Abstract
Annual surveillance coronary angiograpyhy to screen for graft coronary vasculopathy is routine practice after orthotopic heart transplantation. Traditionally, this is performed with direct coronary angiography using static single-plane or biplane angiography. Recently, technological advances have made it possible to perform dual-axis rotational coronary angiography (RA). This technique differs from standard static single-plane or biplane angiography in that a single detector is preprogrammed to swing through a complex 80° arc during a single injection. It has the advantage of providing a perspective of the vessels from a full arc of images rather than from one or two static images per contrast injection. The current study evaluated two coronary angiography techniques used consecutively at a single center to evaluate pediatric heart transplant recipients for graft coronary vasculopathy. A total of 23 patients underwent routine coronary angiography using both biplane static coronary angiography (BiP) and RA techniques at the Children's Hospital of Wisconsin from February 2009 to September 2010. Demographic and procedure data were collected from each procedure and analyzed for significance utilizing a Wilcoxon rank sum test. No significant demographic or procedural differences between the BiP and the RA procedures were noted. Specific measures of radiation dose including fluoroscopy time and dose area product were similar among the imaging techniques. The findings show that RA can be performed safely and reproducibly in pediatric heart transplant recipients. Compared with standard BiP, RA does not increase radiation exposure or contrast use and in our experience has provided superior angiographic imaging for the evaluation of graft coronary vasculopathy.
Keywords: Coronary angiography, Graft vasculopathy, Heart transplantation
Introduction
Graft vasculopathy (GV) is one of the greatest challenges to long-term survival for cardiac transplant recipients. It affects more than one third of transplant recipients within 10 years and is the most frequent cause of death 5 years or more after transplantation [7]. Furthermore, the 5-year survival rate after identification of GV across all age groups is ~50 % [7]. Annual surveillance coronary angiography to screen for GV is routine practice after orthotopic heart transplantation. Traditionally, this is performed with direct coronary angiography using static single-plane or biplane (BiP) angiography.
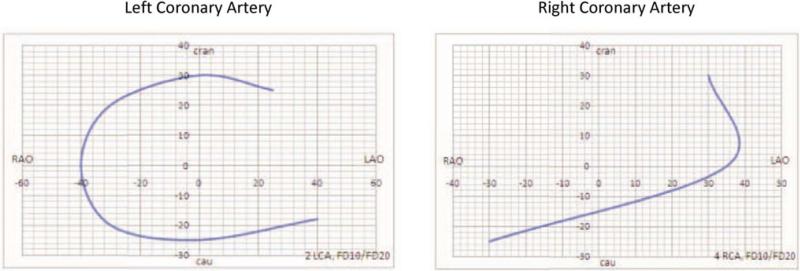
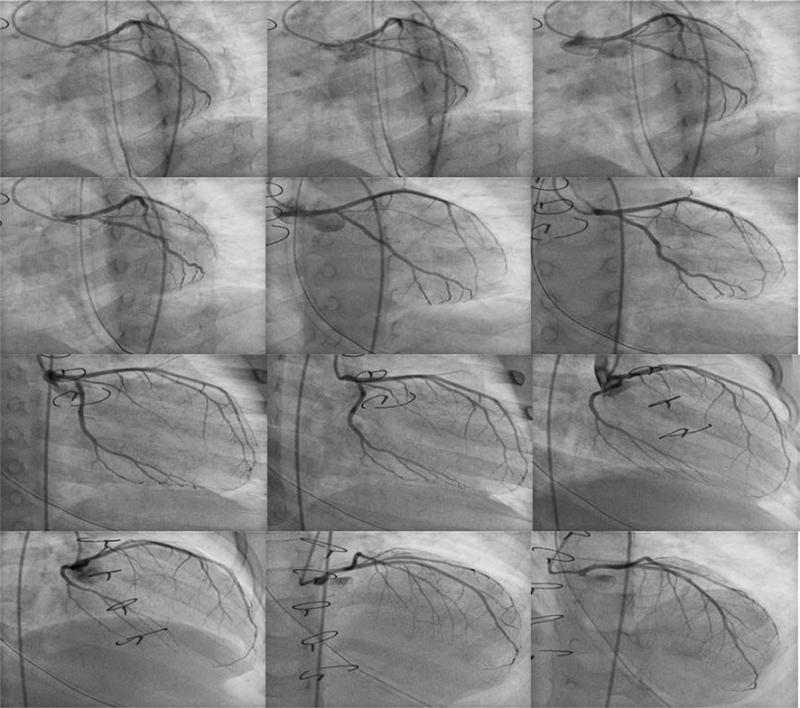
Recently, technological advances have made it possible to perform dual-axis rotational coronary angiography (RA) [5, 6, 8, 9]. This technique differs from standard static single-plane or BiP angiography in that a single detector is preprogrammed to swing through a complex 80° arc during a single injection. The gantry moves through a constantly changing left anterior oblique (LAO)/right anterior oblique (RAO) and cranial/caudal trajectory that encompasses all the traditional coronary angiographic views (Fig. 1). It has the advantage of providing a perspective of the vessels from a full arc of images rather than from one or two static images per contrast injection (Fig. 2).
