Abstract
Bivalent ligands that contain two pharmacophores linked by a spacer are promising tools to investigate the pharmacology of opioid receptor heteromers. Evidence for occupation of neighboring protomers by two phamacophores of a single bivalent ligand (bridging) has relied mainly on pharmacological data. In the present study, we have employed an immunocytochemical correlate to support in vivo biological studies that are consistent with bridging. We show that a bivalent mu agonist/delta antagonist (MDAN-21) that is devoid of tolerance due to possible bridging of mu and delta protomers, prevents endocytosis of the heteromeric receptors in HEK-293 cells. Conversely, a bivalent ligand (MDAN-16) with a short spacer, or monovalent mu agonist give rise to robust internalization. The data suggest that the immobilization of proximal mu and delta protomers is due to bridging by MDAN-21. The finding that MDAN-21 and its shorter spacer homologue MDAN-16 possess equivalent activity in HEK-293 cells, but produce dramatically divergent internalization of mu-delta heteromer, is relevant to the role of internalization and tolerance.
Graphical Abstract
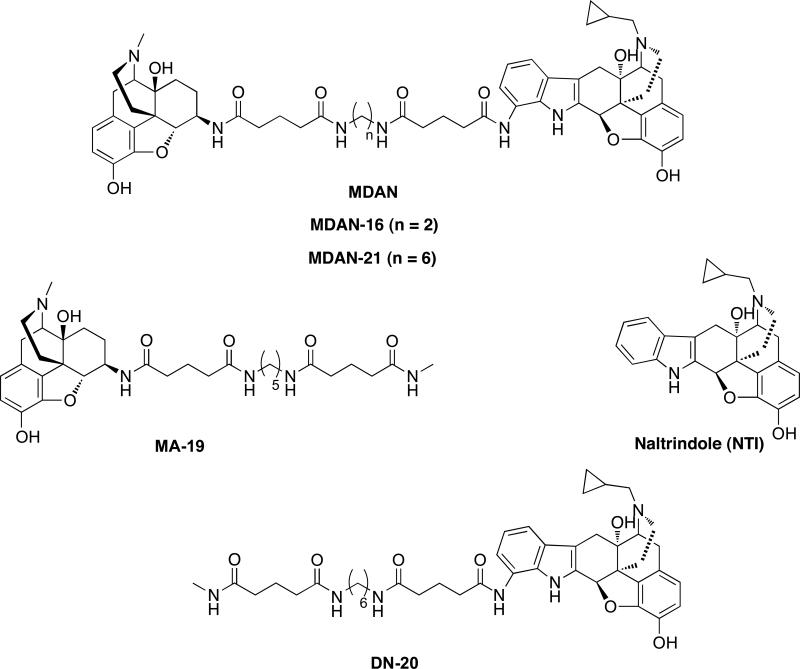
Opioid ligands, such as morphine, produce analgesia via Gi/Go G protein-coupled opioid receptors1,2. There are three receptor types (mu, kappa and delta) in the opioid receptor family that are activated by such ligands.1, 2 Side effects such as tolerance and physical dependence may accompany pharmacotherapy, and several studies have suggested both mu and delta opioid receptors are involved3-6. Notably, it has been shown that co-administration of the delta antagonist, naltrindole7 (NTI) attenuates morphine-induced tolerance and dependence3. These seminal observations, along with the discovery that mu and delta opioid receptors oligomerize to form heteromer8, 9, led to the design of a series of bivalent ligands that contain mu agonist and delta antagonist pharmacophores tethered through different length spacers (MDAN series, Fig 1).10 Significantly, members the MDAN series with spacers containing 18-21 atoms were devoid of tolerance.
Fig 1.
Structures of bivalent ligands (MDAN-16, -21), monovalent ligands (MA-19 and DN-20), and naltrindole (NTI).
