Abstract
We have recently demonstrated that the transcription factor nuclear factor-erythroid 2, which is critical for erythroid maturation and globin gene expression, plays an important role in the pathophysiology of myeloproliferative neoplasms. Myeloproliferative neoplasm patients display elevated levels of nuclear factor-erythroid 2 and transgenic mice overexpressing the transcription factor develop myeloproliferative neoplasm, albeit, surprisingly without erythrocytosis. Nuclear factor-erythroid 2 transgenic mice show both a reticulocytosis and a concomitant increase in iron deposits in the spleen, suggesting both enhanced erythrocyte production and increased red blood cell destruction. We therefore hypothesized that elevated nuclear factor-erythroid 2 levels may lead to increased erythrocyte destruction by interfering with organelle clearance during erythroid maturation. We have previously shown that nuclear factor-erythroid 2 overexpression delays erythroid maturation of human hematopoietic stem cells. Here we report that increased nuclear factor-erythroid 2 levels also impede murine maturation by retarding mitochondrial depolarization and delaying mitochondrial elimination. In addition, ribosome autophagy is delayed in transgenics. We demonstrate that the autophagy genes NIX and ULK1 are direct novel nuclear factor-erythroid 2 target genes, as these loci are bound by nuclear factor-erythroid 2 in chromatin immunoprecipitation assays. Moreover, Nix and Ulk1 expression is increased in transgenic mice and in granulocytes from polycythemia vera patients. This is the first report implying a role for nuclear factor-erythroid 2 in erythroid maturation by affecting autophagy.
Introduction
The transcription factor nuclear factor-erythroid 2 (NF-E2) plays an essential role in erythroid maturation, and is a critical regulator of globin gene expression.1 Patients with polycythemia vera (PV), whose lead symptom is erythrocytosis, show significantly elevated NF-E2 levels both in progenitor cells and in mature compartments.2,3 Recently, we have shown that NF-E2 overexpression in a transgenic (tg) mouse model leads to a myeloproliferative neoplasm (MPN) phenotype characterized by thrombocytosis, leukocytosis, the expansion of stem and progenitor compartments and erythropoietin-independent colony formation.2 In addition, NF-E2 transgenic mice presented with elevated reticulocyte counts, indicating an increased erythroid drive. At the same time, however, tg mice show an increase in iron deposits in the spleen as well as elevated lactate dehydrogenase levels, strongly suggesting augmented red blood cell (RBC) destruction.2 Taken together, these two observations could explain the absence of polycythemia in NF-E2 tg mice.
Erythroid maturation is a complex multistep process in which the erythroid progenitor cell becomes highly specialized for the transport of oxygen. This entails a vast increase in hemoglobin synthesis followed by the elimination of internal organelles as well as the cell nucleus.4 Elimination of organelles like ribosomes and mitochondria, called autophagy in general, or more specifically ribophagy and mitophagy, respectively, is an essential step in the maturation of reticulocytes to erythrocytes.5
Autophagy is an organized process wherein cellular components and organelles are targeted to lysosomes for degradation.6,7 Autophagy occurs either during homeostasis, where the degradation of damaged or dysfunctional mitochondria is essential to protect cells from reactive oxygen species-mediated damage.8 Alternatively, as mentioned above, programmed, maturation-induced autophagy must occur during terminal erythroid differentiation. Signals triggering the elimination of damaged mitochondria during homeostasis include the oxidation of mitochondrial lipids9 and the loss of mitochondrial membrane potential.10,11 In contrast, the signals and molecular mechanisms mediating mitophagy during the course of normal erythrocyte development are incompletely understood. However, impaired mitophagy has been shown to lead to increased red cell destruction.12,13
Given our observation of increased red cell destruction in NF-E2 overexpressing mice, we hypothesized that this transcription factor may play a novel role in mediating mitochondrial and ribosomal clearance during erythroid maturation.
Methods
Mice
The establishment of the VAV-HA-hNF-E2 transgenic animals has been described elsewhere.2 Mice of two independent founder lines were analyzed as detailed in Kaufmann et al.2 All animal experiments were performed in compliance with the German animal protection law. The mice were housed and handled in accordance with good animal practice as defined by FELASA and the national animal welfare body GV-SOLAS. The animal welfare committees of the University of Freiburg as well as the local authorities approved all animal experiments.
Ex vivo maturation of reticulocytes and staining
Cultures were set up according to Zhang et al.14 Briefly, heparinized whole blood was diluted in maturation medium and cultured at 37°C until staining. The cells were stained with 100nM MitoTracker green dye (MTG) (M7514, Invitrogen), Ter119-PE (22155234, ImmunoTools) and CD71-APC (C355, Leinco Technologies) antibodies. Alternatively, cells were stained with thiazole orange (TO) (390062, Sigma-Aldrich), in PBS (2 μg/ml) and Ter119 and CD71 antibodies. JC-1 (65-0851-38, eBioscience) staining was done according to Cossarizza et al.15
Statistical analysis
Statistical analysis was done using GraphPad Prism software. P-values were determined by either one-tailed or two-tailed Student’s t-tests.
Results
NF-E2 overexpression in a tg mouse model led to a significant increase in reticulocyte counts and in the red cell distribution width,2 suggesting an enhanced erythroid drive and increased red cell production. Surprisingly, however, NF-E2 tg mice have a normal hematocrit and normal RBC counts in the peripheral blood (PB).2 We observed increased iron deposits in splenic histiocytes in NF-E2 tg mice, indicating increased red cell destruction,2 which may counteract the increased RBC production, explaining the lack of polycythemia. However, although we expected increased RBC destruction to lead to an increased RBC turnover and a shorter RBC half-life, in fact, NF-E2 tg mice displayed a decreased RBC turnover, manifested by a significantly increased half-life in the PB (Online Supplementary Figure S1). We therefore examined the effect of elevated NF-E2 levels on RBC pathophysiology.
Because of the increase in PB reticulocyte counts2 in the absence of increased RBC turnover, we hypothesized that elevated NF-E2 levels perturb the kinetics of RBC maturation by impeding mitochondrial autophagy. As reticulocytes eliminate internal organelles and mature, they progressively lose CD71 expression and gain Ter119 expression.16 We therefore compared the reticulocyte maturation status of NF-E2 tg mice and wild-type (wt) littermates by FACS staining for CD71, Ter119 as well as for mitochondria and ribosome content.
Ter119 and CD71 staining distinguishes four stages of erythroid maturation,16 the least mature cells (Ter119low/CD71high), gain Ter119 expression to become Ter119+CD71high. Maturing erythrocytes subsequently lose CD71 expression to become Ter119+/CD71med, and finally Ter119+/CD71low cells.
