmosphere with 5% CO2. After 24 h, cells for LRP1 mRNA and LDLR mRNA expression were incubated with actinomycin D (2 mg/ml) in fresh media for 15 min to block transcriptional activity. Cells were then stimulated with UTP (10 μM) and incubated 18 h. Total RNA was isolated from VSMCs using the Qiagen RNeasy Mini Kit. Briefly, cells were washed in ice-cold PBS and collected by scraping following addition of fractionation buffer and lysis buffer. An equal volume of 100% ethanol was added to the lysate, which was then applied to a filter cartridge. Following several washes using different wash solutions with centrifugation, the RNA was eluted with heat-shocked elution buffer or RNase free water. First strand cDNA was synthesized from 1 μg total RNA in 20 μl reaction volume using random hexamers as primers. Synthesis of cDNA was performed using cDNA synthesis mix (10X RT Buffer, 25X dNTP mix, 10X Random Primers, and Multiscribe Reverse Transcriptase) (Invitrogen, Carlsbad CA). RT-PCR was conducted using Fast SYBR Green Master Mix. Two independent PCR reactions were carried out for each cDNA synthesis. The mRNA relative abundance was determined using the ΔΔCt method and GAPDH as a control gene. Oligonucleotide amplification primer sequences for LRP1 were forward 5′-GCCAGCCAGATGTGCCCAAT-3′ and reverse 5′-TGGTGGGGCAGGCGCATTTA-3’. Primer sequences for LDLR were forward 5′-TGCCAATCGACTCACGGGTTCA-3′ and reverse 5′-AGTGTCGACTTCTCTAGGCTGTGT-3’. For P2Y1R, P2Y2R and P2Y4R mRNA expression, cells were incubated with PPADS (2 mg/ml) in media for 15 min, then stimulated with UTP (10 μM) and incubated for 18 h. Sequences for P2Y1R were forward (NM_008772.4) 5′-CGGGAGCGCACTTGCAAACT-3′ and reverse 5′-TGAAGGACGTGCGGGCAACT-3’; sequences for P2Y2R were forward (NM_008773.3) 5′-GGTCGAGTCAGCGCCAAACA-3′ and reverse 5′-AGTTCATCAGCGCACCGGCA-3’; sequences for P2Y4R were forward (NM_020,621.4) 5′-TCGGCTCCGTTCTCTCCGCA-3′ and reverse 5′-CACCCGGCATTCGGCGTTCA-3’. Primer sequences were determined by BLAST (www.ncbi.nlm.nih.gov).
2.7. LDL labeling and aggregation
DiLDL was aggregated by incubation with bacterial sphingomyelinase (SMase), as previously described] [24]. In brief, 1 mg of LDL protein/ml of PBS was incubated with 50 milliunits/ml SMase in the presence of 5 mM MgCl2, for 4 h, under argon, without shaking at 37 °C, followed by 10 mM EDTA to stop the reaction. The LDL was then diluted with binding buffer (10 mM Hepes, pH 7.4, 140 mM NaCl2, 2 mM CaCl2, 2 mM MgCl2 and 3% (w/v) BSA). Unlabeled lipoprotein was fluorescently labeled by Alexa fluor® 546 monoclonal antibody labeling kit according to manufacturer's protocol, then aggregated by combining 500 ml with 500 ml PBS and centrifugation for 10 min at 10,000 × g, followed by collecting and resuspending the pellet in 700 μl PBS.
2.8. Matrix preparation, LDL binding and uptake
To produce SMC-derived matrix, 100 μl of the mouse SMC culture (1 × 106 cells/ml) was plated in 35-mm glass-bottom culture plates and incubated in DMEM with 10% (v/v) FBS. Cells were grown in a monolayer to 100% confluence. Following three washes with DMEM containing 0.2% (w/v) BSA, the SMC monolayer was air dried for 15 min and extracted twice with 3:2 (v/v) hexane:isopropanol for 30 min. The lipid extracts were removed and discarded, and the wells dried for 15 min at room temperature in a laminar flow hood. After washing three times with binding buffer, the matrix was incubated with binding buffer for 1 h at room temperature to block non-specific binding sites. In preparation for LDL-matrix binding, 1 μg of lipoprotein lipase (Sigma-Aldrich, St. Louis, MO) was added to the lipid-extracted SMC matrix for 1 h at room temperature, then 100 μl of SMase-treated LDL was added to the matrix and incubated for 18 h at 37 °C in a humidified atmosphere with 5% CO2. The unbound LDL was removed by washing with DMEM and 0.2% (w/v) BSA.
2.9. UTP-stimulated uptake of agLDL in mouse VSMCs
To investigate the uptake of agLDL in the absence or presence of matrix, 100% (w/v) agLDL solution was vortexed for 4 min and then centrifuged at 10,000 × g for 10 min, and the pellet was recovered. VSMCs were incubated with 50 μg/ml agLDL for 2 h at 37 °C followed by 37 °C for 2 h in the presence or absence of 100 μM UTP. Cells were incubated with 200 μg/ml 1,1'dioctadecyl-3,3,3′,3’-tetramethylindo-carbocyanine (DiI) to stain lipid vacuoles. The nuclei were stained by incubating cells with 1 μg/ml of Hoechst solution (Thermo Scientific, Rockford, IL). Fluorescence microscopy experiments were carried out to visualize the internalization of agLDL by mouse VSMCs using a Zeiss LSM 510 laser scanning confocal microscope.
