Erythropoietin (EPO) is an essential growth factor for red blood cell (RBC) production, and it is mainly produced by renal EPO-producing (REP) cells in the kidneys in an anemia/hypoxia-inducible manner.1,2 Erythropoiesis-stimulating agents (ESAs), including recombinant human EPO (rHuEPO), have been used to treat EPO-deficiency anemia in kidney disease patients for a quarter of a century.3,4 Due to the short plasma halflife of rHuEPO (approx. 1 day after subcutaneous injection), renal anemia patients require rHuEPO injections every 2 or 3 days to maintain their RBC count at non-anemic levels.3 Recently, long-acting ESAs, Darbepoetin alpha (DA, genetically modified EPO) and continuous EPO receptor (EPOR) activator (C.E.R.A., chemically modified rHuEPO), have been developed, with plasma half-lives of approximately 2 days and 5 days, respectively, after subcutaneous injection.3,4 Because of the lack of suitable animal models of EPO-deficiency anemia, it has been difficult to elucidate the detailed profiles of ESA-induced erythropoiesis in vivo. We recently generated a genetically modified mouse model of EPO-deficiency anemia, inherited super anemia mouse/mice (ISAM, EpoGFP/GFP:Tg3.3K-EpoE3 genotype).5 Using ISAM, we were able to obtain comparable measurements of the efficacies of 3 ESAs and demonstrated that the efficacies of these agents on erythropoiesis and iron metabolism depend on their plasma half-lives.
ISAM exhibit severe normocytic-normochromic anemia due to the loss of renal EPO production.5 The EPO-deficiency anemia in ISAM begins approximately two weeks after birth, when the major site of EPO production switches from the liver to the kidneys.5,6 We first confirmed that the hematocrit values and hemoglobin concentrations in the peripheral blood of ISAM were decreased to half of those in control mice at four weeks of age (Online Supplementary Figure S1A). Iron concentrations in the serum and liver of ISAM were higher than those in control mice, suggesting that iron usage for erythropoiesis was suppressed due to anemia in ISAM. Consistently, the unsaturated iron binding capacity of transferrin (UIBC) in the peripheral blood was decreased, and the serum level of hepcidin, a peptide hormone that inhibits iron entry into circulation,7,8 was increased in ISAM. In addition, we found that EPO deficiency seems to be indirectly related to both systemic hypoxia and cardiomegaly through chronic severe anemia in mature ISAM (Online Supplementary Figure S1B and C). Three representative ESAs (rHuEPO, DA and C.E.R.A.) were subcutaneously injected into ISAM at 3.0 μg of EPO-peptide weight per 1 kg body weight (BW), a comparable dose to that in clinical use. Three days after single injections of each ESA (day 3), the hemoglobin and RBC concentrations in the peripheral blood of ISAM were increased, as were the mean corpuscular volume (MCV) of RBCs and the weight of the spleen, the major site of EPO-inducible erythropoiesis in mice (Figure 1A).9 These data indicate that these 3 ESAs similarly induce erythropoiesis in ISAM on day 3. Although C.E.R.A. further increased the levels of hemoglobin and RBC during the last four days of observation, the erythropoietic effects of rHuEPO or DA were eliminated on day 7 (Figure 1A). Thus, the profiles of erythropoietic induction by ESAs largely depend on their plasma half-lives.
Figure 1.