Compared to wt littermates, NF-E2 tg mice display a significant increase in the percentage of immature erythrocytes in both PB and bone marrow (BM), as indicated by the elevated proportion of Ter119low/CD71high, Ter119+/CD71high and Ter119+/CD71med populations (Figure 1A–C). Taken together, these data demonstrate a delay in erythrocyte maturation and an accumulation of immature cell populations in the presence of elevated NF-E2 levels.
Figure 1.
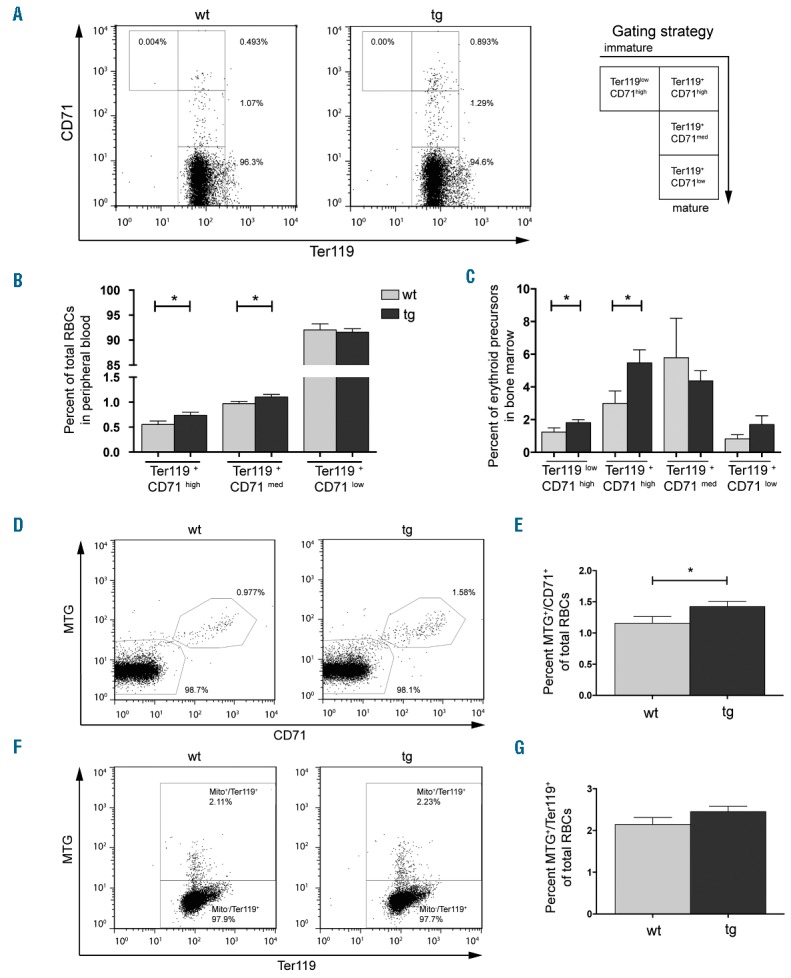
Increased retention of mitochondria in CD71 and Ter119 positive erythrocytes in NF-E2 tg mice. Whole blood or bone marrow from wt and tg animals, as indicated, was stained with CD71 and Ter119 antibodies as well as MitoTracker green (MTG) dye. (A, D, F) Representative FACS analysis and gating strategy for one wt and one tg animal for (A) CD71 and Ter119, (D) MTG and CD71 and (F) MTG and Ter119. (B, C) Percentages of erythroid populations in (B) PB and (C) bone marrow of NF-E2 tg and wt mice. (E, G) Percentages of (E) MTG+/CD71+ and (G) MTG+/Ter119+ stained cells in NF-E2 tg and wt mice. n= 12 wt, n= 21 tg (15 strain “9”, 6 strain “39”). Histograms show mean and SEM. *P<0.05. Statistical significance was calculated by Student’s t-tests.
Since the elimination of mitochondria is a crucial process during erythrocyte maturation, the presence of mitochondria was analyzed in the CD71 and Ter119 positive cell populations. CD71 or Ter119 staining was combined with MTG, a permeable dye that accumulates in active mitochondria.17 NF-E2 tg mice display a significantly increased population of MTG+/CD71+ double positive cells compared to wt controls (Figure 1D,E). A similar trend is seen for the MTG+/Ter119+ population (Figure 1F, G), although the latter did not reach statistical significance. Comparison of the absolute numbers also shows that these two erythroid populations are increased in the tg mice (Online Supplementary Figure S2). Most importantly, within the immature populations (Ter119+/CD71high and Ter119+/CD71med), NF-E2 tg mice show a significant increase in the proportion of MTGhigh cells (Online Supplementary Figure S3A and S3B), which represent cells carrying the largest number of mitochondria.
In order to determine whether cells on average contain more mitochondria per cell, we determined the mean fluorescence intensity (MFI) for MTG in each of the subpopulations (Online Supplementary Figure S3C). Indeed, the MFI for MTG is significantly increased, demonstrating a higher number of mitochondria per cell in NF-E2 tg mice. Increased NF-E2 expression thus impedes mitochondrial elimination in maturing erythrocytes, leading to the accumulation of larger numbers of mitochondria containing red cells, with a higher number of mitochondria per cell in the PB of NF-E2 tg mice.
In a wt adult mouse, the PB contains around 3% reticulocytes, a proportion insufficient for ex vivo maturation analysis. NF-E2 tg and wt mice were therefore treated with phenylhydrazine (PHZ), which induces hemolytic anemia leading to increased reticulocyte production.18 The experimental strategy is detailed in Figure 2A. PB cells isolated after PHZ treatment were cultured and matured ex vivo for 3 days and analyzed. Histological analysis verified that the cells analyzed represent reticulocytes (Online Supplementary Figure S4). Mice were additionally followed up to analyze the recovery of their hematocrit values, as shown in the Online Supplementary Figure S5.
Figure 2.