2.10. UTP-stimulated uptake of agLDL in VSMCs expressing WT or mutant P2Y2R
To determine agLDL uptake by VSMCs, HA-tagged WT P2Y2R- or mutant P2Y2R-transfected VSMCs were incubated with 100 μM UTP for 4 h, then seeded on top of the matrix with 200 μg/ml DiI-oxLDL or Alexa fluor labeled LDL for 0, 1, 2 or 4 h. To visualize the internalization of fluorescently-labeled agLDL, the cells were viewed with a Zeiss LSM 700 confocal microscope. After brief rinsing in DMEM with 0.2% (w/v) BSA, cells were fixed with 3% (v/v) paraformaldehyde. The nucleus was identified using DAPI nuclear stain.
2.11. Statistical analysis
The treatment effects were analyzed using JMP (version 10.0, SAS Institute, Cary, NC), and data were evaluated by Student's t-test and expressed as means ± SEM with statistical significance defined as p < 0.05.
3. Results
3.1. UTP stimulates LRP1 expression in mouse VSMCs
LRP1 is highly expressed in VSMCs and has been shown to be upregulated in vitro by aggregated LDL (agLDL), and in vivo in a hypercholesterolemic porcine model [25,26]. Since agLDL is found in atherosclerotic lesions, our overall goal was to determine how agLDL uptake is regulated in foam cell formation. Therefore, we began our investigation by using western blot analysis to determine what effect UTP had on LRP1 expression in VSMCs from WT mice. Our results revealed that LRP1 expression was the greatest for cells stimulated with the highest concentration of UTP. In addition, there was a dose dependent response for LRP1 expression, with the highest UTP dose showing the greatest response versus the lowest doses with the least response (Fig. 1).
Fig. 1. Protein abundance of LRP1 expression in mouse VSMCs.
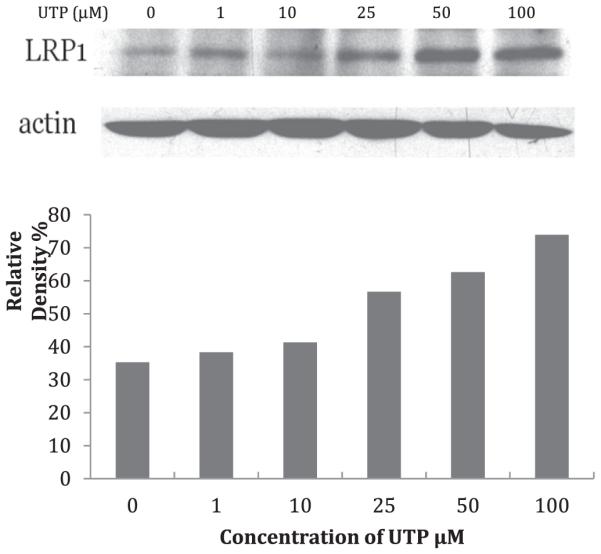
Serum-starved VSMCs were incubated in the absence or presence of UTP (1, 10, 25, 50, 100 μM). (Upper panel) Detection of LRP1 was performed using mouse anti-LRP1 monoclonal antibody (1:3000 dilution; Abcam, Cambridge, MA) followed by horseradish peroxidase-conjugated donkey anti-mouse polyclonal antibody (1:10,000 dilution Abcam, Cambridge, MA). For signal normalization, membranes were probed with polyclonal anti-β-actin antibody (1:2000 dilution). (Lower panel) The results show that LRP1 expression is increased in a UTP dose-dependent manner.
3.2. LRP1 mRNA expression is upregulated in mouse VSMCs with UTP stimulation
We next determined whether LRP1 was transcriptionally regulated in response to UTP stimulation of mouse VSMCs. For this reason, quantitative RT-PCR was carried out to assess LRP1 mRNA relative abundance in VSMCs. As shown in Fig. 2, UTP caused a two-fold increase in LRP1 mRNA expression, as compared to untreated mouse VSMCs expressing the wild type P2Y2R. Addition of UTP to VSMCs from P2Y2R−/− mice showed no effect and was much lower than in WT cells with or without UTP (p < 0.05) (Fig. 2).
Fig. 2. LRP1 mRNA expression in VSMCs stimulated with UTP.
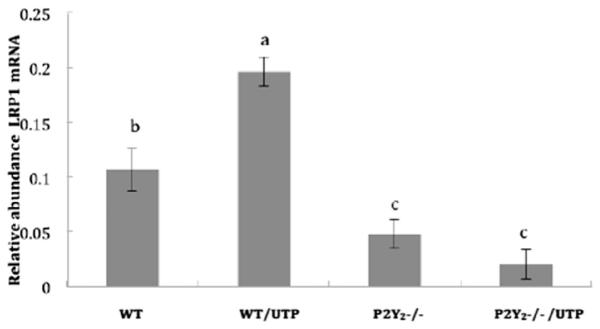
VSMCs isolated from WT and P2Y2R−/− mice were cultured in 12-well plates and grown to at least 80% confluence. The cells were serum-starved overnight followed by treatment with 10 μM UTP. Total RNA was isolated and reverse transcribed to generate cDNA. The mRNA relative abundance was determined by RT-PCR using the ΔΔCt method. Data are shown as means ± SEM of results from 6 independent experiments where p < 0.05 represents a significant difference, as compared to untreated VSMCs from WT mice. GAPDH was used as a control gene to normalize the data.
3.3. UTP stimulates uptake of aggregated LDL in mouse VSMCs
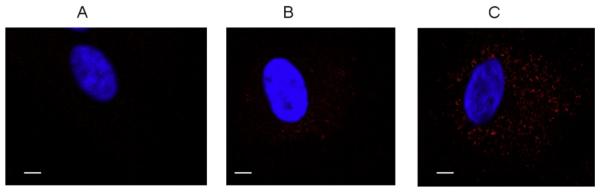
We investigated the uptake of agLDL in the presence and absence of SMC-derived matrix, prepared as mentioned above. Studies of PMA-activated macrophages seeded onto macrophage-derived matrix show significant increases in LDL binding and uptake [24,27], and our previous studies have established a role for the P2Y2R in actin stress fiber formation [16]. Since uptake of matrix-bound agLDL is associated with cytoskeleton reorganization, we hypothesized that stimulation of P2Y2R will increase agLDL uptake in VSMCs. Fluorescence microscopy studies indicate that UTP significantly increased the accumulation of DiL-labeled agLDL in cultured mouse VSMCs (Fig. 3).