Fig. 1.
Gantry trajectory during dual-axis rotational coronary angiography
Fig. 2.
Dual-axis rotational coronary angiography of the LCA. A series of still images from a single injection of the LCA is shown, starting from the LAO caudal trajectory (top left) and ending at the LAO cranial position (bottom right)
We transitioned to RA after moving into a newly equipped hybrid catheterization laboratory in February 2010 because we thought RA had the potential to provide much more information per angiogram. This report describes our experience, comparing RA with BiP angiography in our pediatric transplant recipients.
Methods
A total of 23 patients underwent routine coronary angiography using both BiP and RA techniques at the Children's Hospital of Wisconsin from February 2009 to September 2010. Our practice before February 2010 was to perform BiP angiography at least annually for all pediatric heart transplant recipients.
In February 2010, we moved into a new state-of-the-art biplane hybrid catheterization suite (Phillips Allura bi-plane FD10/10 angiographic equipment with XperSwing rotational angiography software) and began performing RA routinely. Each patient in this series had undergone coronary angiography using both BiP and RA techniques consecutively ~1 year apart.
In our catheterization lab, all procedures are performed with anesthesia administered by a dedicated pediatric cardiac anesthesiologist. The anesthesia technique is determined by the individual anesthesiologist. Generally, younger patients undergo a general anesthetic with airway stabilization using an endotracheal tube or laryngeal mask airway, whereas older patients undergo moderate intravenous sedation without airway stabilization.
Biplane Coronary Angiography
The standard BiP angiography protocol consisted of two biplane angiographic views of the left coronary artery (LCA) using RAO-cranial/LAO-caudal and RAO-caudal/LAO-cranial angulation and one biplane view of the right coronary artery (RCA) using RAO/LAO angulation. This technique requires the patient's arms to be positioned above the head, a common practice in pediatric catheterization laboratories.
All imaging was performed at an acquisition rate of 30 frames per second. The field of view was set by the operator to optimize coronary tree visualization. Two LCA injections and one RCA injection lasting ~3 s each were performed.
Dual-Axis Rotational Coronary Angiography
The standard RA protocol consisted of a single injection in the LCA and RCA while a single gantry moved rapidly through a complex 80° arc that encompassed the traditional coronary angiographic views. Because only one gantry was used, the patient's arms did not need to be elevated above the head and were left at the patient's side. All imaging was performed with an acquisition rate of 15 frames per second. The field of view was set by the operator to optimize coronary tree visualization. An RA of the LCA and RCA requires ~5.3 and 3.7 s, respectively.
Institutional review board approval was obtained before review of the patients’ hospital and outpatient records. Demographic and procedural data were collected and analyzed for significance using a Wilcoxon rank sum test. A p value of 0.05 or lower was defined as significant. All data are presented as medians with 25th and 75th quartiles when appropriate and are summarized in Table 1.
Table 1.
Comparison between biplane and rotational angiography groups
| Biplane median (25th–75th percentile) | Rotational median (25th–75th percentile) | p value | |
|---|---|---|---|
| Age (years) | 11.1 (4.5–16.7) | 12.6 (5.5–17.7) | NS |
| Weight (kg) | 32.6 (15.3–52.8) | 36.0 (17.1–62.0) | NS |
| BSA (m2) | 1.1 (0.6–1.6) | 1.2 (0.7–1.8) | NS |
| Fluro time (min) | 10.2 (8.7–13.4) | 9.3 (7.3–11.9) | NS |
| DAP (mGy/cm2) | 14,000 (4,000–35,000) | 11,711 (6,149–27,295) | NS |
| DAP/kg | 405 (302–683) | 353 (269–615) | NS |
| Total contrast (mL) | 15 (12–15) | 17 (14–20) | NS |
NS not significant, BSA body surface area, DAP dose area product
Results
Patient Characteristics
The patients ranged in age from 1.1 to 27.8 years and weighed 8.3–92.2 kg. Because these represented serial consecutive annual studies for each patient, the BiP group was younger (median age, 11.7 years; range, 4.5–16.7 years) than the RA group (median age, 12.7 years; range, 5.5–17.7 years). The two groups did not differ significantly in terms of weight or body surface area (BSA).