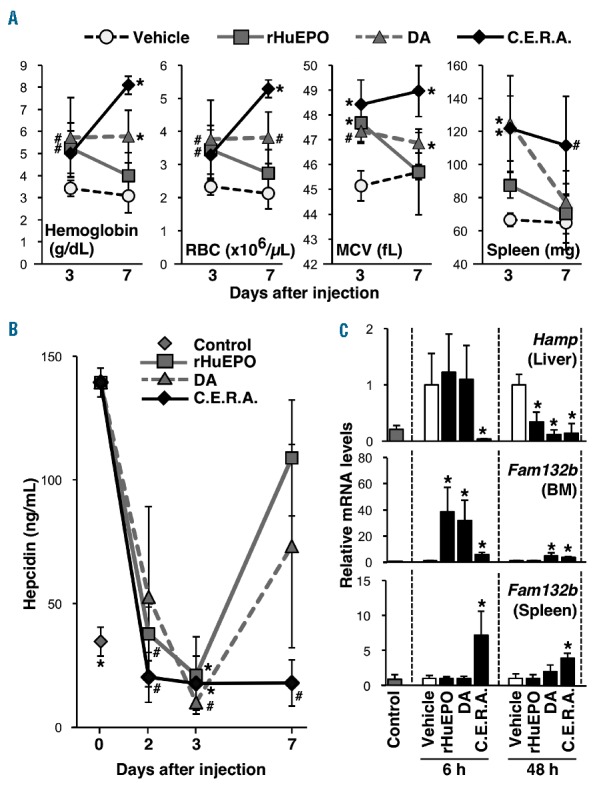
Erythropoiesis-stimulating agents (ESA) administration stimulates erythropoiesis and induces erythroferrone expression in hematopoietic organs, followed by the suppression of hepcidin production in inherited super anemia mouse/mice (ISAM). (A) rHuEPO, DA or C.E.R.A. was subcutaneously injected into 12- to 14-week old ISAM at a 3.0 μg/kg BW dose on day 0, and the hemoglobin concentration, red blood cell (RBC) count and mean corpuscular volume (MCV) in the peripheral blood were measured 3 and 7 days after injection. The spleen weights were also measured; n=4–6. *P<0.01, #P<0.05 compared with vehicle-treated mice (white circles) using Dunnett’s test at each time point. (B) Serum hepcidin concentrations were measured 2, 3 and 7 days after administration of each ESA. Data from the untreated control mice are also shown; n=3 for each point. *P<0.01 compared with ISAM on day 0 using non-parametric Steel test. (C) At 6 and 48 h after the injection of ESAs into ISAM, the expression levels of Hamp (hepcidin) and Fam132b (erythroferrone) mRNA were measured in the livers and hematopoietic organs [bone marrow (BM) and spleen], respectively. Data from untreated control mice are also shown; n=3 for each group. *P<0.01 compared with the vehicle-treated samples using Student’s t-test at each time point.
The elevated concentration of serum hepcidin in ISAM was strongly decreased to levels less than those in normal mice on day 3 (Figure 1B). At day 7, C.E.R.A. continued to suppress hepcidin levels, whereas hepcidin levels increased again in ISAM injected with DA or rHuEPO. The circulating hepcidin concentration is fundamentally regulated at the gene (Hamp) transcription level in hepatocytes.7,8,10 Each ESA significantly suppressed Hamp mRNA expression in ISAM livers 48 hours after administration (Figure 1C). At 6 hours after administration, C.E.R.A. dramatically decreased Hamp mRNA levels, whereas rHuEPO or DA administration did not change the induced Hamp levels. Hepatic Hamp expression is strongly suppressed by erythroferrone, which is secreted by erythroblasts immediately after EPO stimulation.7,11 In the hematopoietic organs of ISAM, erythroferrone gene (Fam132b) expression was induced 6 hours after C.E.R.A. administration, and the induced levels were maintained 48 hours after administration (Figure 1C). DA administration induced Fam132b mRNA expression in the bone marrow of ISAM 6 and 48 hours after administration, suggesting different organ distributions of ESAs. In fact, rHuEPO induced Fam132b expression in the bone marrow of ISAM 6 hours after administration but not in the bone marrow and spleen 48 hours after administration (Figure 1C). These data suggest that EPO gradually and persistently suppresses hepatic hepcidin production through the quick and transient induction of erythroblastic erythroferrone production because rHuEPO decreases the elevated hepcidin levels in ISAM on day 2 and day 3 but not at 6 hours after administration (see Figure 1C). Although the mechanism of erythroferrone-mediated hepcidin suppression is unknown, the efficacies of shorter half-life ESAs on hepcidin suppression are weaker than that of C.E.R.A. by single-dose injection.
