Abstract
Background We developed a decision aid for patients with curable prostate cancer based on Svenson’s DiffCon Theory of Decision Making. This study was designed to determine if surrogate patients using the aid could understand the information presented, complete all tasks, show evidence of differentiation, and arrive at a preferred treatment choice.
Methods Men, at least 50 years old and never diagnosed with prostate cancer, were recruited through local advertisements. Participants were asked to imagine that they were a case‐scenario patient. Then they completed the decision aid interview, which included three components: (i) information presentation, with comprehension questions, (ii) exercises to help identify attributes important to the decision, and (iii) value‐clarification exercises.
Results Sixty‐nine men volunteered. They had a mean age of 61.2 (range 50–83) years, 37% had no formal education beyond high school, and 87% were living with a partner. All participants completed all aspects of the interview. They answered an average of 10 comprehension questions each, with a mean of 94.7% correct without a prompt. Each attribute in the information presented was identified by at least one participant as important to his decision. Participants identified a median of five attributes as important (ranges 1–14) at each of three points during the interview; 75% changed at least one important attribute during the interview. Forty‐nine per cent of participants also identified attributes as important that were not included in the presented information. Participants showed a wide range of values in each of seven trade‐off exercises. Eighty‐eight per cent of participants showed evidence of differentiation; 75% had a clear treatment preference by the end of the interview.
Conclusions Our decision aid appears to meet its goals for surrogate patients and illustrates the strengths of the DiffCon theory. The ability of the aid to accommodate wide variability, both in information needs and in important attributes, is a particular strength of the decision aid. It now requires testing in patients with prostate cancer.
Keywords: decision aids, decision making, informed consent, patient participation, prostate cancer
Introduction
Decision aids are intended to help patients participate in their treatment decisions, but it is not clear what characteristics make the aids most helpful. In a review of decision aids under the auspices of the Cochrane Collaboration, decision aids are described as ‘interventions designed to make deliberative choices among options by providing (at the minimum) information on the options and outcomes relevant to the person’s health status’. 1 , 2 The review noted that some aids also provide other types of information but only a few include explicit values clarification exercises or coaching in decision making. 2 Similarly, a review of decision aids by Molenaar et al. 3 emphasized the role of providing information in their description of decision aids. Thus, although some decision aids aim to assist at least some of the complicated cognitive processing required to arrive at a decision, most focus on information provision and are not theoretically driven.
In contrast, current psychological theories of decision making focus on the cognitive processes that operate on information in order to arrive at a decision. 4 , 5 , 6 , 7 , 8 The theories agree that decision making involves cognitive processing beyond obtaining information and even beyond clarification of values. Decision aids based on such theory therefore would not only strive to facilitate information provision but would also facilitate the cognitive processes that people use on the information in order to arrive at a decision.
One relatively comprehensive psychological theory of decision making is Svenson’s Differentiation and Consolidation Theory (DiffCon). 9 , 10 It is unique among psychological theories because it includes both pre‐ and post‐decision processes as being important to decision making. The goal of decision making, according to DiffCon, is to create an alternative that is sufficiently superior in comparison to its competitor(s) through restructuring and application of one or several decision rules. The restructuring processes, called ‘differentiation’, are derived from a number of different rules contingent on the situation and the person in the situation; ‘consolidation’ includes the same types of processes but they occur post‐decision. Central to DiffCon is the assumption that sufficient differentiation protects the decision maker from external (e.g. poor outcomes) and internal (e.g. changes of own values) threats to the preference for the chosen alternative. It also assumes that people want to minimize their effort and the potential for post‐decision regret and/or cognitive dissonance, and that the attractiveness of attributes varies over time. Decision aids guided by DiffCon, therefore, would aim to reduce the risk that patients’ decisions will cause them regret and/or cognitive dissonance by facilitating pre‐decision differentiation and post‐decision consolidation processes.
Treatment decisions for early stage prostate cancer are among those decisions that are particularly challenging. In Ontario, the standard treatments include three options: surgery (prostatectomy), radiotherapy and watchful waiting (no treatment for now). The decision is further complicated by the fact that there have been no clinical trials providing unequivocal evidence comparing the treatments, although it is clear that the two active treatments have side‐effects that differ. 11 Thus, there is a large amount of information from which pertinent risks and benefits must be weighed. The process can easily become overwhelming as the decision is typically a new type of experience for the patient, and he/she often feels some urgency to make a decision quickly but is in an emotional state that interferes with decision making. 12 , 13
In this paper we describe our development of a decision aid for men with early stage prostate cancer and its theoretical underpinnings. We then describe a study in which we evaluated the aid according to our theoretical expectations in the context of surrogate patients using it to make a treatment decision.
