Abstract
Invasive fungal infections are on the rise due to an increased population of critically ill patients as a result of HIV infections, chemotherapies, and organ transplantations. Current antifungal drugs are helpful, but insufficient in addressing the problem of drug-resistant fungal infections. Thus, there is a growing need for novel antimycotics that are safe and effective. The ebselen scaffold has been evaluated in clinical trials and has been shown to be safe in humans. This makes ebselen an attractive scaffold for facile translation from bench to bedside. We have recently reported a library of ebselen-inspired ebsulfur analogues with antibacterial properties, but their antifungal activity has not been characterized. Herein, we repurposed ebselen, ebsulfur, and 32 additional ebsulfur analogues as antifungal agents by evaluating their antifungal activity against a panel of 13 clinically relevant fungal strains. The effect of induction of reactive oxygen species (ROS) by three of these compounds was evaluated. Their hemolytic and cytotoxicity activities were also determined using mouse erythrocytes and mammalian cells. The MIC values of these compounds were in the ranges of 0.02–12.5 µg/mL against the fungal strains tested. Notably, yeast cells treated with our compounds showed the accumulation of ROS, which may further contribute to the growth inhibitory effect against fungi. This study provides new lead compounds for the development of antimycotic agents.
Keywords: Aspergillus, Benzisothiazolinone, Candida, Ebselen, ROS production
Graphical Abstract
Promising antifungals: The ebselen compound was previously shown to be safe during phase I (U.S.A) and phase III (Japan) clinical trials. Herein, ebselen, ebsulfur, and 32 ebsulfur derivatives were found to display potent antifungal activity against a panel of clinically relevant fungal strains. SAR analysis was done to identify analogues with activity equivalent or better than those of the clinically used antifungal drugs. They were then evaluated and found to be acceptable in terms of cytotoxicity against mammalian cells.
INTRODUCTION
Fungal infections have become an emerging public health threat mainly due to the increasing size of the immunocompromised patient population.1 This population includes patients with AIDS, primary immune deficiency, and those who are immunocompromised due to chemotherapy or organ and bone marrow transplantation. Globally, Candida species are the predominant causes of invasive systemic fungal infections with the prevalence reported at 6.9 cases per 1000 patients.2 In the United States, Candida infections rank fourth among all hospital-acquired systemic infections in intensive care units.3 In most population-based studies, Candida infections represent the seventh to tenth most common bloodstream infections.4 Additionally, many patients are now infected with other fungal species including Aspergillus fumigatus,5 Aspergillus nidulans,6 and Cryptococcus neoformans.7
Common therapeutic classes used to treat systemic fungal infections include azoles (e.g., fluconazole (FLC), itraconazole (ITC), posaconazole (POS), and voriconazole (VOR)), polyenes (e.g., amphotericin B (AmB), nystatin (NYS), and candicidin (CAN)), and echinocandins (e.g., micafungin, caspofungin, and anidulafungin).4 These drugs function by different mechanisms of action: (i) inhibition of the cytochrome P450 enzyme 14α-demethylase (azoles),8 (ii) introduction of transmembrane channel leading to monovalent ion leakage (polyenes),9, 10 and (iii) inhibition of synthesis of glucan in the fungal cell wall via the enzyme 1,3-β-glucan synthase (echinochandins).11
Due to improper usage of these antifungal agents, more drug-resistant fungal strains have evolved.12, 13 Specifically, these improper usages include insufficient dosages and durations of treatment.14 Additionally, new evidence suggests that antibacterials also contribute to this development of fungal resistance.15 Overall, fungal resistance is still relatively uncommon, but this problem is on the rise and expected to become a major healthcare problem. Thus, we have a critical need for the development of novel antifungal compounds.
Currently, three strategies to overcome antifungal drug resistance have been employed. The first strategy is the development of compounds with novel mechanisms of action distinct from previous antifungal agents. For instance, compound E1210 was discovered as a novel first-in-class antifungal compound by the Tsukuba Research Laboratories of Eisai Co., Ltd. This compound was discovered to inhibit fungal glycosylphosphatidylinositol (GPI) biosynthesis and validated in murine models of candidiasis, aspergillosis, and fusariosis.16 The second strategy is the combination of two antifungal agents. In the literature, there have been plenty of examples using two compounds in conjunction to produce synergistic antifungal activity and reduce resistance as well as toxicity.17, 18 Specifically, in patients diagnosed with cryptococcal meningitis, the combination therapy of flucytosine and AmB was shown to be essential for successful clinical outcomes.19 Recently, it was also found that a combination of azoles and analogues of the aminoglycoside antibiotics tobramycin and kanamycin B resulted in favorable synergistic effects against drug-resistant Candida albicans strains.20, 21 Lastly, the third strategy is the repurposing of existing compounds for new applications. For example, the decongestant drug octodrine was identified as a broad-spectrum antifungal compound.22 In this study, we employed a combination of the first and third strategy to address the problem of antifungal drug resistance. We originally attempted to utilize all three strategies, but found that our novel compounds did not display synergy with currently used antifungal agents.
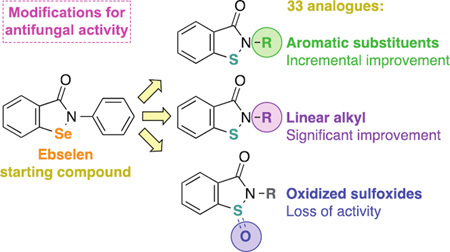
Ebselen (1, Fig. 1) is an organoseleno compound, which has completed phase I clinical trial for general safety in human use. Ebselen (1) has very diverse therapeutic applications and has been studied in several clinical trials.23 During the phase I study, up to 1,600 mg of ebselen (1) was dosed orally and found to be very well tolerated compared to placebo in 32 healthy male and female subjects.24 Ebselen (1) is currently in phase II clinical trials for the treatment of chemotherapy-induced hearing loss and Meniere’s disease (http://clinicaltrials.gov). Furthermore, ebselen (1) completed a 300-patient phase III clinical trial for cerebral ischemia in Japan.25 The ebsulfur or 1,2-benzisothiazol-3(2H)-one scaffold has been demonstrated to have a very narrow spectrum of antibacterial activity (only really being active against methicillin-resistant Staphylococcus aureus (MRSA)), in our previous work.26 We hypothesized that ebsulfur (2a, Fig. 1) would have a similar safety profile compared to that of ebselen (1). This scaffold is interesting because it is structurally very similar to ebselen (1, Fig. 1). Therefore, we hypothesized that ebsulfur (2a, Fig. 1) and its analogues would have a safety profile comparable to that of ebselen (1). In the literature, the antifungal activity of the ebsulfur scaffold had not been well characterized. Herein, we explored the antifungal properties of ebselen (1) as well as ebsulfur (2a) and 32 of its analogues (2b–4n, Fig. 1) by using a combination of minimum inhibitory concentration (MIC) study, time-kill study, and reactive oxygen species (ROS) assays. The safety of these compounds was also assessed and compared to ebselen (1) via mammalian cytotoxicity and hemolytic assays. Our study provides us with a better understanding of the structure-activity-relationship (SAR) of ebselen (1) and the 1,2-benzisothiazol-3(2H)-one scaffold as well as their potential as a new class of antifungal agents.
Figure 1.
Chemical structures of our library featuring ebselen (1), ebsulfur (2a), and 32 ebsulfur analogues.
