Hematopoietic stem cells (HSCs), the source of the entire blood cells repertoire, represent the first stem cells identified in an adult tissue, the bone marrow, and the first stem cells used as a therapy in humans, through bone marrow transplantation.1 Although this therapeutic approach is well established and used to treat a variety of hematological conditions, including leukemia, several aspects limit its application. A lack of matched donors poses a serious barrier, especially for patients from ethnic minority backgrounds. Finding the perfect match between donor and recipient, and obtaining a large number of HSCs, represent two of the unmet major clinical challenges.
Development in a parallel area of stem cells research, induced pluripotent stem cells (iPS) technology, might provide new avenues to circumvent the limitations posed by the scarce number of HSCs available for transplantation. A decade ago the team led by Nobel prize winner Shinya Yamanaka authored a breakthrough study describing the generation of pluripotent stem cells from adult cells.2,3 Since then, many scientists have tried to develop hematopoietic stem cells and blood cells from adult cells via iPS.
Szabo et al. showed that expression of the reprogramming gene and transcription factor OCT4 could induce the generation of HSCs from adult fibroblasts.4 With an analogous approach, Pereira et al. reprogrammed mouse fibroblasts into HSCs by introducing a set of transcription factors, including Gata2, Gfi 1b, Fos and Etv6.5 One of the major obstacles to the reprogramming is the epigenetic code that sets a strong barrier between the differentiation potential of an adult cell and a stem cell. Based on this consideration, Doulatov et al. decided to attempt the reprogramming of human committed myeloid cells into HSCs, identifying 13 essential transcription factors.6 Although these studies showed that the reprogramming of a variety of cells into HSCs is achievable, none of them were able to achieve long-term peripheral blood reconstitution in vivo and to establish the full repertoire of HSCs through serial transplantations. These are hallmarks of the ability of the cells to differentiate and self-renew and represent the gold standards for stem cells. In a more recent study, Riddell and colleagues reprogrammed differentiated somatic blood cells, pro-B cells, by using a cocktail of transcription factors, namely Hlf, Lmo2, Pbx1, Prdm5, Runx1t1, and Zfp37.7
Of course the cocktail used by Riddell and colleagues includes potent leukemic oncogenes that limit the immediate clinical use of HSCs generated by this application. Non-integrating methods and controllable expression systems of the transcription factors are currently under investigation for the clinical translation of these products.
The first clinical trial with iPS derived cells started over two years ago in Japan, where a patient affected by macular degeneration of the retina was injected with iPS derived cells.8 Although the treatment proved safe and effective, last September the trial was halted because of mutations found in the cells prepared for a second patient, and due to new laws regulating regenerative medicine products in Japan.9
However the scientific community strongly believes that the iPS technology holds better promise than embryonic stem cells (ESC) for clinical translation. Currently there is no open source of human ESC (hESC) in the UK10 and hESC products are not patentable in Europe;11,12 moreover, hESC research has been restricted due to ethical consideration regarding the use of human embryos.13 Furthermore, no good manufacturing practices (GMPs) grade hESC lines of O Rh negative blood type are available, a critical aspect in order to develop widely transfusable red blood cells. Novosang is a project born from a consortium between British academic groups and blood services planning to launch the first human clinical trial using red blood cells produced by iPS in 2017.14
Stem cell research is a very hot topic that has led to hope and hype, with exaggerated predictions and claims regarding a potential clinical translation of these products. Considering the steep progress made in iPS technology in less than ten years we can optimistically expect progress for clinically proofed induced hematopoietic stem cells (iHSCs). Moreover, iHSCs hold the promise of becoming potent tools for modelling diseases in vitro and for drug discovery (Figure 1). These are two particularly important aspects in the hematology field, where the lack of appropriate humanized models and ethical concerns over the use of xenotransplantation and in vivo toxicity studies limit both the understanding of the biology of the disease and the screening of new drugs.
Figure 1.
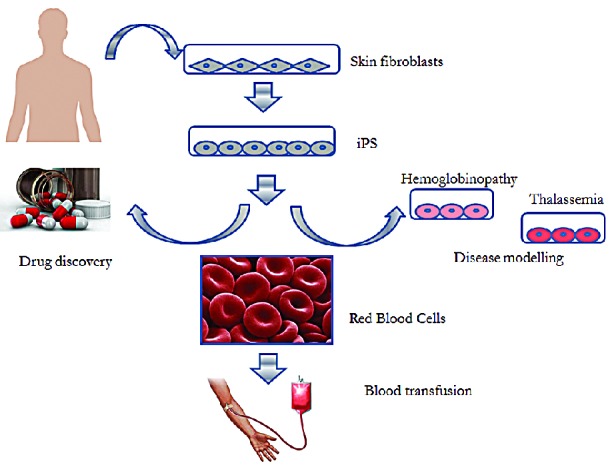
Schematic representation of Red Blood Cells (RBCs) generation from induced pluripotent stem cells (iPS). Fibroblasts are isolated from the skin of the patient and reprogrammed in vitro into iPS. iPS can be differentiated in vitro in RBCs and be used for blood transfusion. Patient-specific iPS can be powerful tools for disease modelling or drug discovery processes.
The marriage between the HSC and iPS technology therefore represents a very promising and interesting area of stem cell research.
References
- 1.Till JE, Mc CE. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. [DOI] [PubMed] [Google Scholar]
- 4.Szabo E, Rampalli S, Risueno RM, et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468(7323):521–526. [DOI] [PubMed] [Google Scholar]
- 5.Pereira CF, Chang B, Qiu J, et al. Induction of a hemogenic program in mouse fibroblasts. Cell Stem Cell. 2013;13(2):205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doulatov S, Vo LT, Chou SS, et al. Induction of multipotential hematopoietic progenitors from human pluripotent stem cells via respecification of lineage-restricted precursors. Cell Stem Cell. 2013;13(4):459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riddell J, Gazit R, Garrison BS, et al. Reprogramming committed murine blood cells to induced hematopoietic stem cells with defined factors. Cell. 2014;157(3):549–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reardon S, Cyranoski D. Japan stem-cell trial stirs envy. Nature. 2014;513(7518):287–288. [DOI] [PubMed] [Google Scholar]
- 9.Garber K. RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nat Biotechnol. 2015;33(9):890–891. [DOI] [PubMed] [Google Scholar]
- 10.Courtney A, de Sousa P, George C, Laurie G, Tait J. Balancing open source stem cell science with commercialization. Nat Biotechnol. 2011;29(2):115–116. [DOI] [PubMed] [Google Scholar]
- 11.Moran N. European court bans embryonic stem cell patents. Nat Biotechnol. 2011;29(12):1057–1059. [DOI] [PubMed] [Google Scholar]
- 12.Moran N. Brustle patent holds up in Germany. Nat Biotechnol. 2013;31(2):94. [DOI] [PubMed] [Google Scholar]
- 13.Hyun I. Policy: Regulate embryos made for research. Nature. 2014;509(7498):27–28. [DOI] [PubMed] [Google Scholar]
- 14.Mittra J, Tait J, Mastroeni M, Turner ML, Mountford JC, Bruce K. Identifying viable regulatory and innovation pathways for regenerative medicine: a case study of cultured red blood cells. N Biotechnol. 2015;32(1):180–190. [DOI] [PubMed] [Google Scholar]