Decision aid – theoretical basis
Our decision aid is an interview, administered on an individual basis, that is intended to be an adjunct to the normal doctor–patient consultations. It fits between an initial consultation when the doctor presents the treatment options and a second consultation that occurs about 1 week later when the treatment decision is made. Thus, the aid is intended to help the patient become clearer about which treatment option he prefers in order to make the decision with his doctor at his next visit. The interview is fundamentally based on Svenson’s DiffCon theory of decision making but other theories underpin aspects of the aid that are beyond decision making, such as strategies for presenting the information. The aid includes three components: the structured presentation of information, exercises designed to help the patient determine which attributes are important to his decision, and exercises designed to help clarify the value of each of his important attributes as they are integrated into the larger picture. In this section, we describe the theoretical underpinnings of the components, highlighting activities that are designed to encourage differentiation.
Information provision
Our information presentation includes several strategies that are designed to reduce the processing burden on the patient because heavy information processing demands necessarily reduce the amount of energy available for decision making. 14 First, we limited the information included in our standard presentation to that identified by a clear majority of patients in our earlier study as being necessary for them to know in order to make this treatment decision. 15 We recognize that patients vary widely in these needs and that the standard presentation would provide incomplete coverage for most individuals, thus, patients were encouraged to add any items not initially presented that are important to them. Providing the information can start the differentiation processes by clarifying/correcting details (fact differentiation) and possibly shifting how the patient thinks about the issues that are important to his decision (problem restructuring).
The information is presented in a matrix so relationships between items are clear. The presentation begins by highlighting the organization, a strategy that provides an ‘advance organizer’ which helps the patient to anticipate the type of information that will be presented and how the pieces will relate to one another. 16 A further strategy to reduce processing burden is that the patient directs the order in which remaining cells of information get added to the table. 17
Important attribute identification
At four different times during the interview, the patient identifies the attributes that he thinks would affect his decision. He is asked to list the attributes in their order of importance, which encourages further differentiation (attractiveness restructuring and attribute importance restructuring). In addition, consistent with Svenson’s assumption that attractiveness and importance of attributes change over time, the exercises provide us with insight into the shifts that occur and help us identify aspects of the interview that are associated with them. Differentiation processes, such as correcting errors or filling in missing facts (fact restructuring), may result in the patient changing the list (attribute importance restructuring).
Later in the interview, the patient is asked to identify the least appealing option in order to drop it from further consideration. The drop is encouraged because the more alternatives people need to compare, the more they resort to simplifying strategies that can result in their ignoring some of the information. 18 Thus, the drop is intended to reduce the processing burden and to encourage further differentiation (including problem restructuring).
Treatment trade‐off exercises
The patient completes treatment trade‐off exercises on each of the quantitative attributes that are important to his choice between the two more preferred options. Each exercise can be seen as a values‐clarification exercise and is designed to help the patient weigh together all information about the options, focusing on one of the quantitative attributes he has identified as being important. 19 , 20 For each quantitative attribute, the likely outcomes associated with each of the two treatment options are presented side by side using vertical bars (each with a scale going from 0 to 100), a format that is among those formats more efficiently and more accurately perceived by patients making a treatment decision. 21 To further reduce processing burden, the likelihoods are presented as frequencies, described as the number of people out of 100 treated with that option who experienced the outcome. 22 , 23 The exercises are designed to encourage further differentiation (attractiveness restructuring).
Insight into the decision processes can be gained by comparing the treatment preferred in the trade‐offs that focus on a particular attribute with the patient’s final treatment preference. If the preferred treatment differs in the two situations, a situation in which there are attributes in conflict, 9 , 10 three outcomes are possible. First, the patient may not be able to arrive at a preferred treatment, and remains in a state of indecision. Secondly, he may decide on his overall preferred treatment and the consolidation processes could reduce the importance of the attribute in conflict so that all important attributes favour the same treatment. Thirdly, he could choose his overall preferred treatment with conflict remaining, which would make the patient vulnerable to cognitive dissonance or regret later on.
Although the patient makes his treatment decision formally with his doctor in the consultation that occurs a couple of days after the interview, it is intended that he be clear about his preferred option by the end of the interview. The extra days between the end of the interview and the consultation therefore provide the patient with time for post‐decision consolidation to occur. The consolidation could potentially allow the patient to be more certain of his decision when it is formally made with the doctor. Alternatively, the consolidation process could fail, in which case the patient would want to change his mind and consolidate the second decision.
Decision aid: a test by surrogate patients
We tested the decision aid with men who were in the age group of most newly diagnosed prostate cancer patients (at least 50 years old). 24 The participants acted as surrogate decision makers as they had never been diagnosed with the disease. While their responses are of interest themselves, they are described in more detail elsewhere 25 and the focus of this paper is on the preliminary evaluation of the aid. The goals of the test were to determine if participants would be able to understand the information presented, to identify what is important to their decisions, to weigh the attractiveness of the treatments on their important attributes, show evidence of differentiation, and be able to identify a preferred treatment option.
Methods
Participants
The participants were a convenience sample of men at least 50 years old, never diagnosed with prostate cancer, who could understand English. We used a purposeful sampling strategy to ensure representation of those with and those without post‐secondary education. Thus, participants were recruited through an advertisement in the local newspaper and one at a local industrial plant, posted on a bulletin board by its health office. The study had ethics approval from the Queen’s University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board.