RESULTS AND DISCUSSION
Antifungal activity
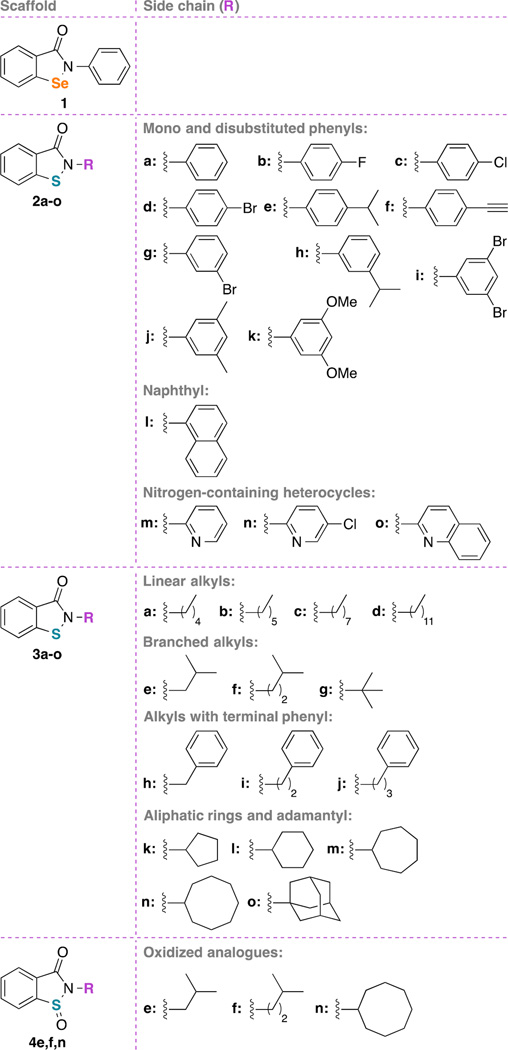
Compounds 1–3a–o and 4e–n were evaluated for whole-cell activity against a panel of clinically relevant fungal strains (Table 1). Our library of compounds featured ebselen (1) and ebsulfur (2a) as the main scaffolds. From the ebsulfur scaffold, the library was further organized into three sub-series: analogues with aromatic substituents (2 series, 2a–o), analogues with aliphatic substituents (3 series, 3a–o), and oxidized sulfoxide analogues (4 series, 4e, 4f, and 4n). Series 2 contained aromatic substituents such as mono- and disubstituted phenyl rings (2a–k), naphthyl (2l), and nitrogen-containing aromatic heterocycles (2m–o). Series 3 contained analogues with substituents such as linear alkyl chains (3a–d), branched alkyl chains (3e–g), alkyl with terminal phenyl ring (3h–j), aliphatic rings (3k–n), and adamantyl (3o). In our previous work, we have verified that all the compounds tested were at least 95% pure by NMR and HRMS.26 We used the commercially available AmB, FLC, ITC, POS, and VOR as positive controls. The MIC values listed for the controls were either tested herein or acquired from some of our previously published manuscripts on unrelated antifungal agents.27 For the controls, AmB, as expected, was the most active against both Candida and Aspergillus strains with MIC values ranging from 0.98–15.6 µg/mL. Despite its potent antifungal activity, it should be noted that AmB, even with the liposomal formulations, has been well known for its severe and potentially lethal side effects such as nephrotoxicity and hypokalemia.28, 29 FLC, the most popular and well-tolerated FDA approved antifungal agent, was fairly inactive against our panel of fungal strains with MIC values mostly from >31.2–>125 µg/mL (except against Candida parapsilosis ATCC 22019 (strain J), MIC = 1.95 µg/mL). ITC, POS, and VOR displayed similar activity against our strains with MIC values mostly ranging from <0.03–31.2 µg/mL. The azole compounds, however, are potent inhibitors of human cytochrome P450 enzymes, which somewhat limit their applications due to drug-drug interactions with co-administered drugs.30 ITC and VOR are also generally not as well tolerated as FLC.31 To effectively evaluate the activities of our compounds, we define poor, good, very good, and excellent activity as ≥12.5 µg/mL, 1.56–6.25 µg/mL, 0.39–0.78 µg/mL, and ≤0.10 µg/mL, respectively. In our broth dilution assays, the concentration of DMSO used is less than 1%, which was previously experimentally determined to not cause any DMSO-related cytotoxicity effect.
Table 1. In vitro susceptibility of Candida and Aspergillus species to ebselen (1) and compounds 2a–4n.
MIC valuesa,b (in µg/mL) (and µM in parentheses)c determined for compounds 1–4n and for five control antifungal agents (AmB, FLC, ITC, POS, and VOR) against various yeast strains and filamentous fungi.
| Yeast strains | Filamentous fungi | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cpd # | A | B | C | D | E | F | G | H | I | J | K | L | M |
| 1 | 12.5 (45.6) |
12.5 (45.6) |
12.5 (45.6) |
1.56 (5.69) |
3.13 (11.4) |
>12.5 (45.6) |
12.5 (45.6) |
1.56 (45.6) |
1.56 (5.69) |
6.25 (22.8) |
6.25 (22.8) |
1.56 (5.69) |
1.56 (5.69) |
| 2a | 12.5 (55.0) |
>12.5 (55.0) |
12.5 (55.0) |
3.13 (13.8) |
6.25 (27.5) |
>12.5 (55.0) |
>12.5 (55.0) |
6.25 (27.5) |
3.13 (13.8) |
12.5 (55.0) |
6.25 (27.5) |
6.25 (27.5) |
6.25 (27.5) |
| 2b | 3.13 (12.8) |
6.25 (25.5) |
3.13 (12.8) |
3.13 (12.8) |
3.13 (12.8) |
6.25 (25.5) |
1.56 (6.36) |
6.25 (25.5) |
3.13 (12.8) |
12.5 (12.8) |
6.25 (25.5) |
12.5 (51.0) |
12.5 (51.0) |
| 2c | 3.13 (12.0) |
>12.5 (47.8) |
12.5 (47.8) |
6.25 (23.9) |
6.25 (23.9) |
6.25 (23.9) |
12.5 (47.8) |
6.25 (23.9) |
3.13 (12.0) |
12.5 (47.8) |
6.25 (23.9) |
6.25 (23.9) |
6.25 (23.9) |
| 2d | 3.13 (10.2) |
3.13 (10.2) |
1.56 (5.10) |
3.13 (10.2) |
0.78 (2.55) |
1.56 (5.10) |
3.13 (10.2) |
6.25 (20.4) |
3.13 (10.2) |
6.25 (20.4) |
12.5 (40.8) |
12.5 (40.8) |
12.5 (40.8) |
| 2e | 3.13 (11.6) |
3.13 (11.6) |
3.13 (11.6) |
3.13 (11.6) |
3.13 (11.6) |
6.25 (23.2) |
3.13 (11.6) |
3.13 (11.6) |
3.13 (11.6) |
3.13 (11.6) |
6.25 (23.2) |
6.25 (23.2) |
6.25 (23.2) |
| 2f | 12.5 (49.7) |
12.5 (49.7) |
>12.5 (49.7) |
12.5 (49.7) |
>12.5 (49.7) |
12.5 (49.7) |
12.5 (49.7) |
6.25 (49.7) |
6.25 (49.7) |
12.5 (49.7) |
12.5 (49.7) |
12.5 (49.7) |
12.5 (49.7) |
| 2g | >12.5 (40.8) |
12.5 (40.8) |
12.5 (40.8) |
12.5 (40.8) |
>12.5 (40.8) |
12.5 (40.8) |
12.5 (40.8) |
12.5 (40.8) |
12.5 (40.8) |
12.5 (40.8) |
12.5 (40.8) |
12.5 (40.8) |
12.5 (40.8) |
| 2h | 3.13 (11.6) |
3.13 (11.6) |
3.13 (11.6) |
3.13 (11.6) |
3.13 (11.6) |
3.13 (11.6) |
3.13 (11.6) |
3.13 (11.6) |
3.13 (11.6) |
3.13 (11.6) |
6.25 (23.2) |
12.5 (46.4) |
12.5 (46.4) |
| 2i | 3.13 (8.13) |
6.25 (16.2) |
6.25 (16.2) |
6.25 (16.2) |
6.25 (16.2) |
3.13 (8.13) |
6.25 (16.2) |
6.25 (16.2) |
6.25 (16.2) |
6.25 (16.2) |
12.5 (32.5) |
3.13 (8.13) |
12.5 (32.5) |
| 2j | 3.13 (12.3) |
6.25 (24.5) |
1.56 (6.11) |
12.5 (49.0) |
0.78 (3.05) |
3.13 (12.3) |
3.13 (12.3) |
12.5 (49.0) |
12.5 (49.0) |
12.5 (49.0) |
12.5 (49.0) |
12.5 (49.0) |
12.5 (49.0) |
| 2k | 12.5 (43.5) |
12.5 (43.5) |
3.13 (10.9) |
3.13 (10.9) |
1.56 (5.43) |
12.5 (43.5) |
12.5 (43.5) |
6.25 (21.8) |
6.25 (21.8) |
6.25 (21.8) |
6.25 (21.8) |
6.25 (21.8) |
6.25 (21.8) |
| 2l | 6.25 (22.5) |
12.5 (45.1) |
>12.5 (45.1) |
3.13(11.3 ) |
>12.5 (45.1) |
>12.5 (45.1) |
6.25 (22.5) |
12.5 (45.1) |
6.25 (22.5) |
12.5 (45.1) |
12.5 (45.1) |
12.5 (45.1) |
12.5 (45.1) |
| 2m | 6.25 (27.4) |
6.25 (27.4) |
12.5 (54.8) |
6.25 (27.4) |
6.25 (27.4) |
6.25 (27.4) |
6.25 (27.4) |
12.5 (54.8) |
6.25 (27.4) |
12.5 (54.8) |
12.5 (54.8) |
12.5 (54.8) |
12.5 (54.8) |