Fig. 3. UTP stimulates uptake of agLDL in mouse VSMCs.
Confocal microscopy of mouse VSMCs incubated with 50 μg/ml agLDL for 2 h at 37 ° C. Lipids were stained red by incubating cells with 1,1′ dioctadecyl-3,3,3′ ,3’-tetramethyl-indocarbocyanine (Dil). VSMCs were incubated with (A) no LDL (B) LDL or (C) UTP (10 μM) þ LDL. Cells were washed and incubated with 5 μg/ml DiI for 30 min in the dark. The red granules indicate lipid uptake. The nuclei were stained by incubating cells with 1 μg/ml Hoechst dye (blue). The images are representative of results from at least 3 independent experiments. Cells were visualized using a Zeiss 510 laser scanning confocal microscope. Scale bar represents 50 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. P2Y2R/FLN A interaction is required for UTP-mediated uptake of matrix-bound agLDL
We next hypothesized that P2Y2R interaction with filamin A was required for the uptake of DiL-labeled agLDL. For this purpose, VSMCs were isolated from P2Y2R−/− mice transduced with adenoviruses encoding either the full length P2Y2R or a mutant P2Y2R defective in FLN-A binding [16] [20]. As shown in Fig. 4, UTP significantly increases matrix-bound agLDL uptake in VSMCs expressing the full length WT P2Y2R. In contrast, UTP failed to stimulate LDL uptake in VSMCs from mice expressing a mutant P2Y2R that does not bind FLN-A (Fig. 4).
Fig. 4. UTP stimulates matrix-bound agLDL uptake in VSMCs expressing WT P2Y2R but not in VSMCs expressing a mutant P2Y2R that does not bind FLN-A.
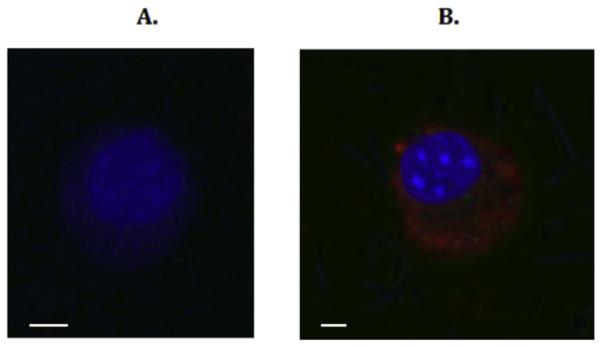
VSMCs from P2Y2R−/− mice were transfected with (A) a mutant P2Y2R that does not bind FLN-A or (B) WT P2Y2R. Cells were grown on top of matrix, incubated with 50 μg/ml agLDL for 4 h at 37 ° C and then stimulated with 10 μM UTP. Lipids were stained by incubating cells with 1,1′ dioctadecyl-3,3,3′ ,3’-tetramethyl-indocarbocyanine (DiI; red) and cells were visualized using a Zeiss LSM 700 confocal microscope. The nuclei were stained by incubating cells with 1 μg/ml Hoeschst dye (blue). The images are representative of results from at least 3 independent experiments. Scale bar represents 50 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. UTP-stimulated LDLR mRNA expression in VSMCs is dependent upon P2Y2R interaction with FLN-A
It has already been established that the LDLR mediates the endocytosis of cholesterol-rich LDL, but not the aggregated form of LDL. However, we wanted to see if the LDLR was transcriptionally regulated by the P2Y2R/FLN-A interaction. We hypothesized that the P2Y2R mediates the mRNA expression of the LDLR through interaction with FLN-A. To evaluate regulation of LDLR mRNA expression by the P2Y2R, we transiently transfected P2Y2R−/− VSMCs with cDNA encoding a hemagglutinin-tagged WT P2Y2R or a mutant P2Y2R that does not bind FLN-A. VSMCs expressing the mutant P2Y2R exhibit 3-fold lower LDLR mRNA expression than VSMCs expressing the WT P2Y2R (Fig. 5). This shows that a full-length P2Y2R capable of FLN-A interaction is necessary for LDLR expression in mouse VSMCs.
Fig. 5. LDL mRNA expression in VSMCs stimulated with UTP. VSMCs from P2Y2R−/− mice were transiently transfected with cDNA encoding a hemagglutinin-tagged WT P2Y2R or a mutant P2Y2R that does not bind FLN-A.
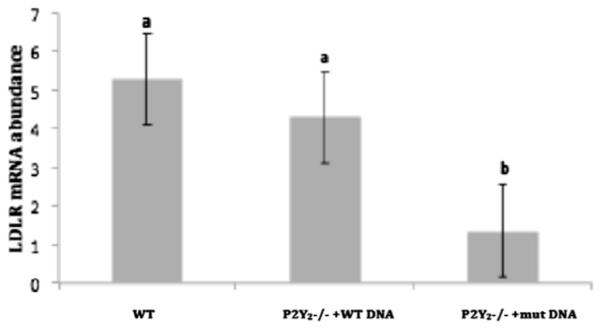
Untransfected VSMCs showed the highest LDLR mRNA relative abundance compared to both transfected groups, although LDLR mRNA abundance was not significantly different between untransfected and WT P2Y2R−/−transfected VSMCs. Additionally, VSMCs expressing the mutant P2Y2R exhibited significantly lower LDLR mRNA relative abundance than VSMCs transfected with the WT P2Y2R. Same letters represent no significant difference between the groups. Different letters represent significant differences between groups (p < 0.05). GAPDH was used as a control gene.