Sample size
Our primary outcome of interest was the proportion of participants that display evidence of differentiation and we wanted our sample to have enough power to provide an estimate of ± 15% (95% confidence interval); we were not interested in a precise estimate but rather a demonstration in principle that a substantial proportion of participants display differentiation. Fifty participants provide such power (β = 0.2, α = 0.05) around 50%, the binomial proportion that has the widest confidence intervals. Thus, we intended to recruit at least 50 participants and included all eligible volunteers.
Procedure
The procedure involved a 11/2‐h interview with a research associate (LVM). It began with the presentation of a case of a 65‐year‐old man just diagnosed with prostate cancer. A brief description of the man’s situation was provided in lay English with the technical details of the disease provided in brackets (Stage T2, PSA 9, Gleason 6). The participant was asked to imagine that he was the patient in the scenario. The flow of the interview activities is provided in Fig. 1, from top to bottom. They began with a baseline assessment of the participant’s attitude towards each of the treatment options (Fig. 1, Study assessments, Treatment preference assessment – 1); the participant rated his inclination towards each treatment option separately on the following 5‐point scale: ‘I probably would not choose this option’, ‘I’m leaning away from this option’, ‘I’m neither for nor against this option’, ‘I may choose this option’ or ‘I’ll probably choose this option’.
Figure 1.
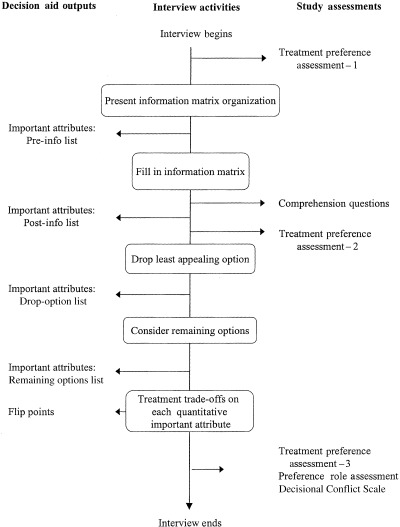
Order of activities in decision aid interview. Activities are identified in the rounded boxes down the central column of the figure; they are listed from the top of the figure down, as they occur chronologically in the interview. Arrows from particular activity boxes to the left identify outputs of the activity. Arrows from the central column to the right identify additional assessments included in the interview in order to evaluate the impact of particular aspects of the interview.
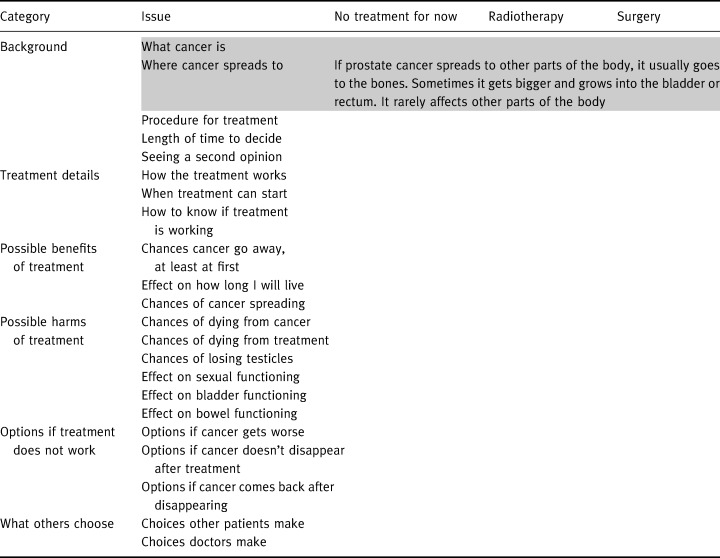
The interview then proceeded to the information provision, beginning with the matrix organization. The information board is a 3′× 5′ metal sheet mounted on the wall. The outline of the information matrix is presented in Table 1. The greyed attributes at the top of the matrix were added to the attributes identified in our previous work as important to a majority of patients making their treatment decisions. The attributes were added because we considered them essential to understanding the remaining attributes and how they relate to one another. In Table 1, we provide the information presented for the attribute ‘where the cancer spreads’, one of the smaller information cells, as an example of the extent of detail provided. The information for each cell of the matrix is printed in large print, laminated and has a magnetic backing (similar to a fridge magnet).
Table 1.
Information board organization
After the matrix labels were introduced, the participant identified his important attributes (pre‐info list). The participant was encouraged to list all important attributes even if they were not included in the information board; the research associate recorded the attributes on a white board so that the list could be referred to when needed.
After the information was presented, the participant was asked standardized comprehension questions (Fig. 1) and was encouraged to look at the board for the answer. If he could not answer a question, he was provided with prompts until the correct response was produced. The interviewer recorded the number of prompts required for the correct response.
Following confirmation of understanding, the participant revisited his list of attributes (post‐info list) and completed a second treatment preference assessment (Fig. 1, Treatment preference assessment – 2). He then identified his least‐preferred option to drop from further consideration and listed the attributes important to the drop (drop‐option list). After the drop, he identified the attributes relevant to the choice between remaining options (remaining‐options list).