The rationale behind the design of the MDAN series was based on the concept that two physically associated GPCR protomers can be bridged through binding of both pharmacophores in a single bivalent ligand.11-14 Subsequent studies have suggested that a variety of opioid bivalent ligands having spacers ranging from 18 to 22 atoms can effectively bridge physically associated protomers.15-17 The most convincing support for the 18-22 atom spacer requirement for bridging employed BRET technology, and involved the bivalent ligand-induced association of mu and CCK2 homomers that do not form constitutive heteromer.18 This study revealed that bivalent ligands containing mu agonist and CCK2 antagonist pharmacophores linked through 18-22 atom spacers efficiently induced physical association of coexpressed mu and CCK2 receptors by shifting the equilibrium from homomers to heteromer, whereas ligands with shorter spacers were not effective in this regard. That the recently reported X-ray crystal structure of the mu opioid receptor reveals transmembrane helices 5 and 6 (TM-5,6) comprise a likely interface for dimerization, 18-22 atoms is consistent with the observed range of spacers for bridging of protomers.19
In the present study, we have performed immunocytochemistry and intracellular calcium release experiments in HEK-293 cells coexpressing mu and delta receptors in the presence of MDAN-21 in an effort to establish an additional correlate for bridging. Given that MDAN-21 has been reported to produce potent antinociception without tolerance, physical dependence, or place preference10, 20, and our suggestion that this is a consequence of bridging, MDAN-21 was compared with its bivalent homologue (MDAN-16) and monovalent opioid agonist (MA-19), both of which possess the aforementioned side effects. MDAN-16 was selected because we had suggested its side effects were related to univalent interaction with opioid receptors due to its shorter spacer (16 atoms). If this is the case, MDAN-21 would be expected to affect endocytosis of mu-delta heteromer differently from MDAN-16 or MA-19.