3.6. UTP-stimulated P2Y1R and P2Y4R mRNA expression in VSMCs
The purpose of this study was to determine the efficiency of the P2Y2R knockout procedure, and whether other P2Y subtypes that respond to UTP are present in the VSMCs from the P2Y2R−/− mice. The results revealed UTP did not increase the abundance of P2Y1R mRNA in either WT or P2Y2R−/− VSMCs (Fig. 6). Although not significant, P2Y2R mRNA relative abundance showed a steady increase with UTP stimulation over time in both WT and P2Y2R−/− cells (Fig. 7), suggesting that the P2Y2R may not have been completely deleted in the P2Y2R−/− mice. The results also showed that UTP stimulation resulted in a significant increase (p < 0.05) in P2Y4R mRNA expression after 2 h in VSMCs from P2Y2R−/− mice versus WT cells, but mRNA expression declined within 4 h (Fig. 8).
Fig. 6. P2Y1R mRNA expression in VSMCs stimulated with UTP.
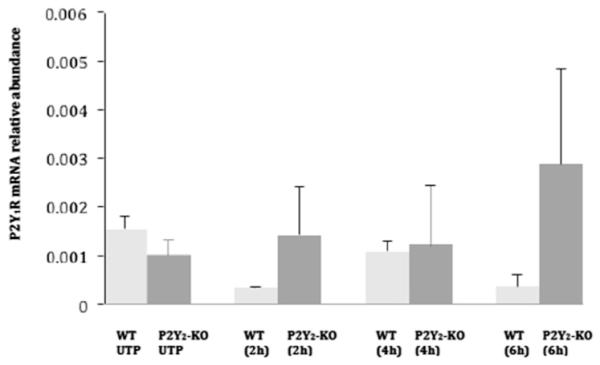
VSMCs isolated from WT or P2Y2R−/− mice were cultured in 12-well plates and grown in DMEM/10% FBS to at least 80% confluence. Cells were serum-starved overnight, followed by stimulation with 10 μM UTP. Total RNA was isolated, and reverse transcribed to cDNA using multiscribe reverse transcriptase and random primers. The mRNA relative abundance was determined by RT-PCR using P2Y1R forward and reverse primers and the ΔΔCt method to calculate fold increase in mRNA levels for results from 6 independent experiments. P2Y1R expression is calculated relative to GAPDH. There were no significant differences in the levels of P2Y1R mRNA detected over time.
Fig. 7. P2Y2R mRNA expression in VSMCs stimulated with UTP.
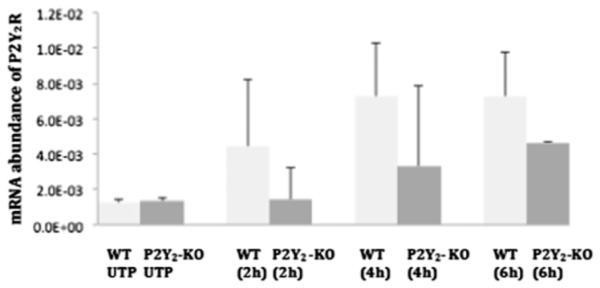
VSMCs isolated from WT or P2Y2R−/− mice were cultured in 12-well plates and grown in DMEM/10% FBS to at least 80% confluence. Cells were serum-starved overnight, followed by stimulation with 10 μM UTP. Total RNA was isolated, and reverse transcribed to cDNA using multiscribe reverse transcriptase and random primers. The mRNA relative abundance was determined by RT-PCR using P2Y2R forward and reverse primers and the ΔΔCt method to calculate fold increase in mRNA levels for results from 6 independent experiments. P2Y2R expression is calculated relative to GAPDH. There were no significant differences in the levels of P2Y2R mRNA detected over time.
Fig. 8. P2Y4R mRNA expression in VSMCs stimulated with UTP.
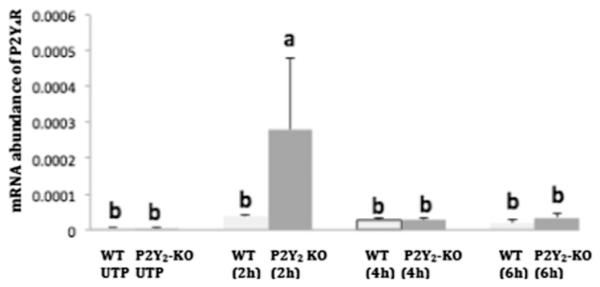
VSMCs isolated from WT or P2Y2R−/− mice were cultured in 12-well plates and grown in DMEM/10% FBS to at least 80% confluence. Cells were serum-starved overnight, followed by stimulation with 10 μM UTP. Total RNA was isolated, and reverse transcribed to cDNA using multiscribe reverse transcriptase and random primers. The relative mRNA abundance was determined by RT-PCR using P2Y4R forward and reverse primers and the ΔΔCt method to calculate fold increase in mRNA levels for results from 6 independent experiments. P2Y4R expression is calculated relative to GAPDH. Different letters represent significant differences between groups (p < 0.05).