Once focused on the remaining options, the participant completed the treatment trade‐off exercises: one trade‐off for each quantitative attribute important to the choice. Figure 2 shows an example focused on the impotence caused by the active treatments: 50 men out of 100 treated with surgery and 50 men out of 100 treated with radiation experience impotence. The patient is then instructed to choose his preferred option of the two, given the presented outcomes and considering everything he had been told about each option. In the Fig. 2 example, the choice is surgery. Then, the frequency associated with one of the options (radiation in the Fig. 2 example) is systematically altered until the participant switches his choice, producing a flip point. The flip point in the Fig. 2 example is 25. The option actually preferred when considering that outcome can be derived by comparing the flip point and the actual estimated probability of the outcome. In the Fig. 2 example, a flip point of 25 for radiation compared with the actual estimated frequency of 50 implies that the patient would prefer surgery when considering this outcome.
Figure 2.
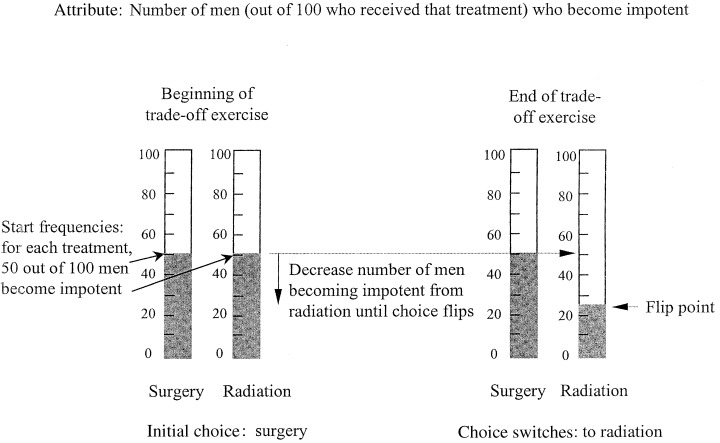
A schematic of an example trade‐off exercise. The two vertical bars on the left show the number of patients (50 out of 100) treated with radiation treatment and (50 out of 100) treated with surgery (left and right, respectively) that become impotent after the respective treatments. The patient then chooses, given those numbers and everything else he has been told about the two treatment options, which treatment he would prefer. In this example, the initial choice is surgery. The number of patients treated with radiation who develop impotence is then systematically dropped until radiation becomes the more appealing option and the patient ‘flips’ his choice. The example shows the ‘flip point’ at 25.
After the trade‐off exercises, final assessments were completed: a final treatment preference assessment (Fig. 1, Treatment preference assessment – 3); decisional role preference, 26 a five‐point scale used to determine how much control the participant would want in making the treatment decision; and the first 10 items (of 16) of the Decisional Conflict Scale. 27 The decisional conflict scale items are statements and the participant indicates the extent that he agrees/disagrees with each statement on a 5‐point ordinal scale. The first three items of the Decisional Conflict Scale are designed to determine how uncertain the participant would feel about making the decision at that point in time; the remaining seven items that we used are intended to determine sources of uncertainty: feeling uninformed (four items) or feeling unclear about important values (three items). The remaining items of the scale were inappropriate for someone making a hypothetical decision. The interview ended with the participant providing demographic information: age, highest level of formal education, partner status, and questions related to his experience with cancer. He was also asked if he had: experienced cancer himself, his PSA tested, a prostate biopsy, or any close family/friends who had experienced prostate cancer.
Results
Participants
Sixty‐nine men participated in the study; 44 recruited through the newspaper and 25 through the industrial plant. The only significant difference between the groups was their ages: the newspaper recruits had a mean age of 63.8 years (range 51–83), while the industrial‐plant recruits had a mean age of 56.8 years (range 50–65) [t(66) = 4.16, P < 0.001]. We were motivated to recruit from the plant to gain more participants with less formal education: 66% of the newspaper recruits had completed some post‐secondary education while 52% of the plant recruits had [χ2 = 3.6, P= 0.17]. The groups did not differ on any other characteristic that we collected data on. The combined group had a mean age of 61.2 years. Four per cent had only grade school education, 33% had completed high school and 61% had some post‐secondary education. Seven per cent were single, 87% were married or living in a common‐law relationship, and 6% were divorced or separated. Their past cancer experience relevant to the study included: 13% had experienced some form of cancer (other than prostate cancer), 58% had had a PSA test, 4.3% had had a prostate biopsy, and 49.3% had close family or friends with prostate cancer.
Information presentation: comprehension
Due to technical difficulties, data were not recorded on the comprehension questions for the first nine participants. Over the remaining 60 participants, 28 different questions were asked (one for each information cell presented on each important attribute identified by each patient) to a total of 779. Of the 779, 94.7% were answered correctly without a prompt, and the remaining answered correctly with a prompt.