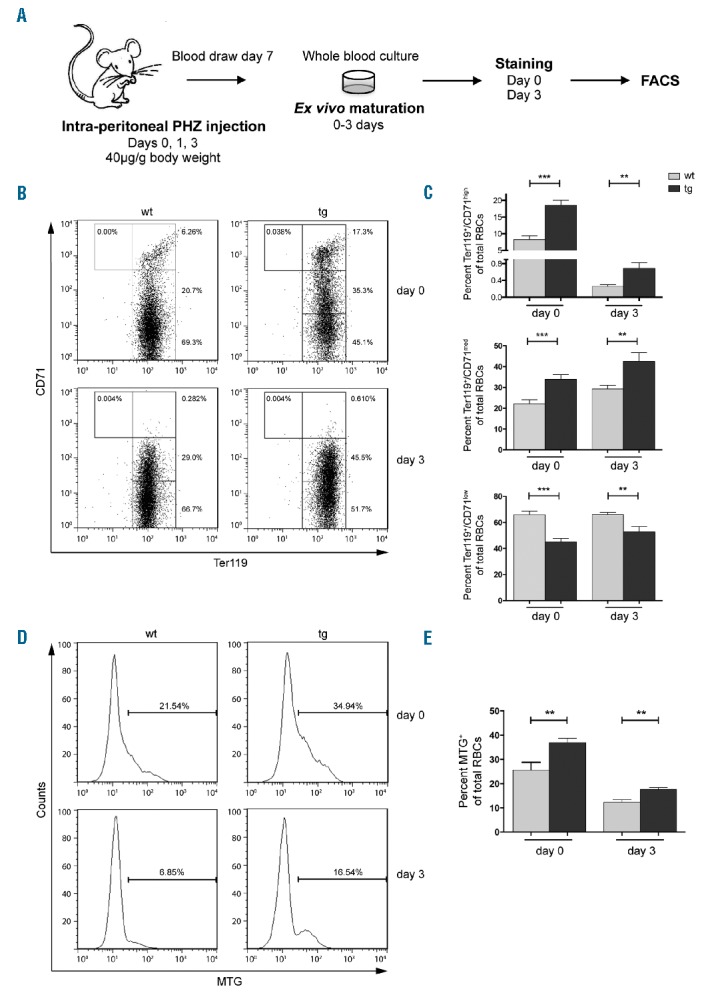
Delayed clearance of mitochondria from newly formed reticulocytes in NF-E2 tg mice. (A) Experimental procedure: mice were treated with phenylhydrazine (PHZ) to induce reticulocytosis, PB obtained on day 7 and matured ex vivo for 3 days. (B, D) Representative FACS analysis for one wt and one tg animal for day 0 and day 3 staining for (B) CD71 and Ter119, (D) MTG. Gating strategy was as detailed in Figure 1A. (C, E) Percentage of stained cells on day 0 and day 3 of ex vivo maturation in NF-E2 tg and wt mice: (C) Ter119+/CD71high, CD71med and CD71low, n= 7 wt, n= 8 tg (5 strain “9”, 3 strain “39”); (E) MTG+, n= 8 wt, n= 8 tg (5 strain “9”, 3 strain “39”). Histograms show mean and SEM. **P<0.01, ***P<0.001. Statistical significance was calculated by Student’s t-tests.
Similar to the untreated mice described above, the NF-E2 tg PHZ treated mice displayed a significantly increased percentage of immature Ter119+/CD71high and Ter119+/CD71med populations compared to controls, and thus a lower percentage of Ter119+/CD71low population (Figure 2B,C). Cells from NF-E2 tg mice thus contained a higher percentage of immature cells at the onset (day 0) of ex vivo maturation. After 3 days in culture, reticulocytes from both the tg and the wt mice had undergone maturation, becoming predominantly Ter119+/CD71low. However, the NF-E2 tg cultures still retained a significantly higher proportion of immature cells, both Ter119+/CD71high and Ter119+/CD71med (Figure 2B,C). NF-E2 overexpressing mice thus retain their less mature phenotype despite following PHZ treatment and ex vivo maturation. Following PHZ treatment, NF-E2 tg mice again display a higher proportion of mitochondria retaining cells, on both day 0 and day 3 of culture (Figure 2D,E). In order to distinguish whether mitochondria are aberrantly retained in more mature populations or whether the increase in mitochondria simply reflects the excess of immature cells, reticulocytes in culture were stained with CD71, Ter119 and MTG. On day 0 of ex vivo culture NF-E2 tg mice contain a significantly increased proportion and absolute number of immature cells retaining mitochondria (MTG+/CD71high) (Figure 3A,B). During culture, these cells mature, giving rise to a MTG+/CD71low population on day 3 (Figure 3A,B). Nonetheless, after 3 days in culture, NF-E2 tg mice still retain a significantly higher percentage of the MTG+/CD71low population as well as a higher absolute number of MTG+/CD71low cells compared to wt controls. Similarly, NF-E2 tg mice contain a higher percentage and a higher absolute number of more mature RBCs with mitochondria (MTG+/Ter119+) (Figure 3C,D). Analysis of defined subpopulations showed that before and after ex vivo maturation, Ter119+/CD71low cells overexpressing NF-E2 contain more mitochondria retaining cells than their wt littermate controls (Online Supplementary Figure S3D and S3E). While ex vivo culture decreases the proportion of cells containing mitochondria in both genotypes, nonetheless, on day 3, NF-E2 overexpressing Ter119+/CD71low cells still contain significantly more MTGhigh cells than wt controls (Online Supplementary Figure S3E, right panel).
Figure 3.
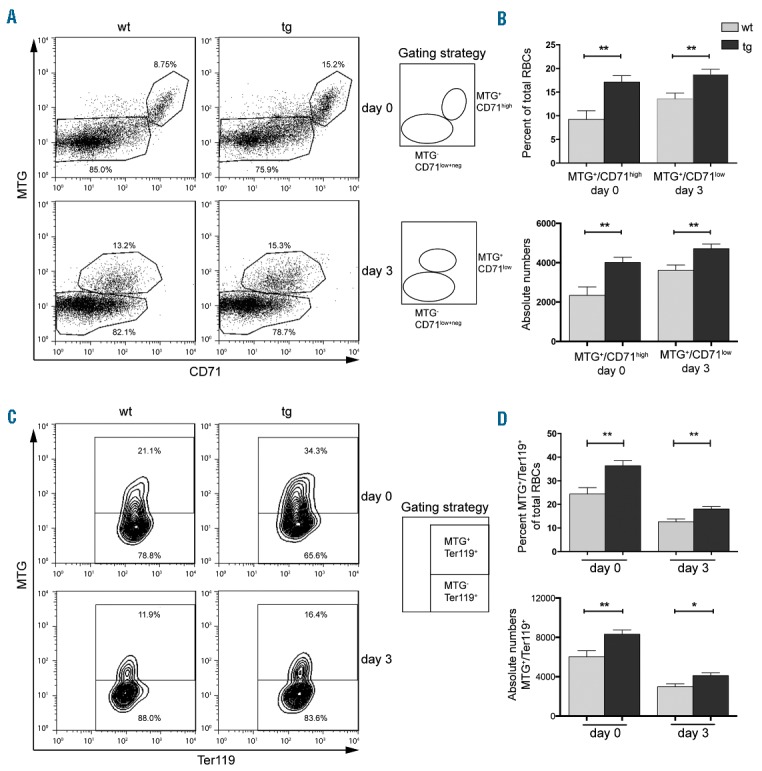
Delayed clearance of mitochondria from Ter119 and CD71 positive newly formed reticulocytes in NF-E2 tg mice. (A, C) Representative FACS analysis and gating strategy for one wt and one tg animal for day 0 and day 3 staining for (A) MTG and CD71, (C) MTG and Ter119. (B, D) Percentage and absolute numbers per 10.000 measured events (per 3 μl whole blood) of stained cells in NF-E2 tg and wt mice on day 0 and day 3 of ex vivo maturation: (B) MTG+/CD71high (day 0) and MTG+/CD71low (day 3), n= 8 wt, n= 7 tg (4 strain “9”, 3 strain “39”); (D) MTG+/Ter119+, n= 8 wt, n= 7 tg (4 strain “9”, 3 strain “39”). Histograms show mean and SEM. *P<0.05, **P<0.01. Statistical significance was calculated by Student’s t-tests.