4. Discussion
4.1. The P2Y2 receptor regulates a protective mechanism during lipid accumulation
A substantial amount of immobilized lipoproteins are present in atherosclerotic lesions [28]. According to the response-to-retention hypothesis, lipoprotein retention in the arterial wall is an initiating event in atherogenesis that triggers an inflammatory response leading to lesion development [29]. Many studies have focused on roles played by activated endothelium and immune cells, such as macrophages and neutrophils, in the development of atherosclerosis. VSMCs and components within the cytoskeleton undoubtedly play a key role in disease progression. However, modifications of lipoproteins retained in the extracellular matrix of VSMCs necessitate a specific mechanism for aggregated lipoprotein uptake. LRP1 is a lipoprotein receptor expressed in arterial SMCs and other cells that is known to regulate the catabolism of lipoproteins [30], and modulates the activities of platelet-derived growth factor (PDGF) receptor-β and integrins [31,32]. The effects of integrins complexing with the P2Y2R was also investigated in a separate study, and it was demonstrated that the P2Y2R, via a arginine-glycine-aspartic acid (RGD) binding domain in its first extracellular loop, is able to bind to specific integrins, which enables ATP and UTP to activate integrin signaling pathways [32]. P2Y2Rs for extracellular ATP and UTP have been suggested to play a significant role in inflammation, responses to injury and the development of atherosclerosis [16,33,34]. UTP, a relatively selective P2Y2R agonist, is shown here to increase LRP1 expression in murine VSMCs at both the mRNA and protein levels (Figs. 1 and 2). Furthermore, the activation of P2Y2Rs in VSMCs increases the uptake of agLDL (Figs. 3 and 4). We suggest that P2Y2R activation mediates LRP1 signaling as a protective mechanism in VSMCs to increase the uptake and degradation of cholesterol trapped within the extracellular matrix. LRP1 has been suggested to prevent the formation of atherosclerotic lesions via interaction with the PDGF-β and the transforming growth factor-β (TGFβ) signaling pathways, both of which have major roles in atherosclerosis development [30,35,36]. Deletion of LRP1 is associated with uncontrolled aortic SMC hyperplasia and increased PDGFRβ expression and phosphorylation, increased Smad 2 phosphorylation and alterations in TGFβ signaling [30]. However, apo-E binding to LRP1 inhibits PDGF-induced SMC migration within the arterial wall [37], suggesting that LRP1 protects the vasculature by suppressing PDGFRβ activation [31].
4.2. P2Y2R association with FLN-A is required for UTP-induced uptake of agLDL
The P2Y2R is associated with the actin cytoskeleton through a C-terminal interaction with FLN-A, and UTP-induced FLN-A phosphorylation is associated with actin polymerization [11]. UTP also induced FLN-A phosphorylation in human 1321N1 astrocytoma cells expressing the full length P2Y2R, but not the mutant P2Y2R defective in FLN-A binding [13]. Thus, we propose that the P2Y2R/ FLN-A interaction is required for UTP-induced agLDL uptake mediated by LRP1. As shown in Fig. 4, UTP significantly increased agLDL uptake in vascular smooth muscle cells expressing the full length WT P2Y2R. In contrast, UTP failed to stimulate LDL uptake in VSMCs from mice expressing the mutant P2Y2R that does not bind FLN-A (Fig. 4). Phosphorylation of FLN-A at Ser/Thr regulates the association between FLN-A and the actin cytoskeleton for the stabilization of caveolae at the plasma membrane [38]. We postulate that FLN-A phosphorylation may be an important factor in UTP-induced LRP1 activation and cytoskeleton dynamics that promotes the uptake of agLDL in VSMCs. Dedieu et al. [39] observed a regulatory role for LRP1 in the architecture and dynamics of the cytoskeleton. In addition, adaptor proteins bind to the cytoplasmic domain of LRP1 to produce a variety of cellular responses. For example, Talin-like protein interacts with LRP1 to promote coupling to the actin cytoskeleton [40]. Some studies report that LRP1 is phosphorylated on both serine and tyrosine residues [41,42]. Li et al. [41] showed that LRP1 phosphorylation both in vitro and in vivo is mediated by cyclic AMP- (cAMP)-dependent protein kinase A (PKA), and used site-directed mutagenesis to identify serine 76 as the site of phosphorylation in the LRP1 cytoplasmic tail. Phosphorylation of tyrosine residues in LRP1 plays a role in association with Shc [42,43], a docking protein whose phosphorylation facilitates protein-protein interactions [42]. Tyrosine phosphorylated LRP1 specifically associates with the Src-homology 2 (SH2) binding domain on Shc [42]. SH2 binding domains mediate interactions between various intracellular signaling proteins, allowing these proteins to locate binding partners to regulate specific signaling pathways [44]. The cytoplasmic tail of the P2Y2R has two consensus proline rich (PXXP) SH3 binding domains that upon activation of the P2Y2R bind Src to promote the transactivation/ phosphorylation of growth factor receptors [45,46]. Both SH2 and SH3 may be involved in modulating interactions with the cytoskeleton, and receptor cross-talk between P2Y2R and LRP1 [42].
LRP1 signaling activity requires the direct interaction with the G stimulatory protein α-subunit (Gsα) of a heterotrimeric Gs protein [47]. Thus, it is possible that cross-talk between LPR1-coupled Gsα-dependent activation of PKA and P2Y2R-coupled Gqα-dependent activation of phospholipase C and protein kinase C [47] coordinately regulates agLDL uptake.