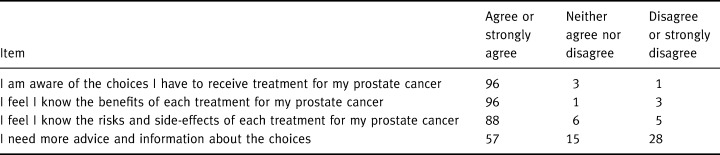
All 69 participants completed the four items of the Decisional Conflict Scale focused on feeling informed. Table 2 shows participant responses to the four statements: almost all participants felt they knew what their treatment choices were and the risks and benefits associated with each of the treatments. Slightly more than half, however, thought they needed more information or advice. Although no personal characteristic was associated with these responses, the location of recruiting did show such a trend: a greater proportion of participants from the industrial plant agreed (or strongly agreed) that they needed more advice/information than of those recruited from the newspaper advertisement [χ2 = 5.1, P = 0.078]. Interestingly, education was not associated with the responses [χ2 = 0.6, P = 0.73].
Table 2.
Responses to decisional conflict items relevant to feeling informed (%), n= 69
Important attributes
In this paper, we focus the report of important attributes on the number and range of what individual participants reported. We report the proportion of participants that thought particular attributes are important elsewhere. 25
Three of the important attribute lists (the pre‐info, post‐info and remaining options lists) were focused on selecting the most preferred option. Table 3 summarizes the number of attributes identified by each participant on those lists and describes the changes between the lists. As the table shows, the median number of attributes was consistently five but there were many attributes dropped and added over the course of the interview. The table shows that the greatest shift in important attributes occurred because of the information presentation, with almost half of the participants dropping attributes and nearly as many adding new ones at that time. The table also shows that generally changes occurred more often because attributes were dropped than because they were added. Fifty‐two participants (75%) changed at least one attribute of those important to selecting their most preferred option. For example, ‘effect on average length of life’ was the attribute dropped most frequently and 14% dropped it after the information was presented and 9% added it at that time because the information was different than they had expected.
Table 3.
A summary of the important attributes identified by each participant pre‐info, post‐info and when considering remaining options
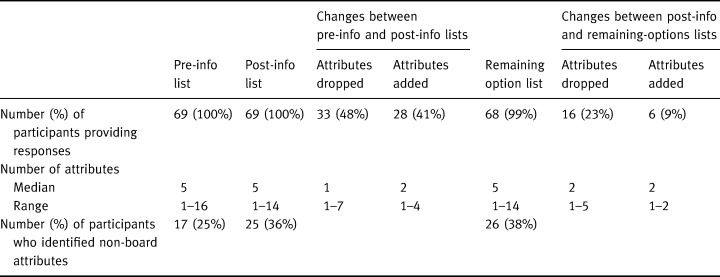
The fourth of the important attribute lists was focused on selecting the least‐preferred option (drop‐option list). Four participants could not choose a least‐preferred option and could not identify attributes that were important to that choice. The remaining 65 (95%) participants identified a median of one attribute (range: 1–6; 25th, 75th percentiles: 1, 2) as important to the choice of option to drop. That is, there was generally only one important attribute that caused the particular option to be dropped.
Table 3 also shows, for each list, the percentage of participants who identified attributes that were not included in the information on the board. Examples of such items are ‘burden on my family’ and ‘effect on my ability to work’. As the table shows, each time important attributes were listed, a significant percentage of participants identified non‐board items. Overall, 34 (49%) participants identified at least one attribute not included on the board at some point during the interview. Those identifying non‐board attributes on the pre‐info, post‐info and remaining options lists identified a median of one non‐board item (range 1–4); all 13 participants identifying non‐board items on the drop‐option list identified only one item each. Neither the participant characteristics nor their recruiting location were associated either with the number of important attributes or with the number of changes in important attributes.
Trade‐off exercises: flip points
Table 4 summarizes the outcomes of the trade‐off exercises. It shows the number of participants whose flip points favoured the respective treatments for each of the trade‐off exercises. Thus, if the participant was making his treatment decision based on this attribute only, the table specifies the number of participants who would choose the option indicated in the column header. As can be seen in the table, each of the trade‐offs was completed by only a few participants, an indication of the wide variation in important attributes and in the options being considered. The table also shows that for every trade‐off, there were some participants who favoured the first listed treatment option while others favoured the second option.
Table 4.
The number of participants whose flip points favoured the respective options of each trade‐off exercise
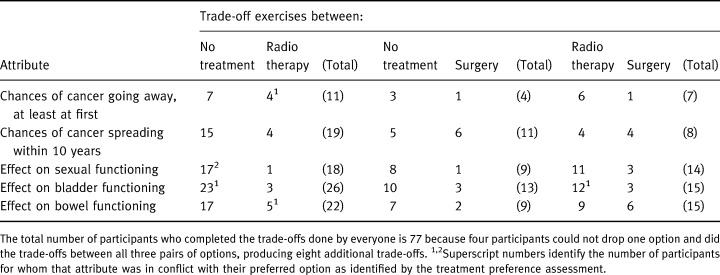
The trade‐off exercises helped us identify attributes in conflict, that is attributes that favoured the treatment not preferred in the treatment preference assessment at the end of the interview. Of the 52 participants who had a preferred treatment at the end of the interview, six (9%) had a quantitative attribute in conflict and they are identified by superscripts in Table 4. Of the 17 who did not have a preferred treatment, six (35%) had at least one quantitative attribute in favour of each of the treatments still being considered.