In order to determine whether these Ter119+/CD71low MTGhigh cells are only more abundant, or also aberrantly retain mitochondria, we determined the MFI for MTG, which reflects the amount of mitochondria per cell (Online Supplementary Figure S3F). The MFI for MTG was significantly increased on day 0 demonstrating that, on average, mature Ter119+/CD71low cells in NF-E2 tg mice contain a higher number of mitochondria per cell. Hence, it is not the presence of increased numbers of immature cells but rather the aberrant retention of mitochondria within phenotypically mature cells that leads to the increased number of MTG+ cells in NF-E2 tg mice.
Interestingly, PHZ altered the characteristics of PB reticulocytes. Under steady state conditions, NF-E2 tg mice display increased mitochondrial retention in the less mature populations (Online Supplementary Figure S3A and S3B). In contrast, under erythropoietic stress, following PHZ treatment, immature cells of both genotypes show large proportions of MTGhigh cells (60–75% MTGhigh in Ter119+/CD71high and Ter119+CD71med, Online Supplementary Figure S3D and S3E, compared to 15–30% MTGhigh in Ter119+/CD71high and Ter119+/CD71med, Online Supplementary Figure S3A and S3B). The increased retention of mitochondria in NF-E2 tg animals following PHZ treatment is seen in the more mature, Ter119+/CD71low population (Online Supplementary Figure S3D and S3E).
Both analyses (native state and PHZ) thus clearly show that elevated NF-E2 levels cause a delay in in vivo and ex vivo maturation of reticulocytes. At the same time, NF-E2 tg erythrocytes are capable of exuding their mitochondria appropriately, as the proportion of Ter119+/CD71low cells remaining positive for MTG on day 3 is less than 5% (Online Supplementary Figure S3E, right panel).
Mitochondrial transmembrane potential plays an important role in mitochondrial autophagy.10,19 Reticulocytes from mice deficient in the mitochondrial outer membrane protein NIX fail to eliminate mitochondria in culture. However, they correctly eliminate their organelles when treated with a depolarizing agent.13,20 We hypothesized that the higher percentage of mitochondria retaining RBCs in NF-E2 tg mice results from a higher mitochondrial membrane potential in these cells. In order to test this hypothesis, reticulocytes were stained with JC-1, a cationic dye that accumulates in mitochondria15 and emits fluorescence at 540nm. The intensity of emission correlates with the membrane potential, hence, depolarization decreases JC-1 fluorescence.21
Reticulocytes from PHZ treated mice were cultured ex vivo and stained with JC-1 at several time points. At culture initiation, no difference in mitochondrial membrane potential between tg and wt mice was observed (Figure 4A,B), indicating that elevated NF-E2 levels do not alter the mitochondrial membrane potential. At subsequent time points, JC-1 fluorescence declines in both the tg and wt reticulocytes (Figure 4A,B). However, the mean fluorescence intensity (MFI) of JC-1 staining in NF-E2 tg reticulocytes remains significantly higher at all measured time points, indicating an aberrant retention of mitochondrial membrane potential during maturation (Figure 4B). This could prevent mitochondrial elimination early during maturation, providing a mechanistic explanation for the observed ex vivo delay in mitochondrial elimination of cells with elevated NF-E2 levels.
Figure 4.
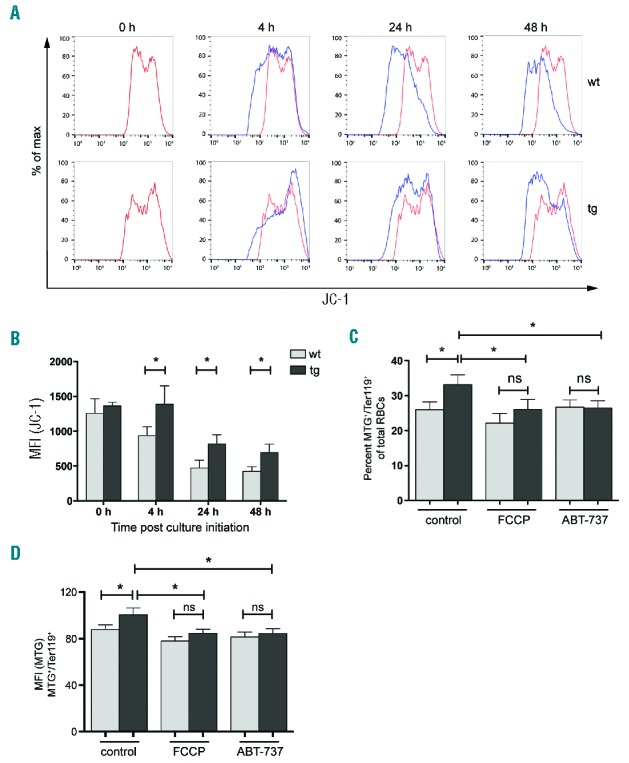
Analysis of mitochondrial membrane potential during maturation of newly formed reticulocytes. PB of PHZ treated mice (as shown in Figure 2A) was stained with JC-1 and analyzed at the indicated time points. (A) Representative JC-1 staining for one wt and one tg animal. Red line: staining for 0 hour control; Blue line: staining after culture for the indicated time points. (B) Mean fluorescence intensity (MFI) of JC-1 staining during ex vivo maturation of newly formed reticulocytes from NF-E2 tg and wt mice. n= 3 wt, n= 3 tg (3 strain “39”). (C) Percentage of MTG+/Ter119+ population of reticulocytes from wt and tg mice cultured in the presence or absence of the depolarizing agents FCCP and ABT-737 for 4 hours. n= 12 wt, n= 12 tg (2 strain “9”, 10 strain “39”). The percentages were determined according to the gating strategy shown in Figure 3C. (D) Determination of mean fluorescence intensity (MFI) for MTG in the MTG+/Ter119+ cells of populations shown in Figure 4C. Histograms show mean and SEM. *P<0.05. Statistical significance was calculated by Student’s t-tests.
