In chronic lymphocytic leukemia (CLL), antigenic stimulation through the B-cell receptor (BcR) and the accumulation of genetic defects critically affect the natural history of the disease.1 The importance of antigen involvement is underscored by the existence of stereotyped BcR in approximately 30% of CLL cases, where patients belonging to different stereotyped subsets share similar biological profile and clinical course.2,3 ATM defects have been associated with CLL evolution and outcome;4 however, their contribution to the pathobiology of individual subsets has not been explored. Therefore, we decided to use targeted next generation sequencing (NGS) to detect variants in the entire coding region of the ATM gene in well-characterized CLL patients assigned to one of 8 major subsets (#1-8). Since ATM is employed in signaling of telomere erosion, we then investigated the potential correlation between ATM defects and telomere length in selected subsets as well as the clinical impact of these parameters.
A total of 249 CLL patients from a large European multicenter cohort, assigned to major subsets #1-8 according to established criteria,2 were included in this study. All patients were diagnosed in accordance with the 2008 International Workshop on Chronic Lymphocytic Leukemia (IWCLL) guidelines,5 and informed consent was obtained according to the Declaration of Helsinki; local review committees granted ethical approval. Clinical and biological characteristics of the cohort are summarized in the Online Supplementary Table S1. The entire coding region (62 exons) and adjacent splicing sites of the ATM gene were investigated using targeted deep-sequencing (n=237 samples) or Sanger sequencing (n=12 samples) with details described in the Online Supplementary Appendix. ATM variants listed only in dbSNP and not in mutation databases COSMIC or HGMD were regarded as non-pathogenic polymorphisms and were excluded from subsequent analyses. A conservative 10% variant allele frequency cut off was applied to avoid false-positives; all mutations (range 10.4%–99.1%) were confirmed by Sanger sequencing.
Within the evaluated cohort of 249 CLL patients, the most populated subset was #2 [n=81, comprising 47 IGHV-mutated (M-CLL) and 34 IGHV-unmutated (U-CLL) cases] followed by the U-CLL subsets #1 (n=68), #7 (n=31), #6 (n=16), #8 (n=14), #5 (n=12) and #3 (n=12). Subset #4 (n=15) was, with a single exception, composed of M-CLL patients. Heat-maps detailing the overall distribution of genomic aberrations and/or recurrent mutations within each subset are illustrated in Figure 1A. The proportions of cytogenetic defects and gene mutations in subsets are shown in Online Supplementary Table S2 and Online Supplementary Figure S1, respectively. Overall, our data correspond well to recent reports showing enrichment of TP53 aberrations in subsets #1, SF3B1 mutations in subset #2, and trisomy 12 in subset #8.3,6,7
Figure 1.
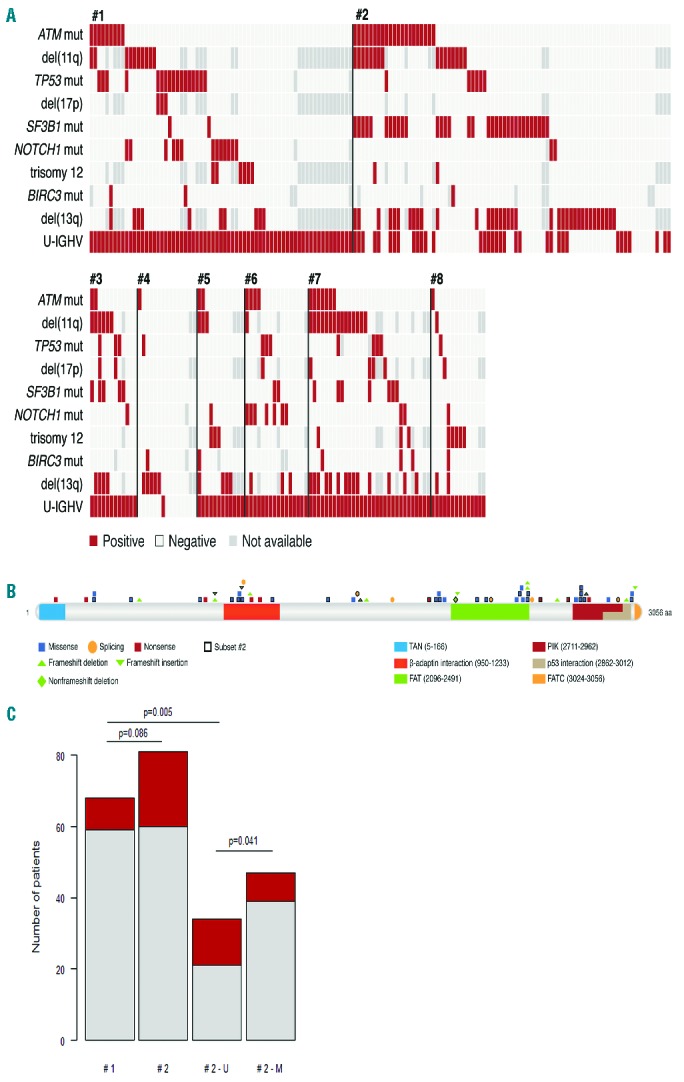