HEK-293 cells containing FLAG tagged mu (FL-mu) and hemagglutinin tagged delta (HA-delta) were incubated with anti-FLAG and anti-HA primary antibodies (Abcam) for 2 hours on ice. The cells were then treated with the respective ligands for 30 minutes at 37°C, washed, and fixed with 4% paraformaldehyde for 10 minutes at room temperature. Staining was performed using the corresponding fluorophore-tagged secondary antibodies (See supplemental methods for full description). Primary antibodies were added to live unfixed cells so as to label receptors distributed on the plasma membrane only; fixing cells with formaldehyde kills cells makes their membranes permeable to antibodies and as a result both plasma membrane and cytoplasmic receptors will become labeled. Antibodies were added to live cells kept on ice to prevent constitutive internalization of receptors.
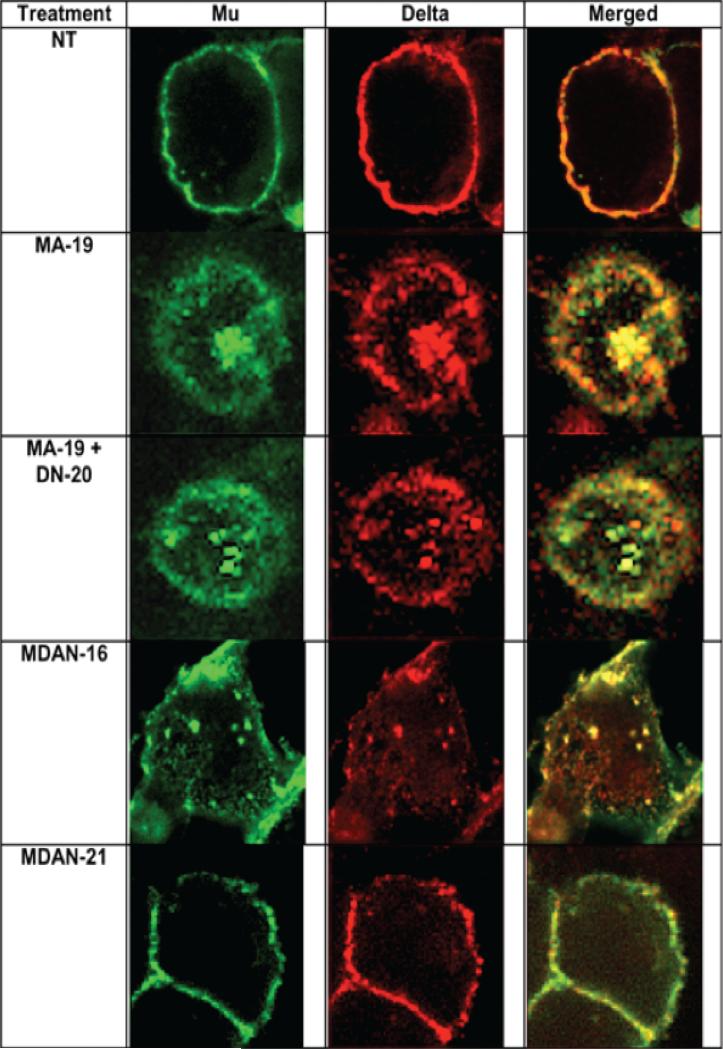
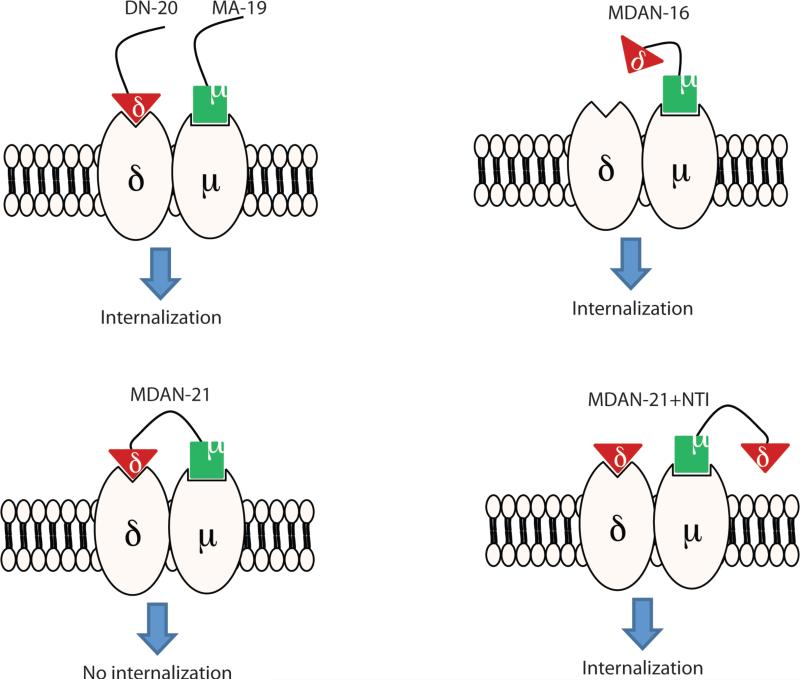
Significantly, MA-19 (1 μM) produced robust co-internalization of mu and delta cell-surface opioid receptors that appear to be co-localized (Fig 2). When taken together with prior reports showing that mu and delta receptors are constitutively expressed as heteromer21, 22, these data suggest that mu and delta receptors are physically coupled and trafficked together. Indeed, the mu agonist, DAMGO, also has been reported to co-internalize mu-delta heteromer22. The fact that co-administration of the monovalent delta antagonist DN-20 with mu agonist MA-19 did not block the co-internalization of mu-delta heteromers (Fig 2), suggests that univalent occupancy of the delta opioid protomer by DN-20 and the mu receptor by MA-19 does not negatively affect trafficking of the heteromer for this combination of ligands.
Fig 2.
High magnification confocal microscopy images depicting effects of bivalent and monovalent ligands on trafficking of mu- and delta opioid receptors co-expressed in HEK-293 cells. NT represents untreated cells. MA19 produces robust internalization. MA-19 + DN-20: Internalization is not antagonized by coadministration of mu agonist and delta antagonist. MDAN-16 also induces endocytosis of both mu and delta receptors. MDAN-21 does not produce significant internalization of either mu or delta receptors.