| 2n | 12.5 (47.6) |
12.5 (47.6) |
12.5 (47.6) |
>12.5 (47.6) |
12.5 (47.6) |
12.5 (47.6) |
12.5 (47.6) |
>12.5 (47.6) |
>12.5 (47.6) |
>12.5 (47.6) |
>12.5 (47.6) |
>12.5 (47.6) |
>12.5 (47.6) |
| 2o | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 3a | 1.56 (7.05) |
0.39 (1.76) |
0.78 (3.52) |
1.56 (7.05) |
0.39 (1.76) |
0.78 (3.52) |
0.78 (3.52) |
1.56 (7.05) |
0.78 (3.52) |
0.78 (3.52) |
≤0.02 (0.09) |
≤0.02 (0.09) |
≤0.02 (0.09) |
| 3b | 1.56 (6.63) |
1.56 (6.63) |
0.78 (3.31) |
1.56 (6.63) |
0.78 (3.31) |
1.56 (6.63) |
0.78 (3.31) |
1.56 (6.63) |
0.78 (3.31) |
0.78 (3.31) |
≤0.02 (0.08) |
≤0.02 (0.08) |
≤0.02 (0.08) |
| 3c | 6.25 (23.7) |
1.56 (5.92) |
6.25 (23.7) |
3.13 (11.9) |
1.56 (5.92) |
1.56 (5.92) |
1.56 (5.92) |
1.56 (5.92) |
1.56 (5.92) |
0.78 (2.96) |
≤0.02 (0.08) |
≤0.02 (0.08) |
≤0.02 (0.08) |
| 3d | >12.5 (39.1) |
>12.5 (39.1) |
>12.5 (39.1) |
>12.5 (39.1) |
>12.5 (39.1) |
>12.5 (39.1) |
>12.5 (39.1) |
12.5 (39.1) |
>12.5 (39.1) |
>12.5 (39.1) |
>12.5 (39.1) |
>12.5 (39.1) |
>12.5 (39.1) |
| 3e | 0.78 (3.76) |
1.56 (7.53) |
0.78 (3.76) |
0.78 (3.76) |
0.78 (3.76) |
0.78 (3.76) |
1.56 (7.53) |
1.56 (7.53) |
0.39 (1.88) |
0.78 (3.76) |
0.20 (0.96) |
0.02 (0.10) |
0.10 (0.48) |
| 3f | 1.56 (7.05) |
0.78 (3.52) |
1.56 (7.05) |
0.78 (3.52) |
0.78 (3.52) |
0.78 (3.52) |
1.56 (7.05) |
1.56 (7.05) |
0.39 (1.76) |
1.56 (7.05) |
0.20 (0.90) |
0.02 (0.09) |
0.10 (0.45) |
| 3g | 0.78 (3.76) |
1.56 (7.53) |
3.13 (15.1) |
1.56 (7.53) |
1.56 (7.53) |
1.56 (7.53) |
1.56 (7.53) |
1.56 (7.53) |
0.78 (3.76) |
1.56 (7.53) |
0.10 (0.48) |
0.10 (0.48) |
0.10 (0.48) |
| 3h | 0.39 (1.62) |
1.56 (6.46) |
1.56 (6.46) |
1.56 (6.46) |
1.56 (6.46) |
1.56 (6.46) |
0.78 (3.23) |
1.56 (6.46) |
0.39 (1.62) |
0.78 (3.23) |
0.78 (3.23) |
0.39 (1.62) |
0.39 (1.62) |
| 3i | 1.56 (6.11) |
1.56 (6.11) |
1.56 (6.11) |
1.56 (6.11) |
1.56 (6.11) |
1.56 (6.11) |
1.56 (6.11) |
1.56 (6.11) |
1.56 (6.11) |
1.56 (6.11) |
0.78 (3.05) |
0.20 (0.78) |
0.20 (0.78) |
| 3j | 6.25 (23.2) |
3.13 (11.6) |
6.25 (23.2) |
3.13 (11.6) |
6.25 (23.2) |
6.25 (23.2) |
6.25 (23.2) |
1.56 (5.79) |
1.56 (5.79) |
6.25 (23.2) |
0.78 (2.90) |
0.20 (0.74) |
0.20 (0.74) |
| 3k | 0.39 (1.78) |
1.56 (7.11) |
0.78 (3.56) |
0.39 (1.78) |
0.39 (1.78) |
0.39 (1.78) |
0.78 (3.56) |
0.78 (3.56) |
0.39 (1.78) |
1.56 (7.11) |
0.20 (0.91) |
0.10 (0.46) |
0.10 (0.46) |
| 3l | 0.78 (3.34) |
1.56 (6.69) |
3.13 (13.41) |
0.78 (3.34) |
1.56 (6.69) |
0.78 (3.34) |
1.56 (6.69) |
1.56 (6.69) |
0.78 (3.34) |
1.56 (6.69) |
0.20 (0.86) |
0.20 (0.86) |
0.20 (0.86) |
| 3m | 1.56 (6.31) |
1.56 (6.31) |
3.13 (12.7) |
1.56 (6.31) |
3.13 (12.7) |
1.56 (6.31) |
3.13 (12.7) |
3.13 (12.7) |
0.39 (1.58) |
3.13 (12.7) |
0.10 (0.40) |
0.20 (0.81) |
0.20 (0.81) |
| 3n | 3.13 (11.2) |
3.13 (11.2) |
6.25 (23.9) |
6.25 (23.9) |
6.25 (23.9) |
6.25 (23.9) |
6.25 (23.9) |
3.13 (11.2) |
0.39 (1.49) |
3.13 (11.2) |
≤0.02 (0.08) |
0.05 (0.19) |
≤0.02 (0.08) |
| 3o | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 4e | >12.5 (56.0) |
>12.5 (56.0) |
>12.5 (56.0) |
>12.5 (56.0) |
>12.5 (56.0) |
>12.5 (56.0) |
>12.5 (56.0) |
>12.5 (56.0) |
>12.5 (56.0) |
>12.5 (56.0) |
>12.5 (56.0) |
>12.5 (56.0) |
>12.5 (56.0) |
| 4f | >12.5 (52.7) |
>12.5 (52.7) |
>12.5 (52.7) |
>12.5 (52.7) |
>12.5 (52.7) |
>12.5 (52.7) |
>12.5 (52.7) |
>12.5 (52.7) |
>12.5 (52.7) |
>12.5 (52.7) |
>12.5 (52.7) |
>12.5 (52.7) |
>12.5 (52.7) |
| 4n | >12.5 (45.1) |
>12.5 (45.1) |
>12.5 (45.1) |
>12.5 (45.1) |
>12.5 (45.1) |
>12.5 (45.1) |
>12.5 (45.1) |
>12.5 (45.1) |
>12.5 (45.1) |
>12.5 (45.1) |
>12.5 (45.1) |
>12.5 (45.1) |
>12.5 (45.1) |
| AmB | 3.9 (4.22) | 3.9 (4.22) |
1.95 (2.11) |
0.98 (1.06) |
1.95 (2.11) |
3.9 (4.22) |
3.9 (4.22) |
1.95 (2.11) |
3.9 (4.22) |
1.95 (2.11) |
15.6 (16.9) |
15.6 (16.9) |
3.9 (4.22) |
| FLC | 62.5 (204)a |
>125 (408)a |
15.6 (51.0)a |
>125 (408)a |
>125 (408)a |
62.5 (204)a |
62.5 (204)a |
>31.2 (102) |
>31.2 (102) |
1.95 (6.37) |
62.5 (204) |
62.5 (204)a |
62.5 (204) |
| ITC | 0.5 (0.71)a |
>62.5 (88.6)a |
7.8 (11.1)a |
31.2 (44.2)a |
31.2 (44.2)a |
31.2 (44.2)a |
31.2 (44.2)a |
7.8 (11.1) |
0.48 (0.68) |
0.12 (0.17) |
0.48 (0.68) |
0.195 (0.28)a |
0.975 (1.38) |
| POS | 0.5 (0.71)a |
>62.5 (89.3)a |
7.8 (11.1)a |
31.2 (44.5)a |
31.2 (44.5)a |
15.6 (22.3)a |
15.6 (22.3)a |
0.12 (0.17) |
0.06 (0.09) |
<0.03 (0.04) |
0.24 (0.34) |
0.195 (0.28)a |
0.48 (0.68) |
| VOR | 7.8 (22.3) |
>31.2 (89.3) |
0.975 (2.79) |
1.95 (5.58) |
1.95 (5.58) |
0.975 (2.79) |
7.8 (22.3) |
0.06 (0.17) |
0.12 (0.34) |
<0.03 (0.09) |
0.24 (0.69) |
0.03 (0.09) |
0.12 (0.34) |
Yeast strains: A = Candida albicans ATCC 10231, B = C. albicans ATCC 64124, C = C. albicans ATCC MYA-2876(S), D = C. albicans ATCC 90819(R), E = C. albicans ATCC MYA-2310(S), F = C. albicans ATCC MYA-1237(R), G = C. albicans ATCC MYA-1003(R), H = Candida glabrata ATCC 2001, I = Candida krusei ATCC 6258, J = Candida parapsilosis ATCC 22019. NOTE: Here, the (S) and (R) indicate that ATCC reports these strains to be susceptible (S) and resistant (R) to ITC and FLC.
Filamentous fungi: K = Aspergillus flavus ATCC MYA-3631, L = Aspergillus nidulans ATCC 38163, M = Aspergillus terreus ATCC MYA-3633.
Known antifungal agents: AmB = amphotericin B, FLC = fluconazole, ITC = itraconazole, POS = posaconazole, and VOR = voriconazole.
These values were previously reported in ref #27.
For yeast strains: MIC-0 values are reported for compounds 1–4n and AmB, whereas MIC-2 values are reported for azoles. For filamentous fungi, MIC-0 values are reported for all compounds.
Values in µM are presented so that the reader can visualize the potential impact of the varied molecular weights of the compounds.
ND indicates that MIC values were not determined due to solubility issues with the compound.