ATM mutations in major stereotyped BcR subsets. (A) Heat map showing incidence of ATM mutations and main genetic defects, IGHV mutational status and their associations in individual patients assigned to subsets #1-8 (rows correspond to aberrations, columns represent individual patients). (B) Schematic localization of identified mutations along the ATM gene displaying different mutation types, affected protein domains and subset #2 cases (framed symbols). (C) Frequency of ATM mutations in the most populated subsets #1 and #2, also considering U-CLL and M-CLL #2 cases separately (red: positive; gray: negative).
We detected 61 ATM mutations in 47 of 249 (19%) patients across all subsets; the mutational spectrum is shown in Figure 1B and mutations are listed in Online Supplementary Table S3. Concerning the functional impact, 54 of 61 (88%) identified mutations were considered to be deleterious based on either mutation type (nonsense, frameshift, abolishing splice sites) or variant effect evaluation using the PredictSNP tool.8 The remaining 7 variants were considered neutral; however, their negative impact on ATM function cannot be completely excluded as this may depend on the status of the other allele (2 of 4 samples with available data harbored 11q-) or ATM properties not included in in silico evaluation. Moreover, PredictSNP confirmed that all presumable polymorphisms were functionally neutral. The highest mutation frequency was observed in subset #2 (21 of 81, 26%) with a significant enrichment in U-CLL versus M-CLL patients (13 of 34 vs. 8 of 47, respectively; P=0.041). Within poor-prognostic U-CLL subsets, ATM mutations predominated in subsets #6 (4 of 16, 25%) and #7 (7 of 31, 23%), while the remaining subsets #3, #5, #1, and #8 showed lower frequencies (2 of 12, 17%; 2 of 12, 17%; 9 of 68, 13%; 1 of 14, 7%, respectively) (Online Supplementary Figure S1A). When comparing the two most populated subsets #1 and #2, ATM mutations were over-represented in the latter. This association did not reach statistical significance (P=0.086); however, when restricting the analysis only to U-CLL subset #2 cases, a significantly higher ATM mutation frequency was seen compared to subset #1 [13 of 34 (38%) vs. 9 of 68 (13%); P=0.005] (Figure 1C). Importantly, this difference remained significant also in a more stringent analysis involving only potentially deleterious ATM mutations (10 of 34 vs. 7 of 68; P=0.023).
Considering that: a) telomere length (TL) reflects the proliferation history of cells; and b) ATM is necessary for launching cellular response to critically short telomeres, we next evaluated the impact of ATM mutations on TL in the context of stereotyped subsets. The prognostic relevance of TL in CLL has been repeatedly reported9,10 and CLL cells with ATM mutations were recently shown to display extreme telomere shortening, allowing telomere fusions and subsequent large-scale genomic rearrangements facilitating disease progression through increased genomic instability.11,12 Relative telomere length (RTL) was investigated by real-time quantitative PCR as originally described by Cawthon et al.13 with certain modifications (Online Supplementary Appendix). The median RTL value 0.4 (range 0.05–2.25) was subsequently used to distinguish between ‘short’ and ‘long’ telomeres.