The mitochondrial membrane undergoes depolarization in the presence of depolarizing agents like FCCP and ABT-737,13,22 which leads to mitochondrial elimination. We therefore investigated whether treatment with depolarizing agents would normalize mitochondrial elimination in NF-E2 tg reticulocytes. Reticulocytes were cultured to undergo ex vivo maturation in the presence or absence of the depolarizing agents FCCP and ABT-737 followed by MTG/Ter119 staining for the detection of mitochondria. Again, untreated reticulocytes from NF-E2 tg mice showed a significantly higher proportion of MTG+/Ter119+ cells (Figure 4C). However, the treatment of NF-E2 tg cells with either FCCP or ABT-737 for 4 hours significantly reduced the number of mitochondria retaining cells, eliminating the difference between the two genotypes and normalizing mitochondrial retention in NF-E2 tg reticulocytes to wt levels (Figure 4C).
In order to determine whether depolarizing agents also normalized the number of mitochondria per cell, we determined the MFI for MTG (Figure 4D). The MFI for MTG was significantly increased in NF-E2 tg MTG+/Ter119+ cells in the absence of depolarizing agents, again demonstrating the increased number of mitochondria per cell in NF-E2 tg cells, but this difference disappeared in the presence of either FCCP or ABT-737. (Figure 4D). Thus, membrane depolarization overcomes the abnormal delay in mitochondrial elimination effected by elevated NF-E2 levels.
Besides mitochondria, the elimination of ribosomes is also essential during RBC maturation.5 In order to assess whether elevated NF-E2 levels specifically delay mitochondrial elimination or also impede the exudation of ribosomes, reticulocytes undergoing ex vivo maturation were stained with thiazole orange (TO), a ribonucleic acid (RNA) binding dye, which, by nature of the vast amount of ribosomal RNA (rRNA) in ribosomes, specifically stains these organelles. In combination with CD71, TO delineated three populations: immature TO+/high/CD71high, maturing to TO+/CD71low+neg, finally becoming mature TO−/CD71low+neg cells (Figure 5A, insert). On day 0, NF-E2 tg mice showed a significantly increased percentage of both the highly immature TO+/high/CD71high population (Figure 5A,B) and the more mature TO+/Ter119+ cells (Figure 5C,D), evidence that elevated NF-E2 levels also lead to an aberrant retention of ribosomes. While the percentage of ribosome containing cells decreased during ex vivo maturation in both the wt and the tg animals, NF-E2 tg mice nonetheless retained a significantly elevated proportion of ribosome retaining reticulocytes on day 3 (Figure 5B,D).
Figure 5.
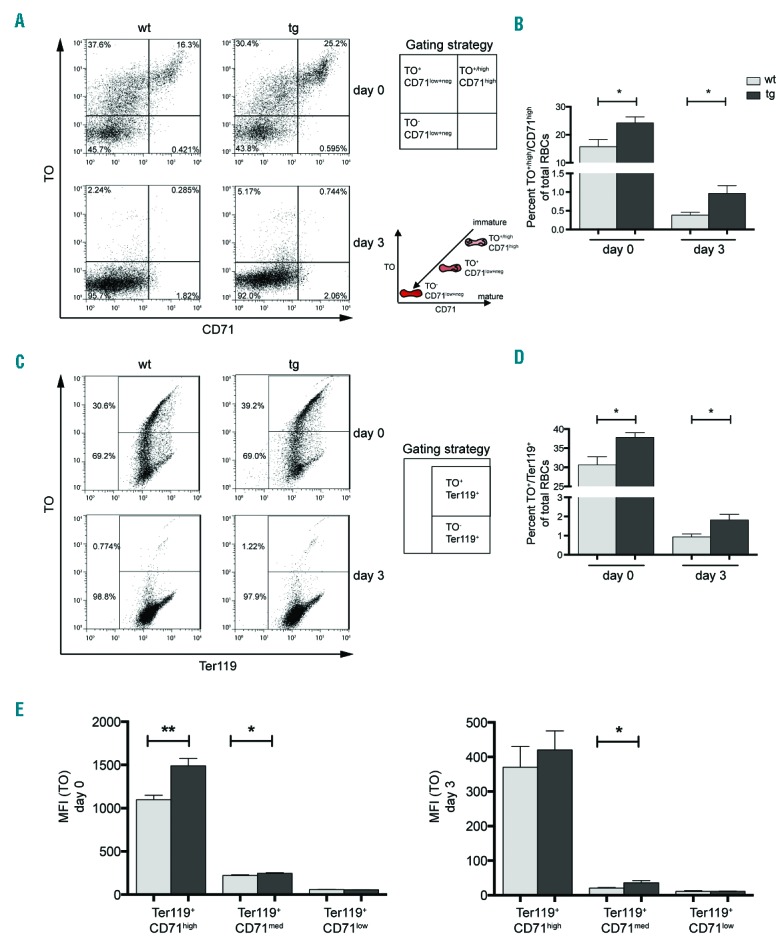
Ribosomal elimination is delayed during maturation of newly formed reticulocytes. PB of PHZ treated mice (as shown in Figure 2A) was stained with thiazole orange (TO) to determine ribosomal content in the maturing reticulocytes. (A, C) Representative FACS analysis and illustration of the gating strategy for one wt and one tg animal for day 0 and day 3 staining for (A) TO and CD71, (C) TO and Ter119. Insert: path of maturation of reticulocytes in culture is shown. (B, D) Percentage of stained cells on day 0 and day 3 of ex vivo maturation in NF-E2 tg and wt mice: (B) TO+/high/CD71high, n= 7 wt, n= 5 tg (3 strain “9”, 2 strain “39”) and (D) TO+/Ter119+, n= 7 wt, n= 6 tg (3 strain “9”, 3 strain “39”). (E) Determination of mean fluorescence intensity (MFI) for TO in the subpopulations determined by CD71 and Ter119 staining (as in Figure 2B) on days 0 and 3. Histograms show mean and SEM. *P<0.05, **P<0.01. Statistical significance was calculated by Student’s t-tests.
Again the question arises as to whether the number of ribosomes per cell is increased. We therefore determined the MFI for TO in all subpopulations determined by CD71/Ter119 staining (Figure 5E). Indeed, the MFI for TO was significantly increased in the less mature subpopulations in NF-E2 tg cells compared to controls, and in Ter119+/CD71med a significant increase remained after 3 days of ex vivo culture (Figure 5E). Hence, at the same level of maturation, NF-E2 tg cells retain significantly more ribosomes per cell, demonstrating a defect in ribophagy.