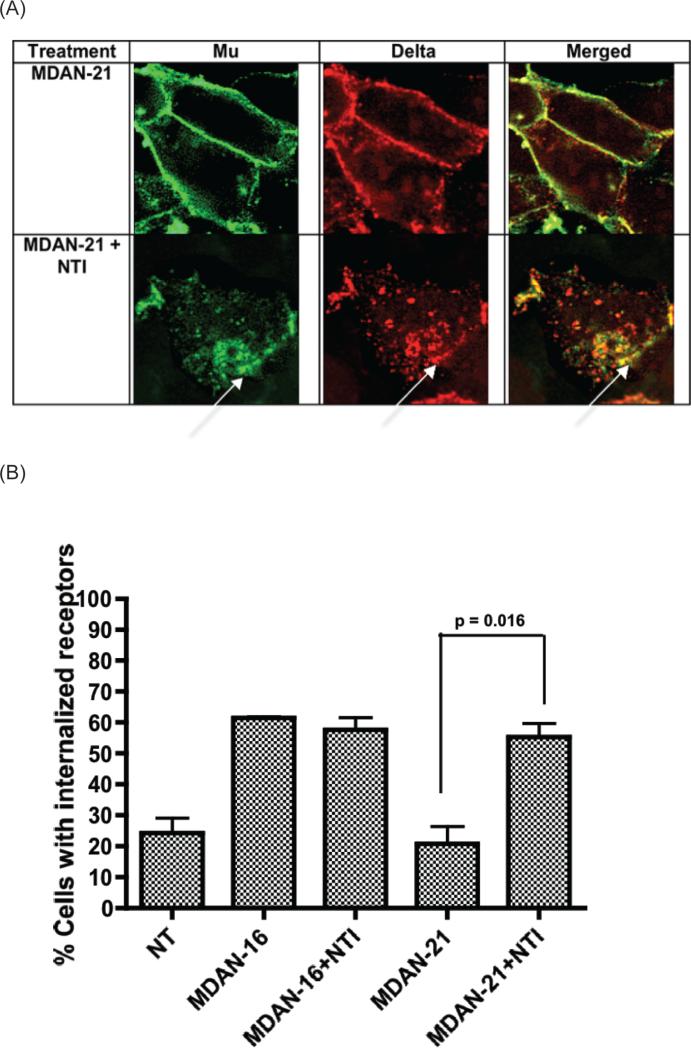
The finding that MDAN-21 (1 μM) did not produce significant internalization of either mu or delta receptors in the mu/delta cell line (Fig 2, Fig 3A) suggests that spacer-mediated bridging of protomers contributes to the dramatic change in trafficking. Since the bivalent ligand with a 16-atom spacer (MDAN-16) produced robust co-internalization of mu and delta receptors, this strongly suggests that MA-19 and MDAN-16 are involved in univalent interaction that leads to co-internalization of mu-delta heteromer.
Fig 3.
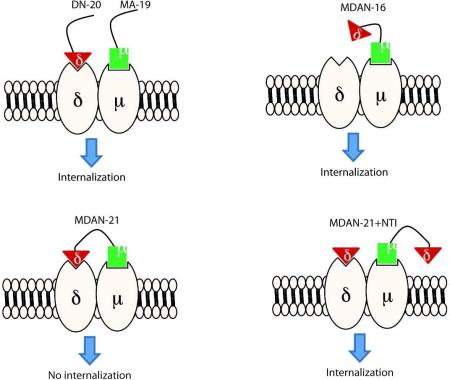
(A) Facilitation of internalization of mu and delta receptors upon pretreatment with delta antagonist, naltrindole (NTI), in the presence of MDAN-21. The bivalent ligand MDAN-21 alone does not produce significant internalization of either mu or delta receptor. However, upon pretreatment with NTI for 10 mins MDAN-21 produced robust co-internalization of both mu and delta receptors. (B) Pretreatment with NTI (1 μM) significantly (p = 0.016, t-test, two-tailed) increases the number of internalized receptors in cells treated with MDAN-21 (1 μM). On the other hand, the number of cells with internalized receptors due to MDAN-16 was unaffected with or without NTI pre-treatment. A minimum of 75 cells were counted for each treatment with two independent experiments.
Additional evidence for bridging of mu-delta protomers was obtained when cells were pretreated with the delta antagonist, naltrindole (NTI) 10 minutes before adding MDAN-21. The fact that several punctate images of co-internalized mu and delta receptors were observed (Fig 3A, Fig 3B), suggests that the bivalent interaction was disrupted due to displacement of the delta antagonist pharmacophore of MDAN-21 by NTI. Thus, due to competition at the delta protomer by NTI, MDAN-21 functions, at least in part, univalently, which promotes endocytosis similar to that of MA-19 and MDAN-16 (Fig 3).