Radiation Dose
Fluoroscopy time, dose area product (DAP), and dose area product per kilogram (DAP/kg) were selected as specific measures of radiation dosing. As a standard measure of radiation dose delivered, DAP was available for all procedures. Because the radiation dose increases exponentially with increased patient mass, we attempted to correct for this by indexing the DAP according to weight in kilograms.
The median fluoroscopy time was shorter in the RA group (9.3 min; range, 7.3–11.9 min) than in the BiP group (10.2 min; range, 8.7–13.4 min), but the difference was not statistically significant. The two groups did not differ significantly in terms of DAP (BiP DAP, 14,000 m Gy cm−2; range, 4,000–35,000 m Gy cm−2 vs. RA DAP, 11,711 m Gy cm−2; range, 6,149–27,295 m Gy cm−2; nonsignifcant difference). When DAP was corrected for patient mass, again, no significant difference was noted (BiP DAP/kg, 405; range, 302–683) vs RA DAP/kg, 353; range, 269–615; nonsignificant difference).
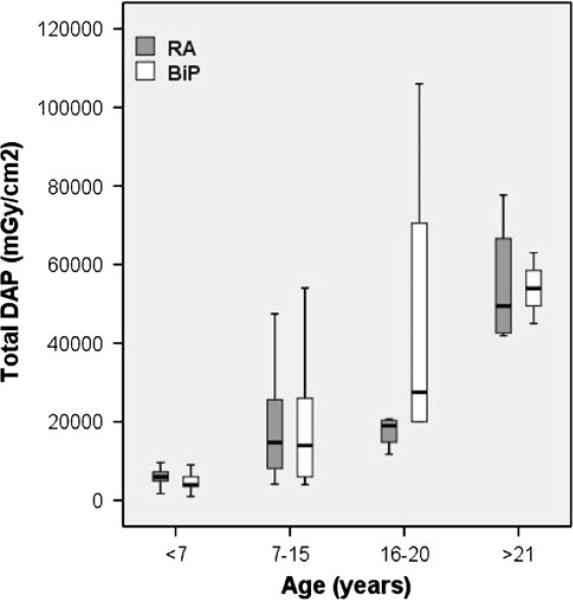
For better detection of small differences in radiation dose between the BiP and RA techniques, we divided our patients into four groups in an attempt to keep body size and mass similar among subgroups. The patients were grouped as follows according to age: younger than 7 years, 7–15 years, 16–20 years, and older than 21 years. Figure 3 demonstrates that although DAP increased markedly in both the BiP and RA groups as age increased, it did not differ significantly between the BiP and RA groups for each individual age group (p > 0.2).
Fig. 3.
Bar graph demonstrating the total dose area product (DAP) stratified by age group. There was no significant difference between the biplane static coronary angiography (BiP) and dual-axis rotational coronary angiography (RA) techniques among the age groups (p > 0.2)
Contrast
The amount of contrast used was similar among the patients undergoing BiP angiography or RA. The BiP median contrast dose was 15 mL (range, 12–15 mL), and the RA median contrast dose was 17 mL (range, 14–20 mL). The difference was not significant.
Graft Vasculopathy
One patient in the RA group was noted to have mild left circumflex coronary artery GV, which had been noted a year earlier with the use of BiP angiography. A second patient in the BiP group had severe multivessel GV noted initially with BiP angiography. This patient underwent retransplantation during the study period and showed normal coronary artery angiography when reimaged with RA.
Arrhythmias
No patients experienced significant arrhythmias or hemodynamic changes during RA or BiP angiography. No episodes of bradycardia or junctional rhythm during or after coronary angiography were noted. A single patient in the RA group exhibited minor and transient ST segment depression on the electrocardiogram (ECG) after RCA angiography. A single patient in the BiP group showed moderate but transient ST segment depression on the ECG after LCA angiography. This patient was noted to have severe multivessel GV and underwent retransplantation during the study period.
Discussion
This report describes our initial experience using a new coronary angiographic technique, dual-axis rotational coronary angiography, to screen for GV in pediatric heart transplant recipients. We performed both standard BiP angiography and RA in 23 heart transplant recipients consecutively over an 18-month period. We found that RA can be performed safely and reproducibly in pediatric patients of all ages. In addition, the use of RA did not increase radiation exposure, as measured by fluoroscopy time, or DAP compared with BiP angiography. Finally, RA did not require the use of additional radiographic contrast compared with BiP angiography.