The RTL was assessed in 213 of 249 patients analyzed for ATM mutations. As expected, indolent M-CLL subset #4 patients had the longest telomeres (median RTL 0.86), which was markedly different not only from U-CLL subsets (range 0.29–0.51) but also from subset #2 (0.45) (Online Supplementary Figure S2), even when restricting the comparison to subset #2 M-CLL cases. In fact, no difference in RTL was noted between M-CLL and U-CLL patients in subset #2 (median 0.45 and 0.37; P=0.51) (Online Supplementary Figure S3); this is well in line with the previous observation that both subgroups have poor outcome independent of IGHV mutation status.14
In order to evaluate the impact of ATM defects on RTL, we decided to focus on the most populated subsets in our study, i.e. #1 and #2. Considering the expected negative impact of TP53 aberrations on RTL,9,15 the samples were divided into five genetic groups based on the hierarchical presence of TP53 and/or ATM defects: ‘Def-TP53’ [TP53 and/or defect(s): 17p- and/or TP53 mutation], ‘Def-ATM’ (biallelic ATM defect: 11q-/mutation or two ATM mutations), ‘Sole 11q-’ (only 11q- with the other ATM allele intact), ‘Sole ATM-mut’ (single ATM mutation without 11q-), and ‘WT’ (wild-type: no TP53 or ATM defect) (patient numbers in Online Supplementary Table S4).
Within subset #1, we observed particularly short telomeres in the ‘Def-TP53’ group (median RTL 0.2 vs. 0.46 in the WT group; P<0.001), while ATM defects had no significant impact. In contrast, the few subset #2 cases harboring TP53 defects showed RTL values similar to the WT group (0.4 vs. 0.49; P=0.47) (Figure 2A). Intriguingly, the ‘Def-ATM’ group demonstrated the shortest RTL of all subset #2 patients (0.23; P=0.003 compared to WT) (Figure 2A); a similar impact of ATM inactivation was observed when analyzing only U-CLL subset #2 cases (0.19 vs. 0.49 in WT; P=0.02) (Online Supplementary Figure S4). Since subset #2 is enriched for SF3B1 mutations, we evaluated the impact of mutations within SF3B1 on TL in patients lacking TP53/ATM mutations; however, both SF3B1-mutated and SF3B1-WT cases showed similar RTLs (median 0.48 and 0.49, respectively; P=0.48) (Figure 2B).
Figure 2.
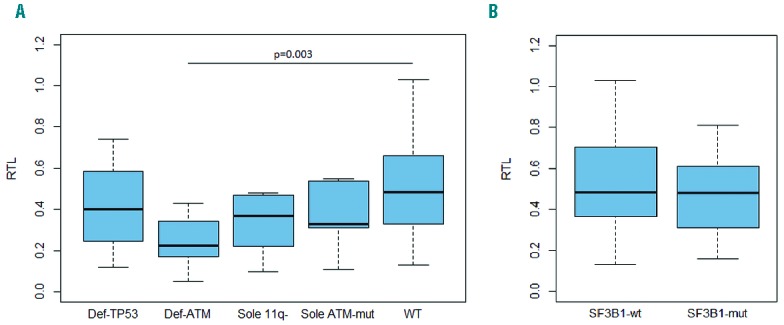
Relative telomere length according to genetic defects presence in subset #2. (A) Impact of TP53 and ATM defects (hierarchical order). (B) Impact of SF3B1 mutation in WT group (without TP53 and ATM defects). Statistically significant differences are marked in the graphs.