4.3. P2Y2R signaling plays a regulatory role in the uptake of agLDL
In conclusion, we have shown for the first time that P2Y2R activation increases LRP1 expression and the uptake of aggregated LDL by murine primary vascular smooth muscle cells. We suggest the existence of a regulatory element within the LRP1 promoter that is responsive to activation of the P2Y2R signaling pathway (Fig. 9). We also propose that UTP-induced uptake of agLDL occurs via activation of the LRP1 signaling pathway and requires the presence of an FLN-A binding domain in the intracellular C-terminal domain of the P2Y2R and possibly FLN-A phosphorylation (Fig. 9). Overall, our novel findings show that increases in LRP1 expression in VSMCs is UTP dose-dependent. Our findings showed that LRP1 expression was transcriptionally upregulated by UTP-induced P2Y2R activation, since a two-fold increase in LRP1 mRNA expression in response to UTP was observed in mouse VSMCs expressing the wild type P2Y2R, as compared to untreated controls, whereas 10-fold lower LRP1 mRNA expression was seen in UTP-treated VSMCs from P2Y2R−/− mice (Fig. 2). Information from this study could prove very useful by providing insights into future pharmacological targets in the treatment of hyperlipidemia and atherosclerosis.
Fig. 9. Predicted pathway for P2Y2R/LRP1 interaction in the regulation of agLDL uptake.
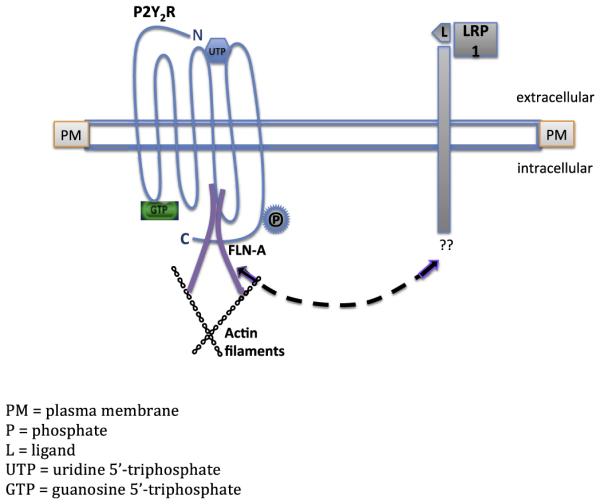
Diagram represents a proposed mechanism for P2Y2R-mediated uptake of agLDL in VSMCs and possible cross-talk between LRP1 and the P2Y2R. The LRP1 extracellular binding domain indicates binding of apo-E ligand (L). In vitro studies show that Apo E binding inhibits platelet-derived growth factor-induced migration and proliferation in smooth muscle cells (Boucher et al., 2003). We predict the existence of a regulatory element within the LRP1 promoter that is responsive to P2Y2R activation. We propose that LRP1 associates with the P2Y2R (dashed line) possibly via a phosphorylated residue in the C-terminus of the P2Y2R or through binding of LRP1 to P2Y2R-associated FLN-A and/or other cytoskeletal proteins. GTP indicates the activated state of the G protein-coupled P2Y2R.
Acknowledgements
We are grateful for the technical assistance in confocal microscopy provided by Joel Sanneman at Kansas State University College of Veterinary Medicine Confocal Core facilities (funded by the College of Veterinary Medicine).
Financial support
This research was funded by a Ruth L. Kirschstein National Service Award to Tixieanna Dissmore for Individual Predoctoral Fellowship F31 HL090029 obtained through the National Heart, Lung and Blood Institute, and by a grant from NIH (1RO1HL112883) to Cheikh I. Seye.
Abbreviations
- LRP1
low-density lipoprotein receptor related protein
- agLDL
aggregated low-density lipoproteins
- P2Y2R
P2Y2 receptor
Footnotes
Conflict of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Reference
- [1].Roger VL, Go AS, Lloyd-James DM, et al. Heart disease and stroke statistics – 2012 update: a report from the american Heart association. Circulation. 2012:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (part 1) N. Eng. J. Med. 1992;326:242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- [3].Fogelstrand P, Borèn J. Retention of atherogenic lipoproteins in the artery wall and its role in atherogenesis. Nutr. Metab. Cardio Dis. 2012;22:1–7. doi: 10.1016/j.numecd.2011.09.007. [DOI] [PubMed] [Google Scholar]
- [4].Erlinge D, Yoo H, Edvinsson L, Reis DJ, et al. Mitogenic effects of ATP on vascular smooth muscle cells vs other growth factors and sympathetic cotransmitters. Am. J. Physiol. 1993;265:H1089–H1097. doi: 10.1152/ajpheart.1993.265.4.H1089. [DOI] [PubMed] [Google Scholar]
- [5].Chaulet H, Desgranges C, Renault MA, Dupuch F, et al. Extracellular nucleotides induce arterial smooth muscle cell migration via osteopontin. Circ. Res. 2001 Oct.89(9):772–778. doi: 10.1161/hh2101.098617. [DOI] [PubMed] [Google Scholar]
- [6].Tertov VV, Sobenin IA, Gabbasov ZA, Popov EG, et al. Lipoprotein aggregation as an essential condition of intracellular lipid accumulation caused by modified low-density lipoproteins. Biochem. Biophys. Res. Commun. 1989;163(1):489–494. doi: 10.1016/0006-291x(89)92163-3. [DOI] [PubMed] [Google Scholar]
- [7].Luis AJ. Atheroscler. Nat. 2000;407(6801):233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ismail NA, Alavi MZ, Moore S. Lipoprotein -proteoglycan complexes from injured rabbit aortas accelerate lipoprotein uptake by arterial smooth muscle cells. Atherosclerosis. 1994;105(1):79–87. doi: 10.1016/0021-9150(94)90010-8. [DOI] [PubMed] [Google Scholar]