Treatment preferences
The final treatment preference assessment (Fig. 1, Treatment preference assessment – 3) indicated that 44% of our participants were inclined towards choosing no treatment for now, 17% towards radiotherapy, 12% towards surgery, and 25% remained undecided at the end of the interview. Neither the participant characteristics nor the location of recruiting were associated with whether or not participants had a preferred treatment at the end of the interview.
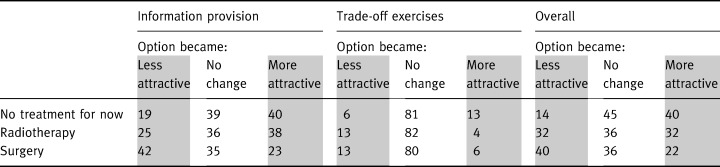
The treatment preference assessments were carried out three times during the interview and Table 5 shows, for each of the three options, the percentage of participants whose assessment shifted to favour it less (shaded), did not change, or shifted to favour it more (shaded). As can be seen in the table, providing information was associated with a shift (becoming either more or less attractive) in attitudes of approximately 67% of our participants’ preferences for each of the treatment options, and the trade‐off exercises were associated with a shift in preferences for each option of about 20% of the participants. Some of the early shift reversed itself, so by the end of the interview just over half of our participants had shifted their preferences regarding no treatment for now and about two‐thirds shifted whether or not they preferred each of the active treatment options. The shifts were largely towards no treatment for now, away from surgery, and about equal shifts towards and away from radiotherapy.
Table 5.
Shifts in treatment preferences (%) associated with:
Evidence of differentiation
Overall, 52 (75%) participants changed the attributes that they identified as important to their decisions at some point during the interview, and the changes reflect attribute importance restructuring. Of the 17 (25%) participants who did not change their listed important attributes, nine (an additional 16% of all participants) showed a shift in their treatment preference assessments. Because those people did not change their important attributes, it is likely that their shifts in a treatment preference assessment reflected a shift in the attractiveness of the treatment on one or more of the important attributes (attribute attractiveness restructuring). Although the treatment preference assessments involved holistic judgements, the deliberate nature of the responses make it unlikely that the shifts reflect holistic differentiation, which is quick and non‐analytical. Using shifts in attribute lists and in treatment preference assessments as indications of changes in how participants were thinking about their options, 88% of all participants showed evidence of differentiation.
Other assessments
The fact that the decision was considered a difficult one was evident by responses to the Decisional Conflict Statement ‘This decision is hard for me to make’, with which 59% either agreed or strongly agreed. Thirty‐three per cent either agreed or strongly agreed with the statement ‘I am unsure of what to do in this situation’. Responses to the decisional conflict statements suggest that the difficulty centres around the weighing of all important risks and benefits together: as Table 2 shows, almost everyone felt that they knew the risks and benefits. However, 53% of participants either agreed or strongly agreed with the statement ‘It’s hard to decide if the benefits are more important to me than the risks, or if the risks are more important’. Not surprisingly with our recruiting strategy, 96% of our participants wanted to participate in their treatment decision: 32% in a shared role, 58% to make the decision after considering the doctor’s opinion, and 6% to make the decision alone. Of the 4% who wanted a passive role in decision making, 1% wanted the doctor to make the decision alone and 3% after considering the patient’s opinion.
Discussion
These results showed that the participants, who were men in the age group of newly diagnosed prostate cancer patients, were able to understand the information provided in the decision aid, felt they were informed about the risks and benefits of the treatment options, and were able to identify attributes important to their decisions. Although some participants had difficulty weighing the important attributes together, most were able to identify a preferred treatment option, and almost all clearly showed evidence of differentiation.
The results showed the wide variability in attributes that were expected to affect the participants’ decisions, similar to the extent of variability in the information considered necessary for the decision that we found in our retrospective survey of patients. 15 Thus, we also expect to find wide variation among patients’ important attributes, and we have demonstrated that the decision aid can accommodate such variability.
Although the interview resulted in three‐quarters of the men in this study stating a preferred treatment, we acknowledge that the interview appeared to provide insufficient support for the remaining participants. One possibility for that impression is the sensitivity of the measure that we used for the treatment preference assessment. These participants may have had weak preferences that we did not detect. Alternately, they may really have been undecided. Being undecided may not be all that surprising for this group considering that although all of the participants had heard about prostate cancer before the study, some may not have known many of the details before the interview. The novelty of the situation for those participants would mean that they would not only have to learn the new information but also have to figure out what values are relevant to their decisions, and decide how to balance it all together within the 1½ h. Because there are many differences between real patients and the surrogates, it is not clear at this point if the decision aid would offer ‘enough’ guidance and support for real patients. For example, real patients will have already been provided with information in their initial consultation with their doctors before the decision aid interview. The advantage may mean that the amount offered in the decision aid interview would be adequate for a larger proportion of patients. On the other hand, real patients often experience intense emotion when given a cancer diagnosis that reduces their processing capacity, they often obtain information from a variety of sources with many types of presentations that make it difficult to integrate, and the information is often conflicting. These differences mean that the real patients might require more support than the surrogates and the amount offered in the decision aid interview might be inadequate for a higher proportion of real patients.