To gain insight into the clinical relevance of our observations, we focused on the impact of RTL on time to first treatment (TTFT) and OS in subset #2 patients. The short telomeres were significantly associated with both reduced TTFT (medians 18 vs. 42 months for long telomeres; P=0.039) (Figure 3A) and overall survival (OS) (medians 88 months vs. not reached for long telomeres; P=0.016) (Figure 3B) in subset #2. Although a previous report showed relatively stable TL after treatment in CLL patients,15 we cannot fully exclude an impact of therapy administration on survival analysis since a proportion of samples (24%) was collected after therapy. Regarding genetic defects, we also assessed their impact on OS. We divided ATM defects according to their type as in the aforementioned RTL analysis; all ATM abnormalities resulted in reduced median OS [71, 82, 89 months in Def-ATM (n=11), Sole ATM-mut (n=6) and Sole 11q- (n=7) groups, respectively] compared to WT patients (121 months, n=40) (Online Supplementary Figure S5); however, none of these comparisons were statistically significant in univariate or multivariate analyses (data not shown). Interestingly, the rare subset #2 patients with TP53 defects had a similar survival (127 months, n=6) as the WT group, underscoring previous observations that TP53 dysfunction plays a minor role in this subset.7 Thus, the very short telomeres in subset #2 patients with biallelic ATM defects imply a synergistic proliferative effect of a distinctive BcR signaling combined with impaired telomere length maintenance. By contrast, the impact of ATM defects themselves seems to be less prominent, possibly due to the heterogeneous nature of mutations affecting distinct parts of ATM protein, their variable association with 11q-, and also probably due to the small number of patients in each subgroup.
Figure 3.
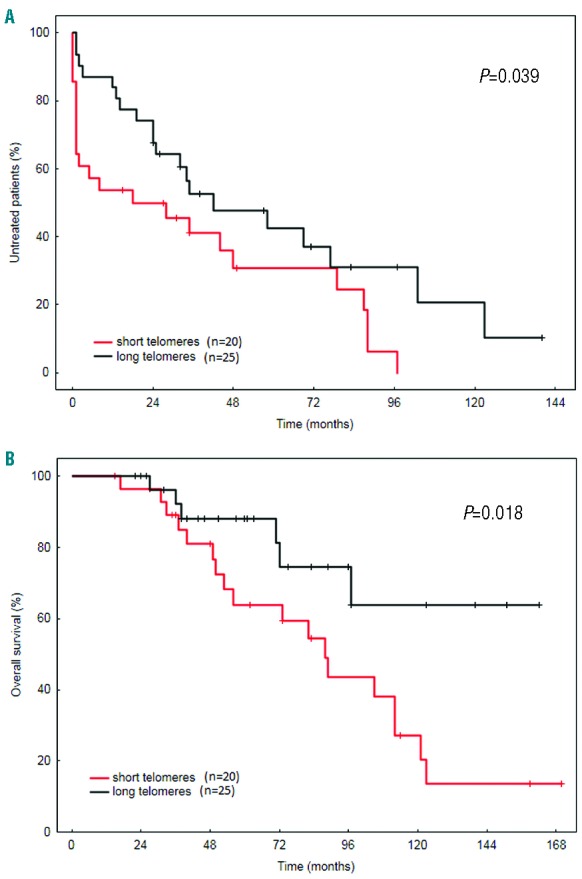
Clinical outcome of subset #2 patients according to telomere length. (A) Time to first treatment and (B) overall survival for patients with short and long telomeres.
To summarize, we demonstrate that ATM mutations can be added to the list of genetic defects with a biased distribution in stereotyped subsets. The enrichment of ATM defects in subset #2 was associated with particularly short telomeres, proposing a role for ATM inactivation in shaping the aggressive phenotype of this subset. This study further reinforces the recent suggestion that CLL development is driven by antigenic selection linked with preferential acquisition of specific genetic defects during disease evolution.