Given that bridging of mu-delta heteromer by MDAN-21 blocks endocytosis, we investigated whether or not the ability of MDAN-21 to activate the heteromer was also attenuated. In this regard, we carried out intracellular calcium release experiments in HEK-293 cells that contain stably expressed mu, delta or mu/delta opioid receptors. These cells were transiently transfected with Δ6-Gαqi4-myr23, a chimeric G protein that has been shown to couple opioid receptors to the calcium release mechanism23. We evaluated the action of bivalent ligands, MDAN-16 and MDAN-21 and compared activity with that of monovalent controls, MA-19 and DN-20. In these experiments we chose to co-administer the monovalent ligands, MA-19 and DN-20, rather than pretreating with DN-20 and then adding MA-19. This was done because both pharmacophores of MDAN-21 would be able to interact with the mu and delta protomers in a concerted manner.
When tested on HEK-293 cells, MA-19 produced strong calcium release (~380 Relative Fluorescence Units (RFU)) that was unaffected by equimolar DN-20 in either mu or mu-delta cells (Supplemental Fig S1, Supplemental Table 1). MDAN-16 produced slightly greater calcium release in mu/delta cells (EC50 = 565.7 nM, ΔRFU = 480 RFU) than in mu cells (EC50 = 796.6 nM, ΔRFU = 321 RFU). However, MDAN-21 was equiactive in both mu/delta (EC50 = 411.6 nM, ΔRFU = 468 RFU) and mu cells (EC50 = 850.6 nM, ΔRFU = 420 RFU). Interestingly, all three ligands had similar peak effect when added at a 10 μM concentration to cells expressing mu-delta heteromers (Supplemental Fig S1). None of the ligands tested showed any significant activity in the delta opioid cells, as all the ligands tested are antagonists at delta receptors. Our data suggest that MDAN-21 does not induce endocytosis of mu-delta heteromer, in spite of activating the heteromer to the same extent as mu homomer. .
In addition to providing a new correlate for bridging of heteromer by MDAN-21, our study is relevant to the debate concerning a possible relationship between receptor endocytosis and the development of tolerance24-28. In this regard, it has been proposed that the regulation of opioid receptors by endocytosis plays a significant role in the development of antinociceptive tolerance24-28. Thus, it has been reported that endocytosis of mu opioid receptors has an inverse relationship to tolerance24, 25, whereas the endocytosis of delta receptors correlates with increased tolerance29.
In this context, our trafficking results of mu-delta heteromer are extremely relevant, as several studies have suggested that mu-delta heteromers play a critical role in tolerance development to clinically employed opioids3-6, 30 such as morphine. Since we have reported that MDAN-21 does not produce tolerance in mice10, and in view of the present finding that it does not promote endocytosis of mu-delta heteromer in cultured cells, this suggests that the lack of internalization of mu receptors by morphine may not be a reliable correlate of tolerance.
In conclusion, immunocytochemical trafficking and receptor activation studies in the presence of MDAN-21 in HEK-293 cells coexpressing mu and delta receptors has revealed a correlation between the absence of receptor trafficking and bridging of mu-delta heteromer. Our study shows that the lack of internalization of mu-delta heteromer in the presence of MDAN-21 is correlated with the reported absence of antinociceptive tolerance and dependence in mice. The fact that both the bivalent MDAN-16 with a shorter spacer and monovalent MA-19 produce tolerance and induce internalization, suggests that the longer spacer (21 atoms) in MDAN-21 permits bridging of mu-delta heteromer. Thus, immunocytochemical trafficking data appears to be a useful approach in assessing whether or not bridging to a heteromer has occurred. The absence of tolerance and internalization of MDAN-21 is inconsistent with the concept that relates the lack of mu-agonist induced internalization to tolerance.
Supplementary Material
Fig 4.
Effect of NTI on trafficking of mu-delta heteromer by MDAN-21. A cartoon illustrating the effect of delta antagonist on the disruption of bridging protomers in mu-delta heteromer.
Acknowledgements
We thank Kostenis E., for providing us with the cDNA for Δ6-Gαqi4-myr chimeric Gα subunit, Whistler J., for stable dual transfected HEK-293 cells, Powers M., for capable technical assistance, and Schnell S., for invaluable input and discussions. This work was supported by NIH grant R01-DA01533.
Footnotes
Author contributions
A.S.Y and P.S.P designed the research while A.S.Y and A.E.K conducted experiments. Al the authors analyzed the data and wrote the manuscript.