We first tested ebselen (1) against our panel of Candida strains (A–J). Ebselen (1) displayed good activity against C. albicans ATCC 90819(R) (strain D), C. albicans ATCC MYA-2310(S) (strain E), C. glabrata ATCC 2001 (strain H), C. krusei (strain I), and C. parapsilosis ATCC 22019 (strain J) (1.56–6.25 µg/mL) and poor activity against strains C. albicans ATCC 10231 (strain A), C. albicans ATCC 64124 (strain B), C. albicans ATCC MYA-2876(S) (strain C), C. albicans ATCC MYA-1237(R) (strain F), and C. albicans ATCC MYA-1003(R) (strain G) (≥12.5 µg/mL). When compared to the controls, these MIC values were generally better than the MIC values of the azoles (except against strains A and H–J), but were worse than those of AmB. Next, we evaluated ebsulfur (2a) to gain insight into the importance of the sulfur atom for antifungal activity. Ebsulfur (2a) displayed a very similar anti-Candida profile to that of ebselen (1). In fact, we noticed that in general, they displayed good and poor activity against the same Candida strains. With the exception of strain J, 2a was active against strains D, E, H, and I (1.56–6.25 µg/mL) and poorly active against strains A, B, C, F, G, and J (≥12.5 µg/mL). This finding demonstrates that replacing the Se atom with the S atom does not compromise antifungal activity.
In search for a chemical modification that would increase the activity of the parent scaffolds, we first decided to assess our analogues with substituents at the para- and meta-positions of the phenyl ring adjacent to the 1,2-benzisothiazol-3(2H)-one (ebsulfur) core. Compounds 2b–d were systematically prepared to contain p-substituted halogen atoms that increased in bulkiness with F < Cl < Br. The SAR comparison for these compounds, however, was flat with all three compounds generally displaying MIC values from 1.56–6.25 µg/mL. We then tested the p-substituted isopropyl analogue (2e), which was previously found to be among the best ebsulfur antibacterial analogues.26 Compound 2e displayed good MIC values (3.13–6.25 µg/mL) similarly to those of 2b–d. Lastly, we tested the p-ethinyl analogue (2f) and found that 2f displayed mostly poor activity against Candida strains (≥12.5 µg/mL). We speculated that substitution at the para-position was not well tolerated due to steric hindrance with the putative target(s) and that a meta-substitution might show a different pattern of activities.
Based on this assumption, we next examined the m-monosubstituted analogues (2g,h) and the 3,5-disubstituted analogues (2i–k). While the m-Br substitution in 2g was not beneficial at all (≥12.5 µg/mL) against Candida strains, the m-iPr (2h), m,m-di-Br (2i), m,m-di-Me (2j), and m,m-di-OMe (2k) analogues were overall better tolerated with good to moderate MIC values (3.13–>12.5 µg/mL). By comparing the p-substituted analogues 2d,e and their m-substituted counterparts 2g,h, we noticed that switching from p-Br (2d) to m-Br (2g) led to loss of activity, whereas switching from p-iPr (2e) and m-iPr (2h) led to compounds which displayed very similar MIC values. Overall, the activity of these compounds appeared to weakly correlate with the number or the positions of the substituents on the phenyl ring.
Moving away from the substituted phenyl strategy, we next explored the analogues with more complex aromatic rings such as the naphthyl (2l), pyridyl (2m,n), and quinolinyl (2o). We found that these relatively bulkier rings were not as well tolerated. Compounds 2l,m displayed good to poor activity (6.25–≥12.5 µg/mL), while compound 2n was poorly active (≥12.5 µg/mL) and 2o could not be evaluated due to solubility issues in our RPMI 1640 medium. In conclusion, the chemical strategy of installing flat aromatic moieties to the core scaffold of the 2 series was able to generate many analogues with mostly good MIC values that are comparable to the parent ebsulfur (2a). We identified compounds 2d, 2e, 2h, and 2i that displayed incrementally improved MIC values when compared to those of ebsulfur (2a), but these improvements were still insufficient. Next, we pondered whether modifications with substituents possessing more geometric freedom and flexibility would be able to generate more substantially potent analogues.
Inspired by the observation that coupling linear alkyl chains to aminoglycoside antibiotics resulted in a significant improvement of their antifungal activity,21, 27, 32–34 we systematically generated and examined the antifungal activities of ebsulfur analogues with linear alkyl chains of 5–12 carbons (C5, C6, C8, and C12, 3a–d). Based on our previous work with aminoglycosides where tobramycin and kanamycin analogues with C12 and C14 alkyl chains displayed the best antifungal activity, we hypothesized that our longer C12 ebsulfur analogue (3d) would be the most active. Surprisingly, we observed an opposite trend than that displayed by the aminoglycosides; we found that our shorter C5 (3a) and C6 (3b) analogues were remarkably effective with very good to good MIC values against all Candida strains (0.39–1.56 µg/mL). The C8 analogue (3c) was slightly worse when compared to the C5 (3a) and C6 (3b) analogues (specifically against strains A and C), and our C12 analogue (3d) displayed poor MIC values (>12.5 µg/mL), which was the worst amongst the analogues.
We were intrigued to identify the C5 (3a) and C6 (3b) linear alkyl analogues as the best anti-Candida agents in our library, thus far. Compared to FLC, 3a was 20- to 320-fold more potent in MIC values (except against strain J). When compared to AmB, 3a was 1.25- to 10-fold more active. Since the C5 and C6 alkyl chains were extremely well tolerated, we speculated whether our putative target(s) could also tolerate branched alkyl chains with similar chain lengths. We went ahead and evaluated the iso-butyl (3e) and iso-amyl (3f) analogues. Interestingly, both 3e and 3f were equally as effective as 3a and 3b (0.39–1.56 µg/mL). We then examined the final branched analogue in our library, the tert-butyl compound 3g. Compound 3g was also as effective as 3a–f (within 2-fold dilution, 0.78–3.13 µg/mL). Against Candida strains, analogues with aliphatic alkyl chains (linear or branched) were found to be very beneficial, which could possibly be attributed to the added rotational flexibility.
As we found that additional methylene groups were highly favorable, we hypothesized that adding methylene linkers (C1 (3h), C2 (3i), and C3 (3j)) in between the 1,2-benzisothiazol-3(2H)-one core and the phenyl ring could provide the added rotational flexibility needed to generate analogues with improved MIC values when compared to the parent compound (2a), which was flat and rigid. The C1 and C2-linker analogues (3h,i) indeed had better MIC values comparing to 2a (0.39–1.56 µg/mL). However, the C3-linker analogue (3j) was not as potent (1.56–62.5 µg/mL). From these observations, we noticed that addition of flexible methylene linkers were well tolerated up to two carbons.
To further understand the correlation between the flexibility of the R group and activity, we tested our non-aromatic analogues (3k–o) as non-aromatic rings are considerably more flexible than their aromatic counterparts. We first tested the cyclopentyl analogue (3k) and found it to be just as active (0.39–1.56 µg/mL) as some of our best analogues 3a–c described above. On the other hand, the cyclohexyl analogue (3l) still displayed very good to good activity (0.78–3.13 µg/mL), but overall was slightly worse relative to 3k. The cycloheptyl (3m) and cyclooctyl (3n) analogues were also not as good as 3k,l. We also attempted to test whether an ultra-bulky ring such as the admantane could still be accommodated, but our adamantyl analogue (3o) was not soluble in the RPMI 1640 medium that we used for determination of MIC values. Collectively, the SAR showed a modest preference for smaller size ring, as systematically expanding the ring size resulted in a gradual loss in activity.
Lastly, we tested the oxidized analogues 4e, 4f, and 4n. Oxidizing the sulfur atom to sulfoxide completely abolish antifungal activity. This finding was in accord with our previous report of these compounds as antibacterials and with other reports in the literature that the biological activity of ebselen (1) and ebsulfur (2a) was highly dependent on the Se-N or S-N bonds.26, 35 Ebselen (1) has been reported to utilize the electrophilic Se-N bond to covalently bind to cysteine residues of multiple enzyme targets.23
Invasive aspergillosis is highly correlated with fulminant development and poor prognosis.36 Compounds with potent anti-Aspergillus activity are considered to be of great valuable. To evaluate the antifungal spectrum of our compounds, we tested them against freshly harvested spores of three Aspergillus strains: A. flavus ATCC MYA-3631 (strain K), A. nidulans ATCC 38163 (strain L), and A. terreus ATCC MYA-3633 (strain M). Overall, our compounds were mostly active against Aspergillus strains and the SAR trends observed from our study with Candida strains were highly translatable to Aspergillus strains. Aromatic analogues (2a–o) remained to have either good to poor activity against strains K–M (1.56–12.5 µg/mL). We were especially intrigued to observe that our linear-chain C5, C6, and C8 analogues (3a–c) displayed excellent activity at ng/mL concentrations (≤0.02–0.20 µg/mL). These results were equivalent or slightly better when comparing them to VOR (0.03–0.24 µg/mL), the gold standard for the treatment of invasive aspergillosis.37 Other analogues (3e–m) displayed very good activity (0.10–0.78 µg/mL), but they were not as effective as 3a–c. We were also surprised to find that our cyclooctyl analogue (3n) displayed excellent activity against Aspergillus strains (≤0.02–0.05 µg/mL). These values were equivalent to our best anti-Aspergillus analogues 3a–c. We realized that many of our potent analogues were lipophilic. Thus, we investigated the log P values of all the compounds by two log P calculators (ChemDraw and molinspiration) (Table S1). In general, we observed that compounds with extremely high or very low lipophilicity did not display good antifungal activity. However, we found that increasing lipophilicity, to a certain extent, generally correlated with increase in antifungal activity.