The hemoglobin levels of ISAM were continuously increased for seven days after administration, and the increased level was maintained for an additional seven days (Figure 2A). Indeed, fixed-point images of the ISAM back skin showed that the thin blood vessels of ISAM were filled with RBCs on day 7 (Figure 2B). Because both the MCV and spleen weights returned to the basal levels of the untreated ISAM at day 10 (Figure 2A), we concluded that C.E.R.A. continuously stimulates erythropoiesis for one week after administration.
Figure 2.
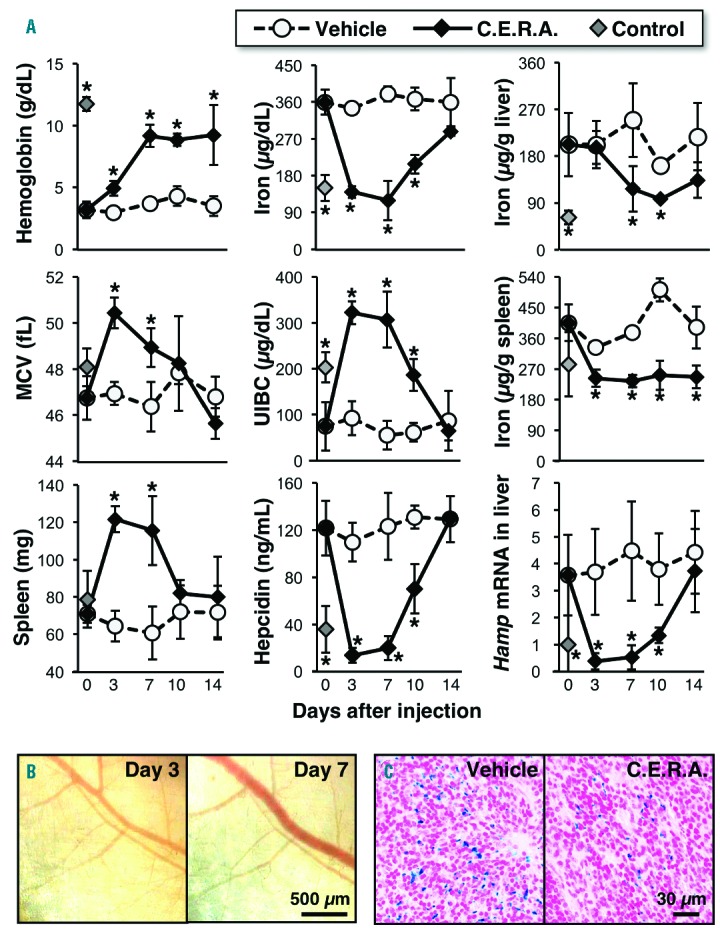
A single dose of C.E.R.A. continuously induces erythropoiesis and iron utilization in inherited super anemia mouse/mice (ISAM). (A) C.E.R.A. was subcutaneously injected at a 3.0 μg/kg BW dose into ISAM on day 0. Changes in the levels of the indicated parameters were measured; n=3 for each point. Data from untreated control mice are also shown. *P<0.01 compared with vehicle-treated ISAM at each time point using Student’s t-test. (B) Fixed-point observation of the inside of the back skin of a living ISAM 3 and 7 days after C.E.R.A. administration. Red blood cells filled the vascular networks of ISAM 7 days after injection. Scale bar 500 μm. (C) Berlin blue staining of the spleen sections from ISAM 7 days after the injection of vehicle or C.E.R.A. shows a decrease in hemosiderin deposition (blue) following C.E.R.A. administration. Scale bar 30 μm. MCV: mean corpuscular volume.