Supporting Information Available: This material is available free of charge via the website http://pubs.acs.org
References
- 1.Cox BM, Borsodi A, Caló G, Chavkin C, Christie MJ, Civelli O, Devi LA, Evans C, Henderson G, Höllt V, Kieffer B, Kitchen I, Kreek M, Liu-Chen L, Meunier J, Portoghese PS, Shippenberg TS, Simon EJ, Toll L, Traynor JR, Ueda H, Wong YW. [13/05/2013];Opioid receptors. Last modified on 13/03/2013. IUPHAR database (IUPHAR-DB), http://www.iuphar-db.org/DATABASE/FamilyMenuForward?familyId=50..
- 2.Gutstein H, Akil H. Opioid analgesics. in Goodman and Gilman's Pharmacological basis of therapeutics. The McGraw Hill companies, Inc.; USA: 2006. [Google Scholar]
- 3.Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J. Pharmacol. Exp. Ther. 1991;258:299–303. [PubMed] [Google Scholar]
- 4.Kest B, Lee CE, McLemore GL, Inturrisi CE. An antisense oligodeoxynucleotide to the delta opioid receptor (DOR-1) inhibits morphine tolerance and acute dependence in mice. Brain Res Bull. 1996;39:185–8. doi: 10.1016/0361-9230(95)02092-6. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez-Blazquez P, Garcia-Espana A, Garzon J. Antisense oligodeoxynucleotides to opioid mu and delta receptors reduced morphine dependence in mice: role of delta-2 opioid receptors. J Pharmacol Exp Ther. 1997;280:1423–31. [PubMed] [Google Scholar]
- 6.Nitsche JF, Schuller AG, King MA, Zengh M, Pasternak GW, Pintar JE. Genetic dissociation of opiate tolerance and physical dependence in delta-opioid receptor-1 and preproenkephalin knock-out mice. J. Neurosci. 2002;22:10906–10913. doi: 10.1523/JNEUROSCI.22-24-10906.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Portoghese PS, Sultana M, Takemori AE. Naltrindole, a highly selective and potent non-peptide delta opioid receptor antagonist. Eur. J. Pharmacol. 1988;146:185–186. doi: 10.1016/0014-2999(88)90502-x. [DOI] [PubMed] [Google Scholar]
- 8.Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J. Neurosci. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, O'Dowd BF. Oligomerization of mu0 and delta-opioid receptors. Generation of novel functional properties. J. Biol. Chem. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- 10.Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc. Nat. Acad. Sci.USA. 2005;102:19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Portoghese PS. From models to molecules: opioid receptor dimers, bivalent ligands, and selective opioid receptor probes. J. Med. Chem. 2001;44:2259–2269. doi: 10.1021/jm010158+. [DOI] [PubMed] [Google Scholar]
- 12.Portoghese PS, Ronsisvalle G, Larson DL, Yim CB, Sayre LM, Takemori AE. Opioid agonist and antagonist bivalent ligands as receptor probes. Life Sciences. 1982;31:1283–1286. doi: 10.1016/0024-3205(82)90362-9. [DOI] [PubMed] [Google Scholar]
- 13.Portoghese PS, Larson DL, Yim CB, Sayre LM, Ronsisvalle G, Lipkowski AW, Takemori AE, Rice KC, Tam SW. Stereostructure-Activity Relationship of Opioid Agonist and Antagonist Bivalent Ligands. Evidence for Bridging Between Vicinal Opioid Receptors. J. Med. Chem. 1985;28:1140. doi: 10.1021/jm00147a002. [DOI] [PubMed] [Google Scholar]
- 14.Portoghese PS, Larson DL, Yim CB, Sayre LM, Ronsisvalle G, Tam SW, Takemori AE. Opioid Agonist and Antagonist Bivalent Ligands. The Relationship of Spacer Length and Selectivity at Multiple Opioid Receptors. J. Med. Chem. 1986;29:1855. doi: 10.1021/jm00160a010. [DOI] [PubMed] [Google Scholar]
- 15.Bhushan RG, Sharma SK, Xie Z, Daniels DJ, Portoghese PS. A bivalent ligand (KDN-21) reveals spinal δ and kappa opioid receptors are organized as heterodimers that give rise to δ1 and κ2 phenotypes. Selective targeting of delta-kappa heterodimers. J. Med. Chem. 2004;47:2969–2972. doi: 10.1021/jm0342358. [DOI] [PubMed] [Google Scholar]