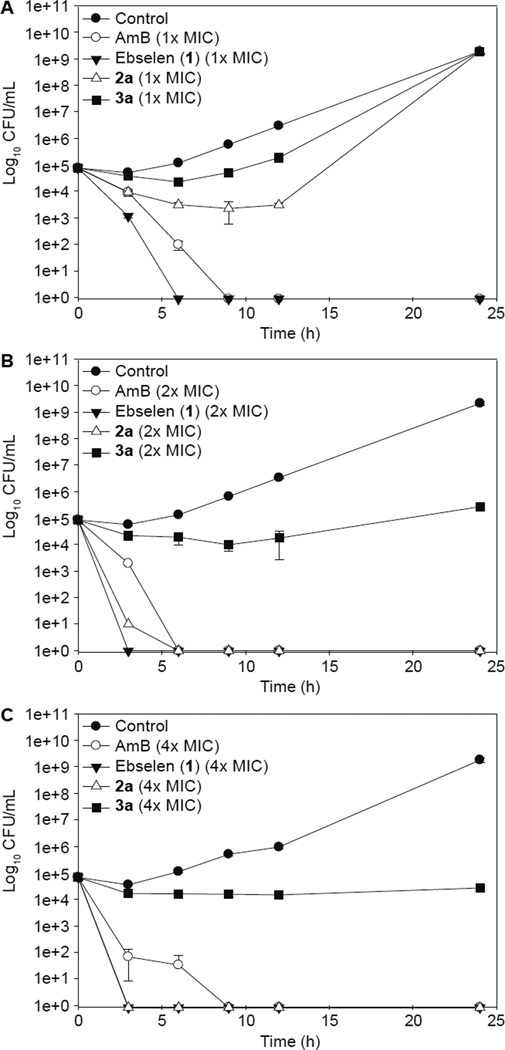
Time-kill curves
To gain insight for the rate of fungicidal activity of our compounds, we performed time-kill assays with ebsulfur (2a) and our most potent analogue 3a (Fig. 2). We then compared the results of our compounds to ebselen (1) and the clinically potent and widely used antifungal agent AmB, which also served as our positive control. We selected ebsulfur (2a) because we previously observed that this compound was bacteriostatic and we pondered whether a similar fungistatic effect would be observed.26 We commenced our study by dosing all of our tested compounds (ebselen (1), ebsulfur (2a), 3a, and AmB) at 1× their respective MIC values (Fig. 2A). For ebsulfur (2a), although the MIC value for ebsulfur (2a) against strain B was greater than 12.5 µg/mL, we decided to test this compound at 12.5 µg/mL because we were concerned that higher concentration may lead to precipitation of the compounds. We observed that ebselen (1) (at 12.5 µg/mL) displayed potent fungicidal activity leading to complete fungal cell death at the 6-h mark, which was even quicker than AmB (at 3.9 µg/mL). Our ebsulfur (2a) (at 12.5 µg/mL) and 3a (at 0.39 µg/mL) displayed fungistatic effects. However, at their 1× MIC, ebsulfur (2a) and 3a were not able to completely inhibit fungal re-growth even after 24 h incubation. Hence, we decided to double the doses of our compounds for our additional time-kill analysis experiments.
Figure 2.
Time-kill analysis of ebselen (1) (black inverted triangles), ebsulfur (2a) (white triangle), compound 3a (black squares) at 0, 3, 6, 9, 12, and 24 h. A. Cultures were exposed to compounds at 1× their respective MIC values. B. Cultures were exposed to compounds at 2× their respective MIC values. C. Cultures were exposed to compounds at 4× their respective MIC values. Untreated culture (black circles) was used as the negative control and AmB (white circles) was used the positive control. Data was combined from two independent experiments. The first experiment was conducted with the standard 1× and 2× respective MIC values. The second experiment was needed to supplement the analysis and was performed at 4× their respective MIC values. Each data points were collected in duplicates. The error bars were reported as ± standard deviations.
At 2× MIC (Fig. 2B), Ebselen (1) (at 25 µg/mL) and AmB (at 7.8 µg/mL) were completely fungicidal from 3 and 6 h, respectively. At the higher concentration, ebsulfur (2a) (25 µg/mL) interestingly became fungicidal. Conversely, compound 3a (at 0.78 µg/mL) remained fungistatic with a 4-log reduction of fungal cells at approximately the 24-h mark. We were very intrigued by this result and pondered whether fungicidal and fungistatic effects could be concentration dependent. Thus, we further increase the concentration of compound 3a to 4× its MIC value with the hope that 3a would also switch to the fungicidal mode. We performed the assay and actually found that 3a at 4× MIC (at 1.56 µg/mL) still remained fungistatic (Fig. 2C). These findings suggested that in order for ebsulfur (2a) and compound 3a to be effective antifungal agents, they would have to be dosed at ≥2× their respective MIC values while albeit at high concentration, ebselen (1) could still be effective at 1× MIC.
Based on the time-kill curves, we observed that ebselen (1) and ebsulur (2a) were fungicidal at high concentrations (12.5 and 25 µg/mL or 45.6 and 110 µM, respectively) and our best compound from the MIC determination assays, 3a was fungistatic at 0.78 and 1.56 µg/mL (3.5 and 7.0 µM), which were much lower than the fungicidal concentrations of the other compounds. Fungistatic property does not necessarily mean that compound 3a is not as good as ebselen (1) and ebsulfur (2a) as an antifungal agent because the most popular antifungal compound (FLC) is also fungistatic. This data, however, gave us hints that compound 3a may potentially not be suitable for some specific fungal infections that absolutely require fungicidal effect for clinical efficacy such as cryptococcal meningitis. Additionally, the fact that these benzisothiazolinone compounds exhibit fungicidal effect at high concentrations may mean that they target a different fungal enzymatic pathway when the concentrations are high.
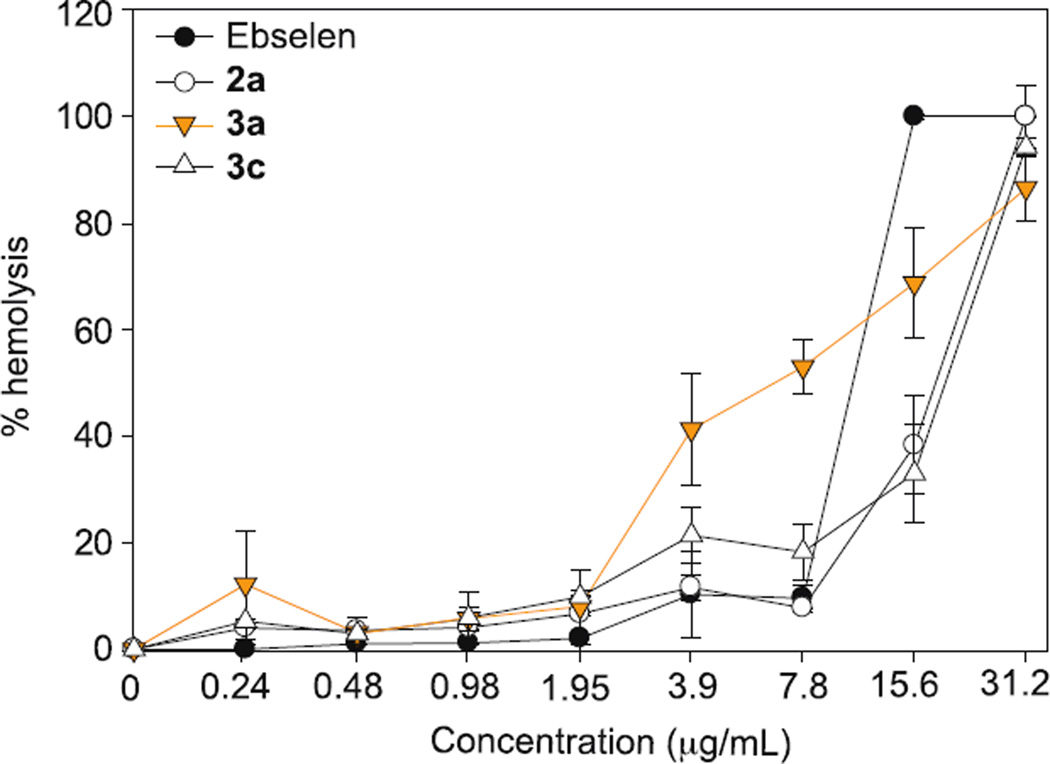
Hemolytic assay
Although we were excited to discover new analogues with improved antifungal activity relatively to ebselen (1), we pondered whether this cytotoxic property could be more selective towards fungal cells than mammalian cells. We were cautiously optimistic that our analogues would still retain some of the good tolerability properties that were highly desirable in the original ebselen (1) scaffold. Previously, our lab studied aminoglycoside analogues with linear alkyl chains and reported that aminoglycoside analogues with linear alkyl chains could potentially be toxic to red blood cells (RBCs) as these RBCs have ultra thin cell membrane and thus, are prone to hemolysis.21 Therefore, we decided to evaluate some of our compounds with linear alkyl chains, the C5 analogue 3a and the C8 analogue 3c against murine red blood cells (mRBCs) and compare their results to ebselen (1) (Fig. 3). Although the C6 analogue 3b was also one of our top analogues, we did not test this analogue because its chain length (C6) was extremely similar structurally to the C5 compound 3a. We also excluded the C12 analogue 3d because it was completely inactive against fungal strains. Ebsulfur (2a) was chosen because we were interested to verify that the linear alkyl chain would be a required feature for hemolysis. Most of the compounds (ebselen (1), ebsulfur (2a), and compound 3c) tested did not show any significant hemolytic activity until 15.6 µg/mL (56.9 µM for ebselen (1), 68.6 µM for ebsulfur (2a), and 59.2 µM for 3c). At first glance, compound 3a appeared to be very hemolytic at approximately 3.9 µg/mL (17.6 µM). However, it should be noted that this compound showed remarkable potency against fungal cells. The MIC values of compound 3a were at least 5- to 195-fold lower than the hemolytic concentrations for Candida and Aspergillus strains, respectively. Thus, we could observe that there was still some cytocidal selectivity towards fungal cells. Initially, we expected the C8 analogue 3c to be more hemolytic because analogues with longer linear alkyl chains tend to perforate cell membranes easily. Thus, it was unexpected to find the C5 analogue 3a to be hemolytic. This observation led us to speculate that the hemolytic activity was not due to disruption of the cell membrane of the mRBCs, but was simply an artifact of general mammalian cytotoxicity. This prompted us to evaluate our three best compounds 3a, 3b, and 3g (in terms of their overall antifungal activity against both Candida and Aspergillus strains) against two different mammalian cell lines, which have normal cell membranes and are not susceptible to membrane-lytic compounds.