We hypothesized that the mechanism by which elevated levels of NF-E2 delay autophagy is by directly modulating the expression of autophagy genes. We therefore initially analyzed NF-E2 binding to autophagy genes by mining in silico ChIP-seq data. In human erythroid K562 cells, NF-E2 bound sites in the genes for NIX and ULK1 (Figure 6A). We verified the in silico data by performing ChIP in human erythroleukemia (HEL) cells. Indeed, NF-E2 binds both a distal site in the NIX locus as well as a site within the ULK1 gene. Control regions within both genes as well as the housekeeping gene myogenin were not bound (Figure 6B). These data demonstrate that these two autophagy genes constitute novel NF-E2 target genes. This observation is corroborated by analysis of gene expression in two large data sets, one from a collection of 200 AML patients, the second from purified erythroid precursor cells (Online Supplementary Figure S6). In both cases, NF-E2 expression correlated highly significantly with the expression of both NIX and ULK1 (P=0.000002 and P=0.000009 and P=0.001 and P=0.00003, respectively, while NF-E2 and ATG7 expression did not correlate, Online Supplementary Figure S6), again suggesting that NF-E2 levels regulate expression of these two autophagy genes.
Figure 6.
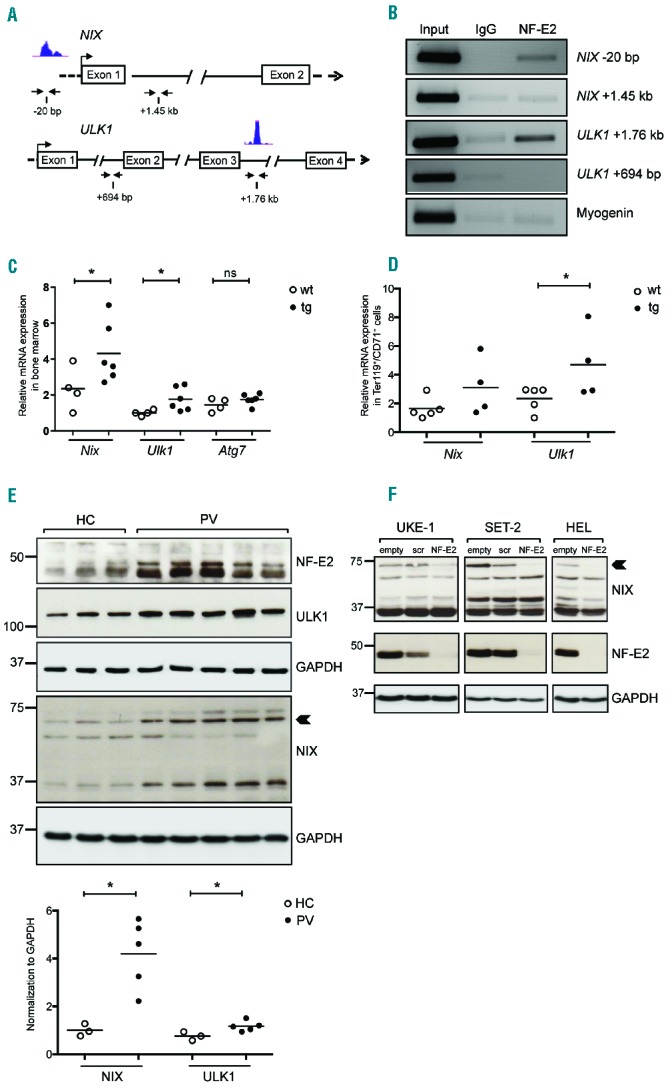
NF-E2 regulates the expression of key mitophagy genes. (A) In silico analysis of NF-E2 binding sites on the NIX and ULK1 gene (UCSC Genome Browser). Blue peaks represent the locus predicted to be bound by NF-E2. Horizontal arrows under the schematic represent primer locations for PCR after ChIP. (B) HEL cell lysates were chromatin immunoprecipitated with antibodies against NF-E2 or an IgG control. PCR was performed with primers flanking the predicted binding sites and the control site, as represented in (A). (C, D) RNA was isolated either from bone marrow cells (C) or from Ter119+/CD71+ sorted cells from PB (D) of wt and NF-E2 tg mice and subjected to qRT-PCR for Nix, Ulk1 and Atg7. Expression levels were normalized to B2m expression. (E) PB granulocytes from HC and PV were analysed by western blotting for NF-E2, NIX and ULK1 protein expression. Quantification and normalization to the loading control GAPDH is depicted underneath. (F) UKE-1, SET-2 and HEL cells were transduced with an empty LeGO-iG lentivirus (“empty”) or with viruses carrying a scrambled, ineffective shRNA (“scr”) or an shRNA directed against NF-E2 (“NF-E2”),23 as indicated. Infected cells were sorted by FACS and protein extracts interrogated by western blotting for expression of the indicated proteins. An arrowhead points to the 75 kDa NIX protein. *P<0.05. Statistical significance was calculated by Student’s t-tests.
An increase in NF-E2 levels alone is sufficient to increase expression of both Nix and Ulk1, as NF-E2 tg mice show significant elevation of Nix and Ulk1 expression in the BM (Figure 6C) as well as in sorted CD71+/Ter119+ cells from PB (Figure 6D). Hence, NF-E2 levels directly affect the autophagy process by altering the expression of critical components.
Because elevated NF-E2 levels in mice are sufficient to increase Nix and Ulk1 expression and PV patients present with NF-E2 overexpression, we determined NIX and ULK1 protein levels in purified primary granulocytes from PV patients and healthy controls (Figure 6E). Expression of both autophagy proteins is significantly increased in PV patients, confirming the fidelity of our mouse model and suggesting alterations in red cell physiology in PV patients.
We determined whether NF-E2 is required for ULK1 and NIX expression by silencing the expression of the transcription factor using a short hairpin RNA (shRNA) against NF-E223 as well as a scrambled control. Three different myeloid cell lines, UKE-1, SET-2 and HEL, all carrying the JAK2V617F mutation prevalent in PV patients, were used. While decreasing NF-E2 expression did not alter ULK1 levels (data not shown), it caused a strong decrease in NIX expression in all three cell lines, particularly of the 75 kDa protein overexpressed in PV patients (Figure 6E,F, arrowheads). These data demonstrate that NF-E2 is required for optimal NIX expression, and show that this autophagy gene is a novel NF-E2 target gene.
Interestingly, PV patients display reticulocytosis, similar to NF-E2 tg mice.24,25 In contrast, the RBC life span has not been reported to be altered in PV patients,26 but these studies were conducted in 1951, and very subtle differences, such as the ones we report (Online Supplementary Figure S1), may not have been evident.
Elevated NF-E2 levels thus cause a delay in both autophagy processes, and the exclusion of mitochondria and ribosomes, an observation that provides a mechanistic explanation for the two observed RBC abnormalities in NF-E2 tg mice. Firstly, delayed autophagy can account for the significant increase in the half-life of NF-E2 tg RBCs (Online Supplementary Figure S1), secondly, abnormal, mitochondria-retaining RBCs may be subject to enhanced elimination in the spleen, explaining the increased splenic RBC destruction observed in NF-E2 tg mice.