C.E.R.A. dramatically reduced serum iron concentrations to the level of normal mice at day 3 (Figure 2A). Because of the rapid reduction in serum iron concentrations, the UIBC of ISAM was higher than that of control mice on day 3 and day 7. The altered levels of both serum iron and UIBC returned to untreated-ISAM levels on day 14. The accumulated iron in the spleens and livers of ISAM were decreased on day 3 and day 7, respectively (Figure 2A). Berlin blue staining also revealed decreased iron deposits in the ISAM spleens on day 7 (Figure 2C). Because the splenic iron was used before the hepatic iron was used, local splenic iron storage may be predominantly utilized for erythropoiesis instead of hepatic iron. The reduced serum hepcidin concentrations and hepatic Hamp mRNA expression in ISAM treated with C.E.R.A. were comparable to those of control mice between day 3 and day 7 and increased again returning to their original levels at day 14 (Figure 2A).
We then investigated the cardiomegaly and systemic hypoxia in ISAM after C.E.R.A. administration. ISAM hearts were enlarged due to severe anemia at 12 weeks of age, and C.E.R.A. administration decreased the size and weight within seven days (Figure 3A and B). The anemia phenotype was reversed 28 days after a single dose of C.E.R.A. (Online Supplementary Figure S2A), and cardiomegaly developed again in ISAM (Figure 3C). These data indicate that severe anemia reversibly causes cardiomegaly in mice. A systemic hypoxic milieu emerged as a result of the severe anemia in ISAM, and the expression levels of the hypoxia-inducible genes Egln3 and Slc2a312 were significantly higher in the hearts and kidneys, respectively, of vehicle-treated ISAM than in those of the control mice (Figure 3D). The induced gene expression levels were reduced to the levels of control mice on day 10 (Figure 3D). These therapeutic effects were similarly observed in rHuEPO-treated ISAM when the hemoglobin level was increased to the normal range by 4 injections per day for seven days (Online Supplementary Figure S3).
Figure 3.
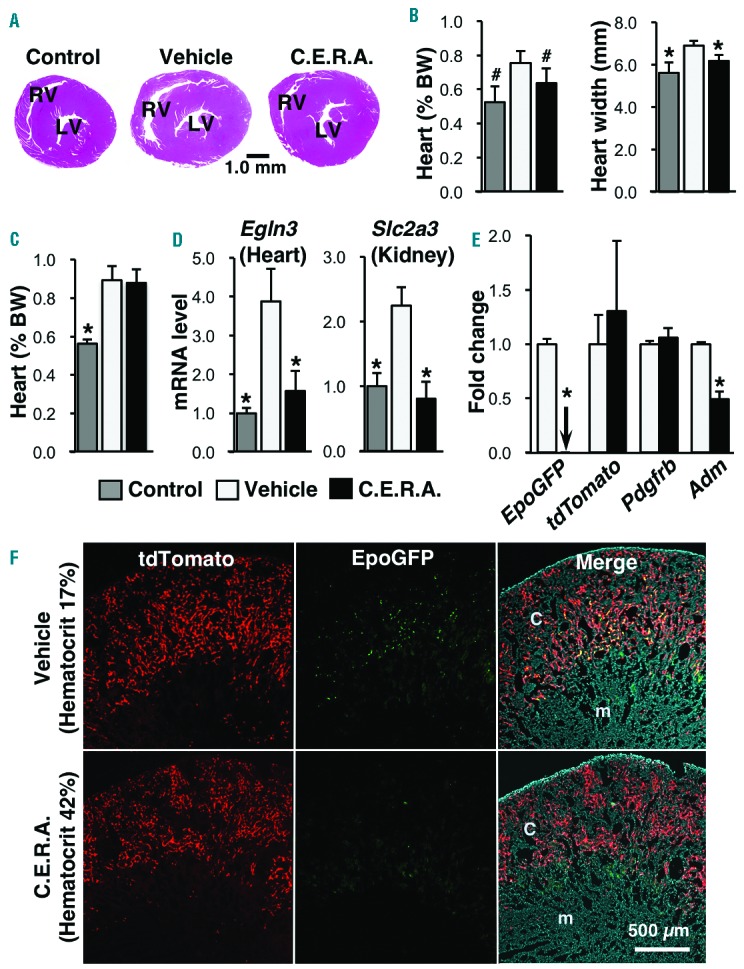
C.E.R.A. administration improves the cardiomegaly and hypoxic milieu of inherited super anemia mouse/mice (ISAM). (A) Hematoxylineosin staining of ISAM heart sections 7 days after C.E.R.A. or vehicle administration. An image from an untreated control mouse is also shown. LV: left ventricle; RV: right ventricle. (B) Weight (% body weight, BW) and maximum width of the ISAM hearts were measured 7 days after C.E.R.A. or vehicle administration. The data from the untreated control mice are also shown; n=3 for each group. (C) Weights of ISAM hearts were measured at 28 days after C.E.R.A. or vehicle administration in ISAM with recurring anemia. Data from the untreated control mice are also shown; n=3 for each group. (D) mRNA expression levels of the Egln3 (Phd3) gene in the heart and the Slc2a3 (Glut3) gene in the kidney were measured 10 days after C.E.R.A. or vehicle injection in ISAM. Data from untreated control