Figure 3.
Hemolytic assays of ebselen (1), ebsulfur (2a), compound 3a, and compound 3c against murine red blood cells (mRBCs). Ebselen (1), ebsulfur (2a), compound 3a, and compound 3c are represented as black circles, white circles, inverted orange triangle, and upright white triangle, respectively. The data points of ebselen (1), ebsulfur (2a), and compound 3c were previously present in one of our publications and are used here for comparison.26 Each data point was collected in duplicates. The error bars were reported as ± standard deviations.
Mammalian cytotoxicity assay
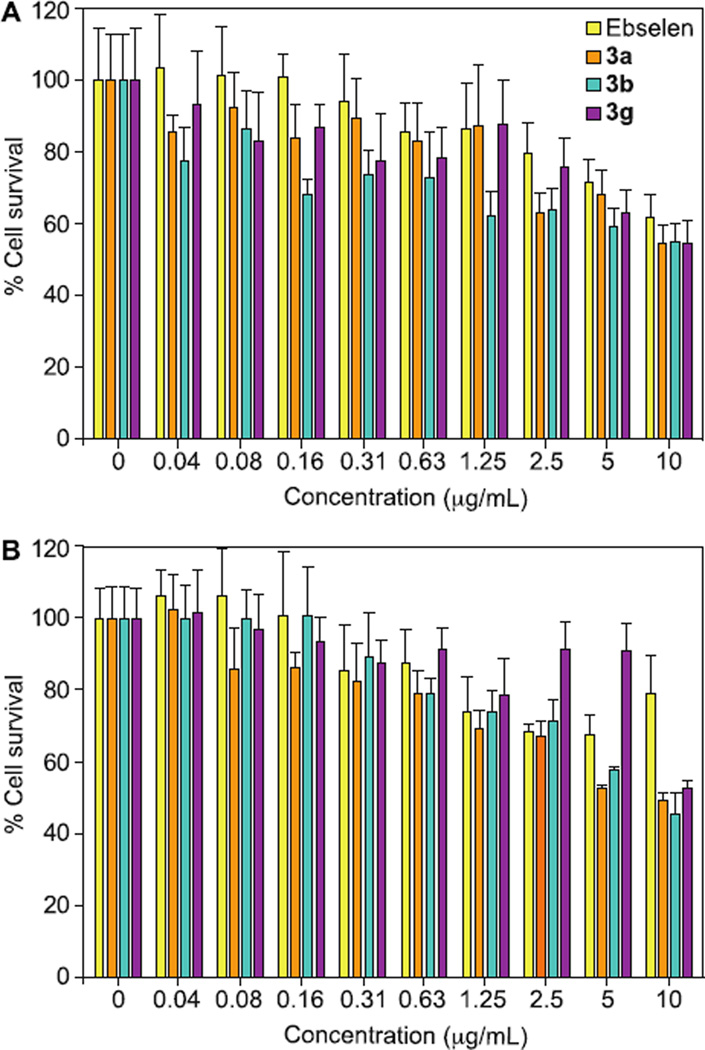
Compounds 3a, 3b, and 3g were evaluated for their cytotoxicity against HEK293 and J774 cell lines using a resazurin assay.20 The concentration of DMSO used in the cytotoxicty assay is 0.1%, which was ensured to not demonstrate any DMSO-related toxicity to the cells. We compared the percentage of surviving cells treated with our analogues versus the percentage of surviving cells treated with ebselen (1) (Fig. 4). Against the HEK293 cell line (Fig. 4A), we noticed that all of our analogues (3a, 3b, and 3g) were slightly more toxic but overall, quite comparable to ebselen (1) at all concentrations tested. Overall, for all the tested compounds (ebselen (1), 3a, 3b, and 3g) the IC50 values were greater than10 µg/mL (Fig. 4A). This corresponds to IC50 values of 36.5 µM for ebselen (1), 45.2 µM for 3a, 42.5 µM for 3b, and 48.2 µM for 3g. We verified that the cytotoxicity data of ebselen (1) found in our study was fairly consistent with other reported in vitro mammalian cytotoxicity studies of ebselen (1).38, 39 Given the good tolerability of ebselen (1) during clinical trials, it was perplexing to us ebselen (1) displayed some in vitro cytotoxicity. The HEK293 cell line was chosen because we were interested to determine whether our compounds could potentially cause kidney injury. The kidney is a highly perfused organ and comes in contact with many compounds due to renal excretion. Thus, many compounds such as AmB are highly nephrotoxic and cause great burden to patients with compromised renal function.
Figure 4.
Mammalian cell cytotoxicity of ebselen (1) (yellow bars), and compounds 3a (orange bars), 3b (turquoise bars), and 3g (purple bars) against A. HEK 293 cell line and B. J774 cell line,. Triton-X 100® (1%, v/v) was used as the positive control (data not shown). Each data point was collected in quadruplicates. The error bars were reported as ± standard deviations.
Next, we evaluated our compounds against J774 (Fig. 4B), a murine macrophage cell line. This cell line was selected because macrophages are the first-line of defense against fungal infection and we were hopeful that our compounds would not interfere with the survival of host macrophages. Against the J774 cell line, we observed a trend similar to the HEK293 cell line and found that our analogues (3a and 3b) were slightly more toxic but still comparable to ebselen (1) with IC50 values approximately at 10 µg/mL. It was interesting that compounds 3g actually did not show any toxicity at all up to 5 µg/mL (24.1 µM). Typically, the difference between the toxic dose in mammalian cells and the fungal MIC value should be at least 10-fold. The fact that this was the case for our most potent antifungal compounds is highly encouraging.
We acknowledge that there are concerns in the literature regarding the highly reactive benzisothiazolinone moiety of the ebsulfur (2a) scaffold, which possibly explains the toxicity effects observed against mammalian cell lines.40 This concern is valid considering that the parent compound ebselen (1) has also been found to target different proteins.23 However, we argue that this scaffold still merits further consideration as a potential antifungal candidate based on two particular reasons. First, since many potent antifungal compounds are only available intravenously, there is currently a dire clinical need for orally active antifungals to assist the azoles as an alternative option for step-down therapy.41 These azoles often complicate drug dosing due to interactions with the metabolism of many drugs and have experienced an increased rate of resistance.42, 43, 44 The ebsulfur analogues would most likely be orally active due to its similarity to ebselen (1), which was successfully administered orally.24, 45 Secondly, while we are also concerned about the high reactivity of the benzisothiazolinone moiety towards non-specific cysteine residues, ebselen (1) with the benzisoselenazolinone moiety has been shown to be well-tolerated during clinical trials. There are also examples of other clinically successful small-molecule drugs with highly reactive pharmacophores within the FDA-approved chemical space. Some of these compounds are penicillin, fosfomycin, or bendamustine.46 Compounds 3a and 3b displayed MIC values against Candida strains at 780 ng/mL and Aspergillus strains at ≤20 ng/mL, which are much lower than their IC50 values against mammalian cells. This could potentially be due to the fact that 3a and 3b may have a fungal-specific mechanism of action at lower concentrations. To gain insights on the mechanism of action, we decided to first look at ROS induction of these compounds.