In their review of decision aids, Molenaar et al. 3 argue that an explicit theoretical model of the important factors related to patients’ decision making should guide decision aid development because it would help delineate the type of information that is important to patients. We agree that decision aid development should be theoretically driven. We are concerned, however, about the focus of the theory that Molenaar suggests would be helpful. In our studies of information needs among patients with early stage prostate cancer we have found that the needs varied substantially from one patient to another, and our search to find predictors of particular information needs did not yield any factors that could predict more than 25% of the variance we found in information needs. 15 , 28 Thus, we are concerned that applying a model that predicts what information to provide will be likely to miss the needs of many individual patients.
We also believe that decision aids would have greater potential to benefit patients if their development was based on theory that recognizes the dynamic nature of decision making (process theories in psychology). We believe that understanding and assisting the dynamics will help patients arrive at a choice that is most consistent with their values. Evidence in prostate cancer shows that patients facing their treatment decisions face a situation that is very new to them, 12 which, in turn, makes it likely that discovering their relevant values is part of their decision making process. 29 Those patients would benefit from decision aids that assist the value‐discovery processes (accommodated in Svenson’s differentiation processes). The dynamics of the processes are also important in attempts to reduce the likelihood of the patient feeling regret about the decision at a later date. According to Svenson’s DiffCon Theory, we naturally differentiate in order to reduce regret. Thus, it is the dynamic differentiation (and consolidation) processes that we want to encourage with decision aids in order to maximize the reduction in the likelihood of regret.
Our use of DiffCon, a process theory of decision making, provides us with a number of benefits. First, we capitalize on the processes that people are inclined to use in decision making, providing an intuitive appeal to participants. It also helps us identify strategies that people use to reduce their burden and that might be compromizing their effectiveness at achieving their ultimate goal; we can then take steps to reduce the likelihood that they will resort to the compromizing strategies. Secondly, we have a framework to guide evaluation of the interview, such as looking for evidence of differentiation. Finally, we can generate testable hypotheses about details of the processes that patients actually use in order to arrive at their preferred treatment that can, in turn, help us to be more effective at encouraging productive strategies and compensating for those that are counter‐productive.
We recognize that, because the focus of this study was to determine in principle if the method warrants further development, we are limited in the extent that we can generalize these results. For example, we used only one interviewer in the study, which leaves open the possibility that the results could be affected by interviewer bias. In addition, our participants are men who volunteered to participate in the project, which leaves open the possibility of volunteer bias. The positive nature of these results suggest that we should continue to develop the aid and address its potential limitations in future studies.
We conclude that our decision aid appears to meet its goals for surrogate patients considering a hypothetical treatment decision well enough to test its ability to help actual patients making real treatment decisions. The ability of the aid to accommodate the variability that we expect from the patients, both in the particular information that they need and in how each of those pieces of information affects their decisions, is a particular strength of the decision aid design. It is also valuable because the method provides some insight into the decision processes that patients use as they are occurring and the insight may, in turn, be useful in making the aid even more helpful to the patients.
Acknowledgements
We would like to thank Dr Ola Svenson for discussions about his theory and for his comments on an earlier draft of this paper. Dr Deb Feldman‐Stewart is supported by an Ontario Ministry of Health Career Scientist Award. The project was supported in part by the National Cancer Institute of Canada and the Canadian Cancer Society. Part of the results were presented at the 68th Annual meeting of the Royal College of Physicians and Surgeons, September 1999 and at the 21st Annual Meeting of the Society of Medical Decision Making, October 1999.
References
- 1. O'Connor AM, Fiset V, Rostom A et al Decision Aids for People Facing Health Treatment or Screening Decisions. Cochrane Collaboration: Consumers and Communication Cochrane Review Group. http://www.cochrane.org/cochrane/revabstr [DOI] [PubMed]
- 2. O'Connor AM, Fiset V, DeGrasse C et al Decision aids for patients considering options affecting cancer outcomes: evidence of efficacy and policy implications. Journal of National Cancer Institute Monographs, 1999; 25 : 67–80. [DOI] [PubMed] [Google Scholar]
- 3. Molenaar S, Sprangers MAG, Postma‐Schuit FCE et al Feasibility and effects of decision aids. Medical Decision Making, 2000; 20 : 112–127. [DOI] [PubMed] [Google Scholar]
- 4. Svenson O. Eliciting and analysing verbal protocols in process studies of judgment and decision making. In: Montgomery H, Svenson O (eds). Process and Structure in Human Decision Making. Chichester: John Wiley & Sons, 1989: 65–81.