- [9].Kowal RC, Herz J, Goldstein JL, Esser V, et al. Low-density lipoprotein receptor-related protein mediates uptake of cholesterol esters derived from apoprotein E-enriched lipoproteins. Proc. Natl. Acad. Sci. U. S. A. 1989;86:5810–5814. doi: 10.1073/pnas.86.15.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kunapuli PS, Daniel JL. P2 receptor subtypes in the cardiovascular system. Biochem. J. 1998;336:513–523. doi: 10.1042/bj3360513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Vaillant N, et al. P2Y1, P2Y2, P2Y4, and P2Y6 receptors are coupled to RHO and rho kinase activation in vascular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2000;278(6):H1751–H1761. doi: 10.1152/ajpheart.2000.278.6.H1751. [DOI] [PubMed] [Google Scholar]
- [12].Pillois X, Chaulet H, Belloc I, Dupuch F, Desgranges C, Gadeau AP. Nucleotidereceptors involved in UTP-induced rat arterial smooth muscle cell migration. Circ Res. 2002 Apr 5;90(6):678–681. doi: 10.1161/01.res.0000013700.98464.8e. [DOI] [PubMed] [Google Scholar]
- [13].Yu N, Erb L, Shivaji R, Weisman GA, Seye CI. Binding of the P2Y2 nucleotide receptor to filamin a regulates migration of vascular smooth muscle cells. Circ. Res. 2008;102:581–588. doi: 10.1161/CIRCRESAHA.107.162271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xu X, Tabas I. Sphingomyelinase enhances low-density lipoprotein uptake and ability to induce cholesterol ester accumulation in macrophages. J. Biol. Chem. 1992;266(36):24849–24858. [PubMed] [Google Scholar]
- [15].Llorente-Cortes V, Martinez-Gonzales J, Badimon L. LDL receptor-related protein mediates uptake of aggregated LDL in human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2000;20:1572–1579. doi: 10.1161/01.atv.20.6.1572. [DOI] [PubMed] [Google Scholar]
- [16].Seye CI, Kong Q, Erb L, Garrad RC, et al. Functional P2Y2 nucleotide receptors mediate uridine 5'-triphosphate-induced intimal hyperplasia in collared rabbit carotid arteries. Circulation. 2002;106:2720–2726. doi: 10.1161/01.cir.0000038111.00518.35. [DOI] [PubMed] [Google Scholar]
- [17].Nakashima Y, Wight TN, Sueishi K. Early atherosclerosis in humans: role of diffuse intimal thickening and extracellular matrix proteoglycans. Cardiovasc Res. 2008;79:14–21. doi: 10.1093/cvr/cvn099. [DOI] [PubMed] [Google Scholar]
- [18].Parr CE, Sullivan DM, Paradiso AM, Lazarowski ER, et al. Cloning and expression of a human P2U nucleotide receptor, a target for cystic fibrosis pharmacotherapy. Proc. Nat. Acad. Sci. U. S. A. 1994;91:3275–3279. doi: 10.1073/pnas.91.8.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Burnstock G. Purine and pyrimidine receptors. Cell Mol. Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liao Z, Seye CI, Weisman GA, Erb L. The P2Y2 receptor requires interaction with αn integrins to access and activate G12. J. Cell Sci. 2007;120:1654–1662. doi: 10.1242/jcs.03441. http://dx.doi.org/10.1242/jcs.03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sakr SW, Eddy RJ, Barth H, Wang F, et al. The uptake and degradation of matrix-bound lipoproteins by macrophages require an intact actin cytoskeleton, rho family GTPases, and myosin ATPase activity. J. Biol. Chem. 2001;276(40):37649–37658. doi: 10.1074/jbc.M105129200. http://dx.doi.org/10.1074/jbc.M105129200. [DOI] [PubMed] [Google Scholar]
- [22].Garrad RC, Otera MA, Theiss PM, Clark LL, et al. Structural basis of agonist-induced desensitization and sequestration of the P2Y2 nucleotide receptor. consequences of truncation of the C terminus. J. Biol. Chem. 1998;273(45):29437–29444. doi: 10.1074/jbc.273.45.29437. [DOI] [PubMed] [Google Scholar]
- [23].Erb L, Garrad R, Wang Y, Quinn T, et al. Site-directed mutagenesis of P2U purinoceptors. Positively charged amino acids in transmembrane helices 6 and 7 affect agonist potency and specificity. J. Biol. Chm. 1995 Mar 3;270(9):4185–4188. doi: 10.1074/jbc.270.9.4185. [DOI] [PubMed] [Google Scholar]
- [24].Tabas I, Yueqing L, Brocia RW, Wen XS, et al. Lipoprotein lipase and sphingomyelinase synergistically enhance the association of atherogenic lipoproteins with smooth muscle cells and extracellular matrix. J. Biol. Chem. 1993;268:20419–20432. [PubMed] [Google Scholar]
- [25].Llorente-Cortes V, Otero-Vinas M, Sanchez S, Rodriguez C, Badimon L. Low-density lipoprotein upregulates low-density lipoprotein receptor-related protein expression in vascular smooth muscle cells: possible involvement of sterol regulatory element binding protein-2-dependent mechanism. Circulation. 2002;106(24):3104–3110. doi: 10.1161/01.cir.0000041434.28573.0b. [DOI] [PubMed] [Google Scholar]
- [26].Llorente-Cortes V, Otero-Vinas M, Camino-Lopez S, Costales P, et al. Cholesterol esters of aggregated LDL are internalized by selective uptake in human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2006;26:117–123. doi: 10.1161/01.ATV.0000193618.32611.8b. [DOI] [PubMed] [Google Scholar]
- [27].Kaplan M, Aviram M. Retention of oxidized LDL by extracellular matrix proteoglycans leads to its uptake by macrophages. Arterioscler. Thromb. Vasc. Biol. 2001;21:386–393. doi: 10.1161/01.atv.21.3.386. [DOI] [PubMed] [Google Scholar]