To the best of our knowledge, this is the first report to describe the use of RA in the pediatric population. According to previous reports, when RA is used in adult catheterization laboratories to screen for coronary artery disease, it provides better imaging with less radiographic contrast and a smaller radiation dose than standard single plane coronary angiography [1–3, 9–11].
We did not demonstrate a similar reduction in radiation dose or contrast use in our patients, but this is explained by the differences in technique between biplane coronary angiography and single-plane coronary angiography. For biplane coronary angiography, we performed two left coronary injections using the anteroposterior (AP) and lateral gantries in two different sets of orthogonal planes and one right coronary injection, again using both the AP and lateral gantries in appropriate orthogonal planes. Altogether, three injections of 3 s duration were performed, and six cine runs were recorded during our standard BiP angiography. This was in contrast to the four to six left coronary and two to three right coronary injections performed during standard single-plane coronary angiography.
Standard BiP imaging reduces the number of coronary injections from as many as nine to three, with a subsequent reduction in radiation dose and contrast administration compared with standard single-plane coronary angiography. Therefore, it is not surprising that no additional reduction in radiation dose or contrast administration occurs during a change from BiP angiography to RA.
In our opinion, RA provides better imaging of the right and left coronary tree than standard BiP angiography. Between 60 and 80 different images are obtained with each coronary angiogram as the gantry rotates through the preprogrammed arc. The operator is able to view each coronary artery and its branches in multiple views, reducing the effects of vessel foreshortening and overlap. We believe these additional images improve our ability to detect early graft coronary disease.
For cases in which suspicious lesions are noted, we have performed static coronary angiograms in the optimal imaging plane, as determined by RA, to confirm suspected graft coronary stenosis. Confirmatory static coronary angiography was not necessary in the current patient series but has been used occasionally for subsequent patients in our experience to date.
An additional benefit of RA over BiP angiography is the convenience of leaving the arms down at the patient's sides rather than needing to raise them above the patient's head. The ability to keep the arms at the patient's sides has led to the unexpected benefit of being able to perform more of our recent procedures in younger children with conscious sedation rather than general endotracheal anesthesia because we no longer need to position the arms uncomfortably above the patient's head.
Initially, we were concerned that the longer coronary injection times could lead to increased hemodynamic or arrythmogenic events. Multiple studies with adult patients have demonstrated the safety of injections up to 7.2 s long [1, 4, 9, 11]. Young children may, however, be more susceptible to these complications due to their smaller coronary arteries. Our patients, including a 2½-year old-girl weighing 12 kg, did not experience any significant hemodynamic changes or arrhythmias during RA. Our experience, however, should be regarded with caution because none of our patients who underwent RA had severe GV. A patient with severe GV may be at greater risk for the development of ischemia-induced bradycardia or ventricular tachycardia due to the longer injection times required for RA. More experience with this technique in children is required for full characterization of this risk.
The limitations of our study relate to its retrospective nature and the relatively small sample size. No objective measurements were made to quantify the improved image quality of the rotational coronary angiograms, but all the operators qualitatively thought the images provided more diagnostic information.
In conclusion, RA can be performed safely and reproducibly for pediatric heart transplant recipients. Compared with standard BiP angiography, RA does not increase radiation exposure or contrast use and in our experience provides superior angiographic imaging for the evaluation of graft coronary vasculopathy.
Supplementary Material
Acknowledgments
The authors thank Pippa Simpson, Ph.D., and Yumi Cao, M.S., for their statistical assistance.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00246-012-0494-2) contains supplementary material, which is available to authorized users.
Contributor Information
Todd M. Gudausky, Division of Pediatric Cardiology, Medical College of Wisconsin, The Herma Heart Center, Children's Hospital of Wisconsin, 9000 W. Wisconsin Avenue, Milwaukee, WI 53226, USA
Andrew N. Pelech, Division of Pediatric Cardiology, Medical College of Wisconsin, The Herma Heart Center, Children's Hospital of Wisconsin, 9000 W. Wisconsin Avenue, Milwaukee, WI 53226, USA
Gail Stendahl, Division of Pediatric Cardiology, Medical College of Wisconsin, The Herma Heart Center, Children's Hospital of Wisconsin, 9000 W. Wisconsin Avenue, Milwaukee, WI 53226, USA.
Kathryn Tillman, Division of Pediatric Cardiology, Medical College of Wisconsin, The Herma Heart Center, Children's Hospital of Wisconsin, 9000 W. Wisconsin Avenue, Milwaukee, WI 53226, USA.