ROS production
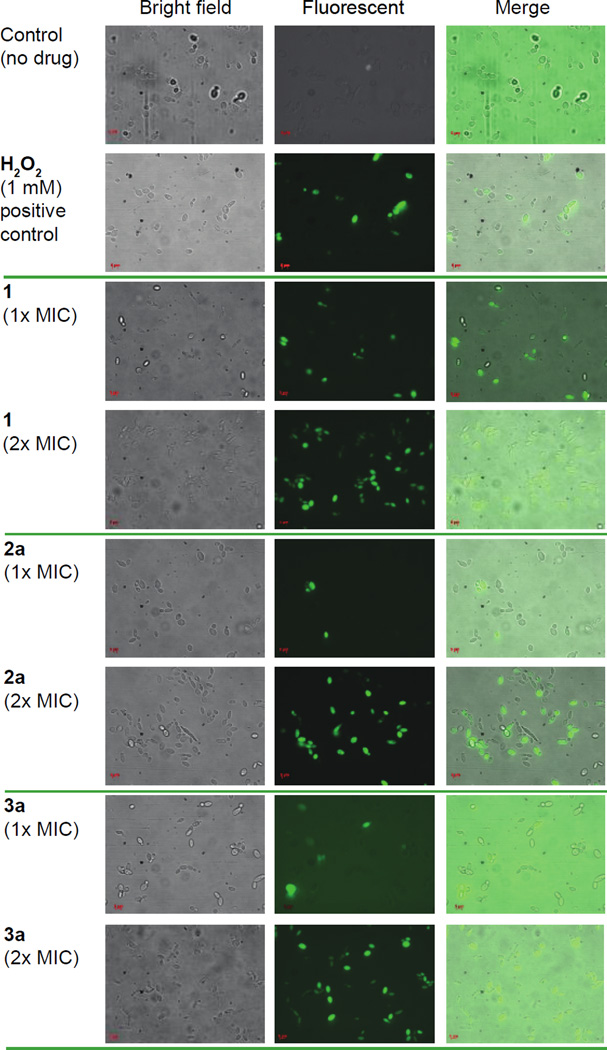
Recently, we showed that ebselen (1) and our ebsulfur analogues with antibacterial activity were highly correlated with ROS production in MRSA bacterial cells.26 Another group independently reported that ebselen (1) induced ROS-mediated cytotoxicity in Saccharomyces cerevisiae via inhibition of glutamate dehydrogenase.47 Thus, we sought to determine whether our analogues would also induce ROS against C. albicans via inhibition of the C. albicans glutamate dehydrogenase. Ebselen (1), ebsulfur (2a), and compound 3a were tested against C. albicans ATCC 10231 cells (strain A) at 1× and 2× their respective MIC values. 2',7'-dichlorodihydrofluorescin diacetate (DCFH-DA) was then used to detect and visualize ROS production (Fig. 5). As a positive control, we treated cells with H2O2, which is an inducer of hydroxyl radical formation. After 1-h treatment, we found that all the compounds tested and the positive control were highly fluorescent, which indicated ROS induction. We also compared the samples that were treated with different doses of compounds (1× and 2× their respective MIC values) and observed that the amount ROS induction could potentially be concentration dependent. It is certainly possible that this ROS induction in C. albicans spp. is due to inhibition of C. albicans glutamate dehydrogenase as we hypothesized. However, this finding still does not rule out other potential drug targets such as fungal enzymes responsible for ROS regulation or it is simply a downstream secondary effect as the ebselen (1) and ebsulfur (2a) scaffolds inhibit enzymes that are unrelated to ROS generation. Assays to determine the specific molecular target(s) of our best compound, 3a, are ongoing and will be the subject of future reports.
Figure 5.
ROS induction assay of ebselen (1), ebsulfur (2a), and compound 3a against C. albicans ATCC 10231 (strain A). Candida cells were treated with no drug (negative control), 1 mM of H2O2 (positive control), or ebselen, 2a, and 3a at their 1× and 2× respective MIC values for 1 h at 37 °C. DCFH-DA (40 µg/mL) was added to detect ROS and the samples were analyzed using a Zeiss Axovert 200M fluorescence microscope.
CONCLUSIONS
In summary, we expanded our knowledge of this scaffold in terms of the antifungal activity against a panel of clinically relevant Candida and Aspergillus strains. In light of our SAR analysis, we identified that the addition of flexible chemical moieties to the 1,2-benzisothiazol-3(2H)-one scaffold is a viable strategy to generate analogues with potent antifungal activities. Interestingly, the majority of our compounds displayed comparable or, in most cases, enhanced antifungal activities against all fungal strains when compared to ebselen (1) and the reference drugs used in this study. Although, our best compound (3a) exhibited some hemolytic activity, its effect on nucleated mammalian cells was found to be in the acceptable range considering their antifungal efficacies. Finally, our preliminary study on mechanism of action indicated that the growth inhibitory effect of fungi by these compounds might be due to the elevating concentration of ROS in the yeast cells. However, the biological activities of the ebselen (1) and ebsulfur (2a) scaffolds are complex due to their general electrophilicity. An extensive study is required to identify the specific mode of action against fungal strains. Once the mechanism of action is established, we feel that this scaffold would have merit for further evaluation in a fungal infected murine model.
Supplementary Material
Acknowledgments
This work was supported by startup funds from the University of Kentucky (to S.G.-T.) and by NIH grant AI90048 (to S.G.-T.). We would like to thank Ms. Taylor A. Lundy for critically reviewing our manuscript, Ms. Emily K. Dennis for helping us conducting some preliminary experiments on drug synergism, and Mr. Joseph M. Eckenrode for helping us with some high-resolution mass spectrometry studies.
Footnotes
ASSOCIATED CONTENT
Supporting Information. The supporting information includes experimental procedures for all assays performed. This material is available free of charge via the Internet at http://pubs.acs.org.”
REFERENCES
- 1.Arendrup MC. Epidemiology of invasive candidiasis. Curr. Opin. Crit. Care. 2010;16:445–452. doi: 10.1097/MCC.0b013e32833e84d2. [DOI] [PubMed] [Google Scholar]
- 2.Kett DH, Azoulay E, Echeverria PM, Vincent JL Extended prevalence of infection in ICU study (EPIC II) group of investigators. Candida bloodstream infections in intensive care units: analysis of the extended prevalence of infection in intensive care unit study. Crit. Care Med. 2011;39:665–670. doi: 10.1097/CCM.0b013e318206c1ca. [DOI] [PubMed] [Google Scholar]
- 3.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 4.Kullberg BJ, Arendrup MC. Invasive candidiasis. N. Engl. J. Med. 2015;373:1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- 5.Mayr A, Lass-Florl C. Epidemiology and antifungal resistance in invasive aspergillosis according to primary disease: review of the literature. Eur. J. Med. Res. 2011;16:153–157. doi: 10.1186/2047-783X-16-4-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Linden JW, Arendrup MC, Warris A, Lagrou K, Pelloux H, Hauser PM, Chryssanthou E, Mellado E, Kidd SE, Tortorano AM, Dannaoui E, Gaustad P, Baddley JW, Uekotter A, Lass-Florl C, Klimko N, Moore CB, Denning DW, Pasqualotto AC, Kibbler C, Arikan-Akdagli S, Andes D, Meletiadis J, Naumiuk L, Nucci M, Melchers WJ, Verweij PE. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg. Infect. Dis. 2015;21:1041–1044. doi: 10.3201/eid2106.140717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sloan DJ, Parris V. Cryptococcal meningitis: epidemiology and therapeutic options. Clin. Epidemiol. 2014;6:169–182. doi: 10.2147/CLEP.S38850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasko MT, Piscitelli SC, Van Slooten AD. Fluconazole: a new triazole antifungal agent. DICP. 1990;24:860–867. doi: 10.1177/106002809002400914. 1990. [DOI] [PubMed] [Google Scholar]
- 9.Zumbuehl A, Jeannerat D, Martin SE, Sohrmann M, Stano P, Vigassy T, Clark DD, Hussey SL, Peter M, Peterson BR, Pretsch E, Walde P, Carreira EM. An amphotericin B-fluorescein conjugate as a powerful probe for biochemical studies of the membrane. Angew. Chem. 2004;43:5181–5185. doi: 10.1002/anie.200460489. [DOI] [PubMed] [Google Scholar]
- 10.Baginski M, Czub J. Amphotericin B and its new derivatives - mode of action. Curr. Drug Metab. 2009;10:459–469. doi: 10.2174/138920009788898019. [DOI] [PubMed] [Google Scholar]
- 11.Morris MI, Villmann M. Echinocandins in the management of invasive fungal infections, part 1. Am. J. Health Syst. Pharm. 2006;63:1693–1703. doi: 10.2146/ajhp050464.p1. [DOI] [PubMed] [Google Scholar]
- 12.Sanguinetti M, Posteraro B, Lass-Florl C. Antifungal drug resistance among Candida species: mechanisms and clinical impact. Mycoses. 2015;58(Suppl 2):2–13. doi: 10.1111/myc.12330. [DOI] [PubMed] [Google Scholar]
- 13.Kanafani ZA, Perfect JR. Antimicrobial resistance: resistance to antifungal agents: mechanisms and clinical impact. Clin. Infect. Dis. 2008;46:120–128. doi: 10.1086/524071. [DOI] [PubMed] [Google Scholar]