- 5. Beach LR & Potter RE. The pre‐choice screening of options. Acta Psychologica, 1992; 81 : 115–126. [Google Scholar]
- 6. Montgomery H. Toward a perspective theory of decision making and judgment. Acta Psychologica, 1994; 87 : 155–178. [DOI] [PubMed] [Google Scholar]
- 7. Reyna VF & Brainerd CJ. Fuzzy‐trace theory and framing effects in choice. Gist extraction, truncation, and conversion. Journal of Behavioral Decision Making, 1991; 4 : 249–262. [Google Scholar]
- 8. Stevenson MK, Busemeyer JR, Naylor JC. Judgement and decision‐making theory. In: Dunnette MD, Hough LM (eds). Handbook of Industrial and Organizational Psychology. Palo Alto, California: Consulting Psychologists Press Inc., 1990: 283–274.
- 9. Svenson O. Differentiation and consolidation theory of human decision making: a frame of reference for the study of pre‐ and post‐decision processes. Acta Psychologica, 1992; 80 : 143–168. [Google Scholar]
- 10. Svenson O & Hill T. Turning prior disadvantages into advantages: differentiation and consolidation in real‐life decision making. In: Rayner R, Crozier WR, Svenson O (eds). Decision Making: Cognitive Models and Explanations. London: Routledge, 1997, 218–232.
- 11. Fleming C, Wasson JH, Albertsen PC, Barry MJ, Wennberg JE. A decision analysis of alternative treatment strategies for clinically localized prostate cancer. Journal of the American Medical Association, 1993; 269 : 2650–2658. [PubMed] [Google Scholar]
- 12. O'Rourke ME & Germino BB. Prostate cancer treatment decisions: a focus group exploration. Oncology Nursing Forum, 1998; 25 : 97–104. [PubMed] [Google Scholar]
- 13. O'Rourke ME. Narrowing the options: the process of deciding on prostate cancer treatment. Cancer Investigation, 1999; 17 : 349–359. [DOI] [PubMed] [Google Scholar]
- 14. Norman DA & Bobrow DG. On data‐limited and resource‐limited processes. Cognitive Psychology, 1975; 7 : 44–64. [Google Scholar]
- 15. Feldman‐Stewart D, Brundage M, McConnell B et al Information that patients with early‐stage prostate cancer need for their treatment decisions? British Journal of Urology International, 2001; 87 : 218–223. [DOI] [PubMed] [Google Scholar]
- 16. Ausabel DP. Educational Psychology: a Cognitive View New York: Holt Rinehart, & Winston, 1968.
- 17. Klayman J. Analysis of predecisional information search patterns. In: Humphreys P, Svenson O, Vari A et al (eds). Analysing and Aiding Decision Processes. Amsterdam: North‐Holland Publishing Co., 1983: 401–414.
- 18. Payne JW. Task complexity and contingent processing in decision making: an information search and protocol analysis. Organizational Behavior and Human Performance, 1976; 16 : 366–387. [Google Scholar]
- 19. Llewellyn‐Thomas HA, McGreal MJ, Thiel EC, Fine S, Erlichman C. Patients’ willingness to enter clinical trials: measuring the association with perceived benefit and preference for decision participation. Social Science and Medicine, 1991; 32 : 35–42. [DOI] [PubMed] [Google Scholar]
- 20. Brundage MD, Davidson JR, Mackillop W, Feldman‐Stewart D, Groome P. Using a treatment‐tradeoff method to elicit preferences for the treatment of locally advanced non‐small cell lung cancer. Medical Decision Making, 1998; 18 : 256–267. [DOI] [PubMed] [Google Scholar]
- 21. Feldman‐Stewart D, Kocovski N, McConnell B, Brundage M, Mackillop W. Perception of quantitative information for treatment decisions. Medical Decision Making, 2000; 20 : 228–238. [DOI] [PubMed] [Google Scholar]
- 22. Gigerenzer G. Why the distinction between single‐event probabilities and frequencies is important for psychology (and vice versa). In: Wright G, Ayton P (eds). Subjective Probability. Chichester: John Wiley & Sons, 1994, 129–161.
- 23. Gigerenzer G & Hoffrage U. How to improve Bayesian reasoning without instruction: frequency formats. Psychological Review, 1995; 102 : 684–704. [Google Scholar]
- 24. National Cancer Institute of Canada . Canadian Cancer Statistics 1999. Toronto: National Cancer Institute of Canada, 1999.
- 25. Van Manen L, Feldman‐Stewart D, Brundage MD. Treatment attributes important to the treatment decision for early‐stage prostate cancer: A comparison between men‐at‐risk and patients. Clinical and Investigative Medicine, 2000; 23 : S7–S7. [Google Scholar]
- 26. Degner LF & Sloan JA. The control preferences scale. Canadian Journal of Nursing Research, 1997; 29 : 21–43. [PubMed] [Google Scholar]
- 27. O'Connor AM. Validation of a decisional conflict scale. Medical Decision Making, 1995; 15 : 25–30. [DOI] [PubMed] [Google Scholar]
- 28. Feldman‐Stewart D, Brundage MD, Hayter C et al What questions do patients with curable prostate cancer want answered? Medical Decision Making, 2000; 20 : 7–19. [DOI] [PubMed] [Google Scholar]
- 29. Slovic P. An Invitation to Cognitive Science: Thinking, Volume 3. Cambridge, Massachusetts: MIT Press, 1990.