Discussion
Programmed removal of internal organelles from maturing reticulocytes constituted an essential step during terminal erythroid differentiation. Several studies have focused on the mechanisms of mitophagy (removal of mitochondria) and ribophagy (removal of ribosomes) in developing erythrocytes.
The Bcl2 related mitochondrial outer membrane protein NIX plays an important role in mitochondrial elimination during reticulocyte maturation.13,20 In reticulocytes from Nix−/− mice, mitochondria fail to enter the autophagosomes. However, the autophagy process itself remains functional, as the addition of depolarizing agents can correct the defect and force mitophagy.13,20 NIX has been shown to bind LC3/GABARAP proteins on autophagosomes, tethering the outer mitochondrial membrane to the autophagosomes. NIX thus functions as a mitophagy receptor, targeting superfluous mitochondria to the autophagosome for degradation.27
Several other proteins have been implicated in mitophagy during erythroid differentiation, including ULK1 and ATG7.28,29 Mice deficient in the kinase ULK1 display a defect in reticulocyte maturation. Ulk1−/− mice show impaired clearance of both mitochondria and ribosomes.28 Again, the depolarizing agent carbonyl cyanide m-chlorophenylhydrazone (CCCP) was able to rescue mitophagy in ULK1 deficient mice. Since ULK1 functions downstream of mitochondrial depolarization,30 these data suggest the presence of ULK1-independent pathways of mitochondrial clearance in RBCs.
ATG7 is an E1-activating enzyme essential for the ubiquitin-like conjugation of autophagosomal proteins to each other and to phosphatidylethanolamine and, ultimately, for autophagosome formation.31 ATG7 deficient reticulocytes show impaired mitochondrial clearance.29 Interestingly, even during ex vivo maturation, ATG7 deficient reticulocytes do not depolarize. Since ATG7 is required for autophagosome formation, these data suggest that mitochondrial depolarization is a consequence, rather than a cause, of autophagosome formation. However, a transient depolarization, which is undetectable in ex vivo cultures, may normally precede and cause autophagosome formation. Alternatively, novel, unidentified signals may trigger or contribute to autophagy in maturing reticulocytes.
Overexpression of the transcription factor NF-E2 in our transgenic mouse model led to a significant increase in the number of erythroid precursor cells in the BM.2 Furthermore, NF-E2 tg mice present with both reticulocytosis and a concomitant increase in red cell destruction in the spleen, manifested by increased iron deposits. As a result, NF-E2 tg mice do not display polycythemia, since the increased RBC destruction appears to outweigh the increased red cell production. Nonetheless, the absence of polycythemia in our mice appears paradoxical given the observed NF-E2 overexpression in more than 90% of patients with polcythemia vera. We therefore sought to determine the molecular effect of elevated NF-E2 levels on red cell physiology.
We hypothesized that elevated NF-E2 levels may perturb the kinetics of erythroid differentiation, leading either to an accelerated or to a delayed maturation. To date, a possible role for NF-E2 in programmed organelle clearance during erythroid differentiation has not been investigated. Therefore, we sought to analyze the role of NF-E2 in erythroid maturation, mitophagy and ribophagy using our NF-E2 overexpressing transgenic mouse model.
We first assessed whether the NF-E2 tg reticulocytes displayed a maturation defect. Indeed, increased NF-E2 levels led to an excess of immature erythroid cells in the PB and a delay in their ex vivo maturation. This maturation delay can explain both the observed reticulocytosis and the increased RBC half-life, as both observations result from immature cells remaining in the peripheral circulation for a longer time. Delayed erythroid maturation has previously been observed by elevating NF-E2 levels in human CD34+ hematopoietic stem cells.32 Our current data thus corroborates the importance of correct NF-E2 levels for physiological erythroid maturation.
Delayed maturation can result from inefficient or absent elimination of internal organelles from maturing reticulocytes. Our analyses showed a significantly increased proportion of cells retaining mitochondria and ribosomes in both the CD71 and the Ter119 positive populations in NF-E2 tg mice, indicating a defect in organelle elimination. The measurement of mitochondrial membrane potential revealed significantly increased potential in NF-E2 tg mice compared to wt controls, even after 48 hours of ex vivo culture, indicating that membrane depolarization is inefficient or completely absent (Figure 4B). As in NIX and ULK1-deficient mice,13,28 the addition of depolarizing agents rescued the defect (Figure 4C), demonstrating that in the presence of elevated NF-E2 levels, the autophagy process itself remains functional.
The mitophagy defect observed in NF-E2 tg mice is less severe than that of Nix−/− mice,13,20 as the organelles are retained in more than 70% of Ter119+ NIX deficient cells after ex vivo maturation, compared to the 20% retention in NF-E2 tg mice (Figure 3D). Our observations are more similar to those in Atg7−/− mice, which also show a delay in maturation but undergo organelle clearance if given enough time during ex vivo maturation.29 The relatively mild autophagy defect in NF-E2 tg mice is exacerbated during stress erythropoiesis following PHZ treatment (compare Figure 1 and Figure 2). Stress erythropoiesis occurs in the spleen and liver, rather than in the BM, where steady state erythropoiesis takes place. Moreover, stress erythropoiesis utilizes a population of stress erythroid progenitor cells, which are distinct from the BM, steady state erythroid progenitors.33 The effect of NF-E2 overexpression appears to be more pronounced in these stress erythroid progenitor cells.
We investigated the mechanism underlying the maturation defect. Two critical autophagy genes, NIX and ULK1 are directly regulated by NF-E2 (Figure 6B). Increased NF-E2 activity in tg mice led to a significant increase in Nix and Ulk1 expression in the BM (Figure 6C) as well as in sorted CD71+/Ter119+ cells from PB (Figure 6D). Randhawa and colleagues have recently published in silico data that likewise suggests an important role for NF-E2 in ULK1 regulation, albeit this paper does not contain functional data.34 Ours is the first report demonstrating a role for NF-E2 in erythroid maturation by affecting autophagy genes. Because NIX and ULK1 interact with partner proteins in the autophagy process,35 their increased expression may disrupt complex formation. If the levels of interacting proteins are not raised concomitantly, ineffective complexes may be formed.