Judy Mattice, The Herma Heart Center, Children's Hospital of Wisconsin, 9000 W. Wisconsin Avenue, Milwaukee, WI 53226, USA.
Stuart Berger, Division of Pediatric Cardiology, Medical College of Wisconsin, The Herma Heart Center, Children's Hospital of Wisconsin, 9000 W. Wisconsin Avenue, Milwaukee, WI 53226, USA.
Steven Zangwill, Division of Pediatric Cardiology, Medical College of Wisconsin, The Herma Heart Center, Children's Hospital of Wisconsin, 9000 W. Wisconsin Avenue, Milwaukee, WI 53226, USA.
References
- 1.Akhtar M, Vakharia KT, Mishell J, Gera A, Ports TA, Yeghiazarians Y, Michaels AD. Randomized study of the safety and clinical utility of rotational vs standard coronary angiography using a flat-panel detector. Catheter Cardiovasc Interv. 2005;66:43–49. doi: 10.1002/ccd.20442. [DOI] [PubMed] [Google Scholar]
- 2.Empen K, Kuon E, Hummel A, Gebauer C, Dorr M, Konemann R, Hoffmann W, Staudt A, Weitmann K, Reffelmann T, Felix SB. Comparison of rotational with conventional coronary angiography. Am Heart J. 2010;160:552–563. doi: 10.1016/j.ahj.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Garcia JA, Agostoni P, Green NE, Maddux JT, Chen SY, Messenger JC, Casserly IP, Hansgen A, Wink O, Movassaghi B, Groves BM, van den Heuvel P, Verheye S, Van Langenhove G, Vermeersch P, Van den Branden F, Yeghiazarians Y, Michaels AD, Carroll JD. Rotational versus standard coronary angiography: an image content analysis. Catheter Cardiovasc Interv. 2009;73:753–761. doi: 10.1002/ccd.21918. [DOI] [PubMed] [Google Scholar]
- 4.Garcia JA, Chen SY, Messenger JC, Casserly IP, Hansgen A, Wink O, Movassaghi B, Klein AJ, Carroll JD. Initial clinical experience of selective coronary angiography using one prolonged injection and a 180 degrees rotational trajectory. Catheter Cardiovasc Interv. 2007;70:190–196. doi: 10.1002/ccd.21054. [DOI] [PubMed] [Google Scholar]
- 5.Garcia JA, Movassaghi B, Casserly IP, Klein AJ, Chen SY, Messenger JC, Hansgen A, Wink O, Groves BM, Carroll JD. Determination of optimal viewing regions for x-ray coronary angiography based on a quantitative analysis of 3D reconstructed models. Int J Cardiovasc Imaging. 2009;25:455–462. doi: 10.1007/s10554-008-9402-5. [DOI] [PubMed] [Google Scholar]
- 6.Hudson PA, Klein AJ, Kim MS, Wink O, Hansgen A, Casserly IP, Messenger JC, James Chen SY, Carroll JD, Garcia JA. A novel dual-axis rotational coronary angiography evaluation of coronary artery disease: case presentation and review. Clin Cardiol. 2010;33:E16–E19. doi: 10.1002/clc.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirk R, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dobbels F, Rahmel AO, Stehlik J, Hertz MI. The registry of the International Society for Heart and Lung Transplantation: fourteenth pediatric heart transplantation Report–2011. J Heart Lung Transplant. 2011;30:1095–1103. doi: 10.1016/j.healun.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Klein AJ, Garcia JA. Rotational coronary angiography. Cardiol Clin. 2009;27:395–405. doi: 10.1016/j.ccl.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Klein AJ, Garcia JA, Hudson PA, Kim MS, Messenger JC, Casserly IP, Wink O, Hattler B, Tsai TT, Chen SY, Hansgen A, Carroll JD. Safety and efficacy of dual-axis rotational coronary angiography vs standard coronary angiography. Catheter Cardiovasc Interv. 2011;77:820–827. doi: 10.1002/ccd.22804. [DOI] [PubMed] [Google Scholar]
- 10.Kuon E, Niederst PN, Dahm JB. Usefulness of rotational spin for coronary angiography in patients with advanced renal insufficiency. Am J Cardiol. 2002;90:369–373. doi: 10.1016/s0002-9149(02)02491-8. [DOI] [PubMed] [Google Scholar]
- 11.Maddux JT, Wink O, Messenger JC, Groves BM, Liao R, Strzelczyk J, Chen SY, Carroll JD. Randomized study of the safety and clinical utility of rotational angiography versus standard angiography in the diagnosis of coronary artery disease. Catheter Cardiovasc Interv. 2004;62:167–174. doi: 10.1002/ccd.20036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.