mice are also shown. Male mice at 12–16 weeks of age were analyzed; n=3 for each group. (E) Changes in the mRNA expression of the indicated genes were examined in the kidneys of ISAM-REC mice injected with C.E.R.A. or vehicle every week for 28 days; n=3 for each group. The arrow indicates an undetectable level. *P<0.01, #P<0.05 compared with vehicle-treated ISAM-REC mice using Student’s t-test. (F) EpoGFP (green) and tdTomato (red) fluorescence was detected in kidney sections from ISAM-REC mice injected with C.E.R.A. or vehicle every week for 28 days. (Right) Merged images of EpoGFP and tdTomato expression, with DAPI counterstaining. C: cortex; m: medulla.
In the ISAM-REC mice (EpoGFP/GFP:Tg3.3K-EpoE3:Rosa26LSL-tdTomato:TgEpoCre genotype), tdTomato expression permanently labels all REP cells, and EpoGFP expression is a marker for REP cells in which the transcription of the Epo allele is activated.5 To characterize REP cells under stable non-anemic conditions, C.E.R.A. was injected into ISAM every week for four weeks. The hematocrit values of ISAM remained in the normal range after the second injection (Online Supplementary Figure S2B), and EpoGFP mRNA expression was dramatically decreased, whereas the expression of REP-cell markers (tdTomato and Pdgfrb) were unaffected on day 28 (Figure 3E).13 The expression of the hypoxia-inducible Adm (Adrenomedullin) gene in ISAM kidneys was significantly reduced by the weekly C.E.R.A. administration,12 indicating that the hypoxic milieu of the ISAM kidneys was ameliorated. Analyses of tissue sections from the ISAM-REC kidneys also demonstrated that the increased expression of EpoGFP in the REP cells of ISAM disappeared after the weekly C.E.R.A. administration, without a loss of tdTomato-positive REP cells (Figure 3F). These results demonstrate that Epo transcription is activated in the REP cells, which sense hypoxia/anemia, and that the total number of REP cells is stable in the kidneys regardless of the oxygen conditions.
This study proposes ISAM as a remarkable experimental system to assess ESA efficacy and to elucidate the in vivo mechanisms of erythropoiesis that are linked to iron metabolism. The increased serum and tissue iron levels, which are considered to be the source of cytotoxic hydroxyl radicals,14 were decreased immediately and sustainably after C.E.R.A. administration. Both the hypoxic milieu and cardiomegaly in ISAM were ameliorated by ESA administration. Renal anemia in chronic kidney diseases is often linked to chronic heart failure [cardio-renal-anemia syndrome (CRAS)].15 We propose that ESA may interfere with the CRAS linkage by inducing erythropoiesis, and ISAM may help elucidate the molecular basis of CRAS.
Acknowledgments
We thank Atsuko Konuma, Aina Fukuda (Tohoku University) and Yukari Matsuo-Tezuka (Chugai Pharmaceutical) for their technical support. We are also grateful to Sakura Motion Picture Co. Ltd., the Biomedical Research Core, and the Center for Laboratory Animal Research of Tohoku University for technical support.