We recently described that, surprisingly for a transcription factor, NF-E2 is found predominantly in the cytoplasm of maturing, nucleated erythrocytic cells.36 Only 10% of all erythroid cells, mainly the early erythroblasts, showed nuclear NF-E2 staining. So far, no function has been attributed to the cytoplasmically located NF-E2 protein. Our data presented herein support the hypothesis that cytoplasmically located NF-E2 may have a function in the elimination of internal organelles. The mild autophagy defect observed in our mice may be due to the requirement for additional proteins in the formation of larger complexes to exert this function. The elevation of NF-E2 levels in isolation may not completely disrupt the function of this complex.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/9/1054
References
- 1.Armstrong JA, Emerson BM. NF-E2 disrupts chromatin structure at human beta-globin locus control region hypersensitive site 2 in vitro. Mol Cell Biol. 1996;16(10):5634–5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaufmann K, Gründer A, Hadlich T, et al. A novel murine model of myeloproliferative disorders generated by overexpression of the transcription factor NF-E2. J Exp Med. 2012;209(1):35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goerttler PS, Kreutz C, Donauer J, et al. Gene expression profiling in polycythaemia vera: overexpression of transcription factor NF-E2. Br J Haematol. 2005;129(1):138–150. [DOI] [PubMed] [Google Scholar]
- 4.Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112(3):470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koury MJ, Koury ST, Kopsombut P, Bondurant MC. In vitro maturation of nascent reticulocytes to erythrocytes. Blood. 2005;105(5):2168–2174. [DOI] [PubMed] [Google Scholar]
- 6.Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10(7): 458–467. [DOI] [PubMed] [Google Scholar]
- 7.Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22(2):124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexeyev MF, Ledoux SP, Wilson GL. Mitochondrial DNA and aging. Clin Sci (Lond). 2004;107(4):355–364. [DOI] [PubMed] [Google Scholar]
- 9.Kissova I, Deffieu M, Samokhvalov V, et al. Lipid oxidation and autophagy in yeast. Free Radic Biol Med. 2006;41(11):1655–1661. [DOI] [PubMed] [Google Scholar]
- 10.Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001;15(12):2286–2287. [DOI] [PubMed] [Google Scholar]
- 11.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183(5):795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holm TM, Braun A, Trigatti BL, et al. Failure of red blood cell maturation in mice with defects in the high-density lipoprotein receptor SR-BI. Blood. 2002;99(5):1817–1824. [DOI] [PubMed] [Google Scholar]
- 13.Sandoval H, Thiagarajan P, Dasgupta SK, et al. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454(7201):232–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Kundu M, Ney PA. Mitophagy in mammalian cells: the reticulocyte model. Methods Enzymol. 2009;452:227–245. [DOI] [PubMed] [Google Scholar]
- 15.Cossarizza A, Salvioli S. Flow cytometric analysis of mitochondrial membrane potential using JC-1. Curr Protoc Cytom. 2001;Chapter 9:Unit9.14. [DOI] [PubMed] [Google Scholar]
- 16.Socolovsky M, Nam H, Fleming MD, Haase V, Brugnara C, Lodish HF. Ineffective erythropoiesis in Stat5a(−/−)5b(−/−) mice due to decreased survival of early erythroblasts. Blood. 2001;98(12):3261–3273. [DOI] [PubMed] [Google Scholar]
- 17.Sutovsky P, Navara CS, Schatten G. Fate of the sperm mitochondria, and the incorporation, conversion, and disassembly of the sperm tail structures during bovine fertilization. Biol Reprod. 1996;55(6):1195–1205. [DOI] [PubMed] [Google Scholar]
- 18.Vannucchi AM, Bianchi L, Cellai C, et al. Accentuated response to phenylhydrazine and erythropoietin in mice genetically impaired for their GATA-1 expression (GATA-1(low) mice). Blood. 2001;97(10): 3040–3050. [DOI] [PubMed] [Google Scholar]
- 19.Priault M, Salin B, Schaeffer J, Vallette FM, di Rago JP, Martinou JC. Impairing the bioenergetic status and the biogenesis of mitochondria triggers mitophagy in yeast. Cell Death Differ. 2005;12(12):1613–1621. [DOI] [PubMed] [Google Scholar]
- 20.Schweers RL, Zhang J, Randall MS, et al. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc Natl Acad Sci USA. 2007;104(49):19500–19505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reers M, Smiley ST, Mottola-Hartshorn C, Chen A, Lin M, Chen LB. Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol. 1995;260:406–417. [DOI] [PubMed] [Google Scholar]
- 22.Gottlieb E, Vander Heiden MG, Thompson CB. Bcl-x(L) prevents the initial decrease in mitochondrial membrane potential and subsequent reactive oxygen species production during tumor necrosis factor alpha-induced apoptosis. Mol Cell Biol. 2000; 20(15):5680–5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roelz R, Pilz IH, Mutschler M, Pahl HL. Of mice and men: human RNA polymerase III promoter U6 is more efficient than its murine homologue for shRNA expression from a lentiviral vector in both human and murine progenitor cells. Exp Hematol. 2010;38(9):792–797. [DOI] [PubMed] [Google Scholar]
- 24.Anger B, Haug U, Seidler R, Heimpel H. Polycythemia vera. A clinical study of 141 patients. Blut. 1989;59(6):493–500. [DOI] [PubMed] [Google Scholar]
- 25.d’Onofrio G, Tichelli A, Foures C, Theodorsen L. Indicators of haematopoietic recovery after transplantation: the role of reticulocyte measurements. Clin Lab Haematol. 1996;18 Suppl 1:45–53. [PubMed] [Google Scholar]
- 26.Berlin NI, Lawrence JH, Lee HC. The life span of the red blood cell in chronic leukemia and polycythemia. Science. 1951;114(2963):385–387. [DOI] [PubMed] [Google Scholar]
- 27.Novak I, Kirkin V, McEwan DG, et al. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 2010; 11(1):45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kundu M, Lindsten T, Yang CY, et al. Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood. 2008; 112(4):1493–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Randall MS, Loyd MR, et al. Mitochondrial clearance is regulated by Atg7-dependent and -independent mechanisms during reticulocyte maturation. Blood. 2009;114(1):157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itakura E, Kishi-Itakura C, Koyama-Honda I, Mizushima N. Structures containing Atg9A and the ULK1 complex independently target depolarized mitochondria at initial stages of Parkin-mediated mitophagy. J Cell Sci. 2012;125(Pt 6):1488–1499. [DOI] [PubMed] [Google Scholar]
- 31.Komatsu M, Waguri S, Ueno T, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169(3):425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mutschler M, Magin AS, Buerge M, et al. NF-E2 overexpression delays erythroid maturation and increases erythrocyte production. Br J Haematol. 2009;146(2):203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paulson RF, Shi L, Wu DC. Stress erythropoiesis: new signals and new stress progenitor cells. Curr Opin Hematol. 2011;18(3):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randhawa R, Sehgal M, Singh TR, Duseja A, Changotra H. Unc-51 like kinase 1 (ULK1) in silico analysis for biomarker identification: a vital component of autophagy. Gene. 2015;562(1):40–49. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Wu K, Xiao X, et al. Autophagy as a regulatory component of erythropoiesis. Int J Mol Sci. 2015;16(2):4083–4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aumann K, Frey AV, May AM, et al. Subcellular mislocalization of the transcription factor NF-E2 in erythroid cells discriminates prefibrotic primary myelofibrosis from essential thrombocythemia. Blood. 2013;122(1):93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]