- 16.Daniels DJ, Kulkarni A, Xie Z, Bhushan RG, Portoghese PS. A bivalent ligand (KDAN-18) containing δ-antagonist and κ-agonist pharmacophores bridges δ2 and κ1 opioid receptor phenotypes. J. Med. Chem. 2005;48:1713–1716. doi: 10.1021/jm034234f. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S, Yekkirala A, Tang Y, Portoghese PS. A bivalent ligand (KMN-21) antagonist for μ/κ heterodimeric opioid receptors Bioorg. Med. Chem. Lett. 2009;19:6978–6980. doi: 10.1016/j.bmcl.2009.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng Y, Akgün E, Harikumar KG, Hopson J, Powers MD, Lunzer MM, Miller LJ, Portoghese PS. Association of μ Opioid (MOP) and Type 2 Cholecystokinin (CCK2) Receptors by Novel Bivalent Ligands. J. Med. Chem. 2009;52:247–258. doi: 10.1021/jm800174p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manglik A, Kruse AC, Andrew C, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK, Granier S. Crystal structure of themu opioid receptor bound to a morphinan antagonist. Nature. 2012;485:321–326. doi: 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenard NR, Daniels DJ, Portoghese PS, Roerig SC. Absence of conditioned place preference or reinstatement with bivalent ligands containing mu-opioid receptor agonist and delta-opioid receptor antagonist pharmacophores. European journal of pharmacology. 2007;566:750–82. doi: 10.1016/j.ejphar.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Sun X, Bohn LM, Sadee W. Opioid receptor homo- and heterodimerization in living cells by quantitative bioluminescence resonance energy transfer. Mol/ Pharmacol. 2005;67:2173–2184. doi: 10.1124/mol.104.010272. [DOI] [PubMed] [Google Scholar]
- 22.Hasbi A, Nguyen T, Fan T, Cheng R, Rashid A, Alijaniaram M, Rasenick MM, O'Dowd BF, George SR. Trafficking of preassembled opioid mu-delta heterooligomer-Gz signaling complexes to the plasma membrane: coregulation by agonists. Biochemistry. 2007;46:12997–13009. doi: 10.1021/bi701436w. [DOI] [PubMed] [Google Scholar]
- 23.Kostenis E. Is G alpha16 the optimal tool for fishing ligands of orphan G-protein-coupled receptors? Trends Pharmacol. Sci. 2001;22:560–564. doi: 10.1016/s0165-6147(00)01810-1. [DOI] [PubMed] [Google Scholar]
- 24.Martini L, Whistler JL. The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr Opin Neurobiol. 2007;17:556–64. doi: 10.1016/j.conb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Koch T, Hollt V. Role of receptor internalization in opioid tolerance and dependence. Pharmacol Ther. 2008;117:199–206. doi: 10.1016/j.pharmthera.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 26.von Zastrow M. Regulation of opioid receptors by endocytic membrane traffic: mechanisms and translational implications. Drug Alcohol Depend. 108:166–71. doi: 10.1016/j.drugalcdep.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JA, Bartlett S, He L, Nielsen CK, Chang AM, Kharazia V, Waldhoer M, Ou CJ, Taylor S, Ferwerda M, Cado D, Whistler JL. Morphine-induced receptor endocytosis in a novel knockin mouse reduces tolerance and dependence. Curr Biol. 2008;18:129–35. doi: 10.1016/j.cub.2007.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charlton JJ, Allen PB, Psifogeorgou K, Chakravarty S, Gomes I, Neve RL, Devi LA, Greengard P, Nestler EJ, Zachariou V. Multiple actions of spinophilin regulate mu opioid receptor function. Neuron. 2008;58:238–47. doi: 10.1016/j.neuron.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Massotte D, Gaveriaux-Ruff C, Kieffer BL. In vivo delta opioid receptor internalization controls behavioral effects of agonists. PLoS One. 2009;4:e5425. doi: 10.1371/journal.pone.0005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yekkirala AS, Kalyuzhny AE, Portoghese PS. Standard opioid agonists activate heteromeric opioid receptors: evidence for morphine and [D-Ala2-MePhe4-Glyol5]enkephalin as selective μ-∂ agonists. ACS Chem. Neurosci. 2010;1:146–154. doi: 10.1021/cn9000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.