- 14.Shah DN, Yau R, Lasco TM, Weston J, Salazar M, Palmer HR, Garey KW. Impact of prior inappropriate fluconazole dosing on isolation of fluconazole-nonsusceptible Candida species in hospitalized patients with candidemia. Antimicrob. Agents Chemother. 2012;56:3239–3243. doi: 10.1128/AAC.00019-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben-Ami R, Olshtain-Pops K, Krieger M, Oren I, Bishara J, Dan M, Wiener-Well Y, Weinberger M, Zimhony O, Chowers M, Weber G, Potasman I, Chazan B, Kassis I, Shalit I, Block C, Keller N, Kontoyiannis DP, Giladi M Israeli candidemia study group. Antibiotic exposure as a risk factor for fluconazole-resistant Candida bloodstream infection. Antimicrob. Agents Chemother. 2012;56:2518–2523. doi: 10.1128/AAC.05947-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hata K, Horii T, Miyazaki M, Watanabe NA, Okubo M, Sonoda J, Nakamoto K, Tanaka K, Shirotori S, Murai N, Inoue S, Matsukura M, Abe S, Yoshimatsu K, Asada M. Efficacy of oral E1210, a new broad-spectrum antifungal with a novel mechanism of action, in murine models of candidiasis, aspergillosis, and fusariosis. Antimicrob. Agents Chemother. 2011;55:4543–4551. doi: 10.1128/AAC.00366-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kontoyiannis DP, Lewis RE. Toward more effective antifungal therapy: the prospects of combination therapy. Br. J. Haematol. 2004;126:165–175. doi: 10.1111/j.1365-2141.2004.05007.x. [DOI] [PubMed] [Google Scholar]
- 18.Day JN, Chau TT, Wolbers M, Mai PP, Dung NT, Mai NH, Phu NH, Nghia HD, Phong ND, Thai CQ, Thai le H, Chuong LV, Sinh DX, Duong VA, Hoang TN, Diep PT, Campbell JI, Sieu TP, Baker SG, Chau NV, Hien TT, Lalloo DG, Farrar JJ. Combination antifungal therapy for cryptococcal meningitis. N. Engl. J. Med. 2013;368:1291–1302. doi: 10.1056/NEJMoa1110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen MH, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2010;50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shrestha SK, Fosso MY, Garneau-Tsodikova S. A combination approach to treating fungal infections. Sci. Rep. 2015;5:17070. doi: 10.1038/srep17070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fosso MY, Shrestha SK, Green KD, Garneau-Tsodikova S. Synthesis and bioactivities of kanamycin B-derived cationic amphiphiles. J. Med. Chem. 2015;58:9124–9132. doi: 10.1021/acs.jmedchem.5b01375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim K, Zilbermintz L, Martchenko M. Repurposing FDA approved drugs against the human fungal pathogen, Candida albicans. Ann. Clin. Microbiol. Antimicrob. 2015;14:32. doi: 10.1186/s12941-015-0090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azad GK, Tomar RS. Ebselen, a promising antioxidant drug: mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014;41:4865–4879. doi: 10.1007/s11033-014-3417-x. [DOI] [PubMed] [Google Scholar]
- 24.Lynch E, Kil J. Development of ebselen, a glutathione peroxidase mimic, for the prevention and treatment of noise-induced hearing lost. Semin. Hear. 2009;30:047–055. [Google Scholar]
- 25.Parnham MJ, Sies H. The early research and development of ebselen. Biochem. Pharmacol. 2013;86:1248–1253. doi: 10.1016/j.bcp.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 26.Ngo HX, Shrestha SK, Green KD, Garneau-Tsodikova S. Development of ebsulfur analogues as potent antibacterials against methicillin-resistant Staphylococcus aureus. Bioorg. Med. Chem. 2016 doi: 10.1016/j.bmc.2016.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shrestha SK, Fosso MY, Green KD, Garneau-Tsodikova S. Amphiphilic tobramycin analogues as antibacterial and antifungal agents. Antimicrob. Agents Chemother. 2015;59:4861–4869. doi: 10.1128/AAC.00229-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deray G. Amphotericin B nephrotoxicity. J. Antimicrob. Chemother. 2002;49(Suppl 1):37–41. doi: 10.1093/jac/49.suppl_1.37. [DOI] [PubMed] [Google Scholar]
- 29.Moen MD, Lyseng-Williamson KA, Scott LJ. Liposomal amphotericin B: a review of its use as empirical therapy in febrile neutropenia and in the treatment of invasive fungal infections. Drugs. 2009;69:361–392. doi: 10.2165/00003495-200969030-00010. [DOI] [PubMed] [Google Scholar]
- 30.Dvorak Z. Drug-drug interactions by azole antifungals: Beyond a dogma of CYP3A4 enzyme activity inhibition. Toxicol. Lett. 2011;202:129–132. doi: 10.1016/j.toxlet.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 31.Wang JL, Chang CH, Young-Xu Y, Chan KA. Systematic review and meta-analysis of the tolerability and hepatotoxicity of antifungals in empirical and definitive therapy for invasive fungal infection. Antimicrob. Agents Chemother. 2010;54:2409–2419. doi: 10.1128/AAC.01657-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang CW, Takemoto JY. Antifungal amphiphilic aminoglycosides. Med Chem Comm. 2014;5:1048–1057. doi: 10.1039/C4MD00078A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fosso M, AlFindee MN, Zhang Q, Nziko Vde P, Kawasaki Y, Shrestha SK, Bearss J, Gregory R, Takemoto JY, Chang CW. Structure-activity relationships for antibacterial to antifungal conversion of kanamycin to amphiphilic analogues. J. Org. Chem. 2015;80:4398–4411. doi: 10.1021/acs.joc.5b00248. [DOI] [PubMed] [Google Scholar]
- 34.Fosso MY, Li Y, Garneau-Tsodikova S. New trends in aminoglycosides use. Med Chem Comm. 2014;5:1075–1091. doi: 10.1039/C4MD00163J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu J, Vodnala SK, Gustavsson AL, Gustafsson TN, Sjoberg B, Johansson HA, Kumar S, Tjernberg A, Engman L, Rottenberg ME, Holmgren A. Ebsulfur is a benzisothiazolone cytocidal inhibitor targeting the trypanothione reductase of Trypanosoma brucei. J. Biol. Chem. 2013;288:27456–27468. doi: 10.1074/jbc.M113.495101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin. Infect. Dis. 2001;32:358–366. doi: 10.1086/318483. [DOI] [PubMed] [Google Scholar]
- 37.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF Infectious Diseases Society of America. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 2008;46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 38.Miorelli ST, Rosa RM, Moura DJ, Rocha JC, Lobo LA, Henriques JA, Saffi J. Antioxidant and anti-mutagenic effects of ebselen in yeast and in cultured mammalian V79 cells. Mutagenesis. 2008;23:93–99. doi: 10.1093/mutage/gem048. [DOI] [PubMed] [Google Scholar]
- 39.Bueno DC, Meinerz DF, Allebrandt J, Waczuk EP, dos Santos DB, Mariano DOC, Rocha JBT. Cytotoxicity and genotoxicity evaluation of organochalcogens in human leucocytes: A comparative study between ebselen, diphenyl diselenide, and diphenylditelluride. BioMed Res. Int. 2013:537279. doi: 10.1155/2013/537279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasan M, Neres J, Williams J, Wilson DJ, Teitelbaum AM, Remmel RP, Aldrich CC. Inhibitors of the salicylate synthase (MbtI) from Mycobacterium tuberculosis discovered by high-throughput screening. Chem Med Chem. 2010;5:2079–2087. doi: 10.1002/cmdc.201000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. Clinical practice guideline for the management of candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016;62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cronin S, Chandrasekar PH. Safety of triazole antifungal drugs in patients with cancer. J. Antimicrob. Chemother. 2010;65:410–416. doi: 10.1093/jac/dkp464. [DOI] [PubMed] [Google Scholar]
- 43.Hughes CA, Foisy M, Tseng A. Interactions between antifungal and antiretroviral agents. Expert Opin. Drug Saf. 2010;9:723–742. doi: 10.1517/14740331003752694. [DOI] [PubMed] [Google Scholar]
- 44.Lortholary O, Desnos-Ollivier M, Sitbon K, Fontanet A, Bretagne S, Dromer F French Mycosis Study Group. Recent exposure to caspofungin or fluconazole influences the epidemiology of candidemia: a prospective multicenter study involving 2,441 patients. Antimicrob. Agents Chemother. 2011;55:532–538. doi: 10.1128/AAC.01128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, Yasuhara H. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Ebselen Study Group. Stroke. 1998;29:12–17. doi: 10.1161/01.str.29.1.12. [DOI] [PubMed] [Google Scholar]
- 46.Singh J, Petter RC, Baillie TA, Whitty A. The resurgence of covalent drugs. Nat. Rev. Drug Discov. 2011;10:307–317. doi: 10.1038/nrd3410. [DOI] [PubMed] [Google Scholar]
- 47.Azad GK, Singh V, Mandal P, Singh P, Golla U, Baranwal S, Chauhan S, Tomar RS. Ebselen induces reactive oxygen species (ROS)-mediated cytotoxicity in Saccharomyces cerevisiae with inhibition of glutamate dehydrogenase being a target. FEBS Open Bio. 2014;4:77–89. doi: 10.1016/j.fob.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.