- [28].Smith EB, Massie IB, Alexander KM. The release of an immobilized lipoprotein fraction from atherosclerotic lesions by incubation with plasmin. Atherosclerosis. 1976;25(1):71–84. doi: 10.1016/0021-9150(76)90049-6. [DOI] [PubMed] [Google Scholar]
- [29].Williams KJ, Tabas I. The response-to retention hypothesis of early atherogenesis. Atheroscler. Thromb. Vasc. Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Boucher P, Herz J. Signaling through LRP 1: protection from atherosclerosis and beyond. Biochem. Pharmacol. 2011;81(1):1–10. doi: 10.1016/j.bcp.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Boucher P, Gotthardt M, Li WP, et al. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300(5617):329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- [32].Erb L, Liu J, Ockerhausen J, Kong Q, Garrad R.c., et al. An RGD sequence in the P2Y2 receptor interacts with an b3 integrins and is required for Go-mediated signal transduction. J. Cell Biol. 2001 Apr 30;153(3):491–502. doi: 10.1083/jcb.153.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mercier N, Kiviniemi O, Saraste A, Miiluniemi M, et al. Impaired ATP-induced coronary blood flow and diminished aortic NDPDase activity precede lesion in apolipoprotein E-deficient mice. Am. J. Pathol. 2012;180(1):419–428. doi: 10.1016/j.ajpath.2011.10.002. http://dx.doi.org/10.1016/j.ajpath.2011.10.002. Epub 2011 Nov 7. [DOI] [PubMed] [Google Scholar]
- [34].Ferrari D, Vitiello L, Idzko M, La Sala A. Purinergic signaling in atherosclerosis. Trends Mol. Med. 2015;21(3):184–192. doi: 10.1016/j.molmed.2014.12.008. [DOI] [PubMed] [Google Scholar]
- [35].Loukinova E, Ranganathan S, Kuznetsoy S, Gorlatova N, et al. PDGF-induced tyrosine phosphorylation of the LDL receptor-related protein (LRP): evidence for integrated co-receptor function between LRP and the PDGF receptor. J. Biol. Chem. 2002;277:15499–15506. doi: 10.1074/jbc.M200427200. [DOI] [PubMed] [Google Scholar]
- [36].Boucher P, Liu P, Gotthardt M, Heisberger T, et al. Platelet-derived growth factor mediates tyrosine phosphorylation of the cytoplasmic domain of the low-density lipoprotein receptor-related protein in caveolae. J. Biol. Chem. 2002;277:15507–15513. doi: 10.1074/jbc.M200428200. [DOI] [PubMed] [Google Scholar]
- [37].Swertfeger DK, Bu G, Hui DY. Low-density lipoprotein receptor-related protein mediates apolipoprotein E inhibition of smooth muscle cell migration. J. Biol. Chem. 2002;277:4141–4146. doi: 10.1074/jbc.M109124200. [DOI] [PubMed] [Google Scholar]
- [38].Muriel O, Echarri A, Hellriegel C, Pavon DM. Phosphorylated filamin A regulates actin-linked caveolae dynamics. J. Cell Sc. 2011;124:2763–2776. doi: 10.1242/jcs.080804. [DOI] [PubMed] [Google Scholar]
- [39].Dedieu S, Langlois B. LRP-1: a new modulator of cytoskeleton dynamics and adhesive complex turnover in cancer cells. Cell Adh Migr. 2008 Apr-May;2(2):77–80. doi: 10.4161/cam.2.2.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lillis AP, van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: functions revealed by selective gene knockout studies. Physiol. Rev. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li Y, van Kerkhof P, Paz Marzolo M, Strous GJ, et al. Identification of a major cyclic amp-dependent protein kinase A phosphorylation site within the cytoplasmic tail of the low-density lipoprotein receptor-related protein: implication for receptor-mediated endocytosis. Mol. Cell Biol. 2001;21(4):1185–1195. doi: 10.1128/MCB.21.4.1185-1195.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].van der Geer P. Phosphorylation of LRP1: regulation of transport and signal transduction. Trends cardiovasc. Med. 2002 May;12(4):160–165. doi: 10.1016/s1050-1738(02)00154-8. [DOI] [PubMed] [Google Scholar]
- [43].Barnes H, Larsen B, Tyers M, van Der Geer P, et al. Tyrosine-phosphorylated low-density lipoprotein receptor-related protein 1 (LRP1) associates with the adapter protein SHC in src-transformed cells. J. Biol. Chem. 2001;276(22):19119–19125. doi: 10.1074/jbc.M011437200. [DOI] [PubMed] [Google Scholar]
- [44].Alberts B, Johnson A, Lewis J, Raff M, et al. Molecular Biology of the Cell. fourth 2002. pp. 847–848. [Google Scholar]
- [45].Weisman GA, Wang M, Kong Q, Chorna NE. Molecular determinants of P2Y2 receptor nucleotide function: implications for proliferative and inflammatory pathways in astrocytes. Mol. Neurobiol. 2005;31(1–3):169–183. doi: 10.1385/MN:31:1-3:169. [DOI] [PubMed] [Google Scholar]
- [46].Soltoff SP. Related adhesion focal tyrosine kinase and the epidermal growth factor receptor mediate the stimulation of mitogen-activated protein kinase by the G-protein-coupled P2Y2 receptor. Phorbol ester or [Ca2] elevation can substitute for receptor activation. J. boil Chem. 1998;273(36):23110–23117. doi: 10.1074/jbc.273.36.23110. [DOI] [PubMed] [Google Scholar]
- [47].Goretzki L, Mueller BM. Low-density-lipoprotein-receptor-related protein (LRP) interacts with a GTP-binding protein. Biochem. J. 1998;336:381–386. doi: 10.1042/bj3360381. [DOI] [PMC free article] [PubMed] [Google Scholar]