Footnotes
Funding: this work was supported by the research projects MSMT CR CEITEC2020 (LQ1601) and CZ.1.07/2.3.00/30.0009, the Ministry of Health CR - conceptual development of research organization (FNBr, 65269705), grant TACR-TE02000058/2014; project IGA MZCR NT13493-4/2012; Horizon2020 Programme Twinning (MEDGENET/2016-2018/no.692298); the Swedish Cancer Society, the Swedish Research Council, Uppsala University, Uppsala University Hospital, Selander’s Foundation (Uppsala) and Lion’s Cancer Research Foundation (Uppsala); Grant EMCR 2014-6564 by Dutch Cancer Society; Associazione Italiana per la Ricerca sul Cancro AIRC (Investigator Grant #15189 and Special Program Molecular Clinical Oncology – 5 per mille #9965), Milano, Italy and Ricerca Finalizzata 2010 (RF-2010-2318823) – Ministero della Salute, Roma, Italy; H2020 AEGLE, an analytics framework for integrated and personalized healthcare services in Europe by the European Commission; Bloodwise (11052, 12036, 14027), the Kay Kendall Leukaemia Fund (873) and the Bournemouth Leukaemia Fund, with infrastructure support from a Cancer Research-UK center grant (C34999/A18087).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Sutton L-A, Rosenquist R. The complex interplay between cell-intrinsic and cell-extrinsic factors driving the evolution of chronic lymphocytic leukemia. Semin Cancer Biol. 2015;34:22–35. [DOI] [PubMed] [Google Scholar]
- 2.Agathangelidis A, Darzentas N, Hadzidimitriou A, et al. Stereotyped B-cell receptors in one-third of chronic lymphocytic leukemia: a molecular classification with implications for targeted therapies. Blood. 2012;119(19):4467–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baliakas P, Hadzidimitriou A, Sutton L-A, et al. Clinical effect of stereotyped B-cell receptor immunoglobulins in chronic lymphocytic leukaemia: a retrospective multicentre study. Lancet Haematol. 2014;1(2):e74–84. [DOI] [PubMed] [Google Scholar]
- 4.Stankovic T, Stewart GS, Fegan C, et al. Ataxia telangiectasia mutated-deficient B-cell chronic lymphocytic leukemia occurs in pregerminal center cells and results in defective damage response and unrepaired chromosome damage. Blood. 2002;99(1):300–309. [DOI] [PubMed] [Google Scholar]
- 5.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strefford JC, Sutton L-A, Baliakas P, et al. Distinct patterns of novel gene mutations in poor-prognostic stereotyped subsets of chronic lymphocytic leukemia: the case of SF3B1 and subset #2. Leukemia. 2013;27(11):2196–2199. [DOI] [PubMed] [Google Scholar]
- 7.Rossi D, Spina V, Bomben R, et al. Association between molecular lesions and specific B-cell receptor subsets in chronic lymphocytic leukemia. Blood. 2013;121(24):4902–4905. [DOI] [PubMed] [Google Scholar]
- 8.Bendl J, Stourac J, Salanda O, et al. PredictSNP: robust and accurate consensus classifier for prediction of disease-related mutations. PLoS Comput Biol. 2014;10(1):e1003440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roos G, Kröber A, Grabowski P, et al. Short telomeres are associated with genetic complexity, high-risk genomic aberrations, and short survival in chronic lymphocytic leukemia. Blood. 2008;111(4):2246–2252. [DOI] [PubMed] [Google Scholar]
- 10.Strefford JC, Kadalayil L, Forster J, et al. Telomere length predicts progression and overall survival in chronic lymphocytic leukemia: data from the UK LRF CLL4 trial. Leukemia. 2015;29(12):2411–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin TT, Letsolo BT, Jones RE, et al. Telomere dysfunction and fusion during the progression of chronic lymphocytic leukemia: evidence for a telomere crisis. Blood. 2010;116(11):1899–1907. [DOI] [PubMed] [Google Scholar]
- 12.Britt-Compton B, Lin TT, Ahmed G, et al. Extreme telomere erosion in ATM-mutated and 11q-deleted CLL patients is independent of disease stage. Leukemia. 2012;26(4):826–830. [DOI] [PubMed] [Google Scholar]
- 13.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baliakas P, Agathangelidis A, Hadzidimitriou A, et al. Not all IGHV3-21 chronic lymphocytic leukemias are equal: prognostic considerations. Blood. 2015;125(5):856–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansouri L, Grabowski P, Degerman S, et al. Short telomere length is associated with NOTCH1/SF3B1/TP53 aberrations and poor outcome in newly diagnosed chronic lymphocytic leukemia patients. Am J Hematol. 2013;88(8):647–651. [DOI] [PubMed] [Google Scholar]