Footnotes
Funding: this work was supported in part by Grants-in-Aid from MEXT/JSPS KAKENHI (grant ns. 26111002 and 24249015 for MY; 26116702 and 25670157 for NS), the Platform for Drug Discovery, Informatics, and Structural Life Science from MEXT, Japan (MY and NS), SENSHIN Medical Research Foundation (NS), Japan Anti-Doping Agency (NS and MY) and Senri Life Science Foundation (NS). The funders had no role in the study design, data collection and analysis, the decision to publish or the preparation of the manuscript.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Suzuki N, Yamamoto M. Roles of renal erythropoietin-producing (REP) cells in the maintenance of systemic oxygen homeostasis. Pflugers Arch. 2015;468(1):3–12. [DOI] [PubMed] [Google Scholar]
- 2.Souma T, Nezu M, Nakano D, et al. Erythropoietin synthesis in renal myofibroblasts is restored by activation of hypoxia signaling. J Am Soc Nephrol. 2015;27(2):428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macdougall IC, Robson R, Opatrna S, et al. Pharmacokinetics and pharmacodynamics of intravenous and subcutaneous continuous erythropoietin receptor activator (C.E.R.A.) in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2006;1(6):1211–1215. [DOI] [PubMed] [Google Scholar]
- 4.Jelkmann W. The ESA scenario gets complex: from biosimilar epoetins to activin traps. Nephrol Dial Transplant. 2015;30(4):553–559. [DOI] [PubMed] [Google Scholar]
- 5.Yamazaki S, Souma T, Hirano I, et al. A mouse model of adult-onset anaemia due to erythropoietin deficiency. Nat Commun. 2013;4:1950. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki N, Obara N, Pan X, et al. Specific contribution of the erythropoietin gene 3′ enhancer to hepatic erythropoiesis after late embryonic stages. Mol Cell Biol. 2011;31(18):389603905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koury MJ, Haase VH. Anaemia in kidney disease: harnessing hypoxia responses for therapy. Nat Rev Nephrol. 2015;11(7):394–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutschow P, Schmidt PJ, Han H, et al. A competitive enzyme-linked immunosorbent assay specific for murine hepcidin-1: correlation with hepatic mRNA expression in established and novel models of dysregulated iron homeostasis. Haematologica. 2015;100(2):167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiang J, Wu DC, Chen Y, Paulson RF. In vitro culture of stress erythroid progenitors identifies distinct progenitor populations and analogous human progenitors. Blood. 2015;125(11):1803–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasaki Y, Noguchi-Sasaki M, Yasuno H, Yorozu K, Shimonaka Y. Erythropoietin stimulation decreases hepcidin expression through hematopoietic activity on bone marrow cells in mice. Int J Hematol. 2012;96(6):692–700. [DOI] [PubMed] [Google Scholar]
- 11.Kuhrt D, Wojchowski DM. Emerging EPO and EPO receptor regulators and signal transducers. Blood. 2015;125(23):3536–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tausendschön M, Rehli M, Dehne N, et al. Genome-wide identification of hypoxia-inducible factor-1 and -2 binding sites in hypoxic human macrophages alternatively activated by IL-10. Biochim Biophys Acta. 2015;1849(1):10–22. [DOI] [PubMed] [Google Scholar]
- 13.Pan X, Suzuki N, Hirano I, Yamazaki S, Minegishi N, Yamamoto M. Isolation and characterization of renal erythropoietin-producing cells from genetically produced anemia mice. PLoS One. 2011;6(10):e25839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arosio P, Levi S. Cytosolic and mitochondrial ferritins in the regulation of cellular iron homeostasis and oxidative damage. Biochim Biophys Acta. 2010;1800(8):783–792. [DOI] [PubMed] [Google Scholar]
- 15.Attanasio P, Ronco C, Anker SD, Cicoira M, von Haehling S. Role of iron deficiency and anemia in cardio-renal syndromes. Semin Nephrol. 2012;32(1):57–62. [DOI] [PubMed] [Google Scholar]


