Abstract
Objective To assess the feasibility and acceptability of a patient workbook for self‐assessing coronary risk.
Design Pilot study, with post‐study physician and patient interviews.
Setting and subjects Twenty southern Ontario family doctors and 40 patients for whom they would have used the workbook under normal practice conditions.
Interventions The study involved convening two sequential groups of family physicians: the first (n=10) attended focus group meetings to help develop the workbook (using algorithms from the Framingham Heart Study); the second (n=20) used the workbook in practice with 40 patients. Follow‐up interviews were by interviewer‐administered questionnaire.
Main outcome measures Physicians' and patients' opinions of the workbook's format, content, helpfulness, feasibility, and potential for broad application, as well as patients' perceived 10‐year risk of a coronary event measured before and after using the workbook.
Results It took an average of 18 minutes of physician time to use the workbook: roughly 7 minutes to introduce it to patients, and about 11 minutes to discuss the results. Assessments of the workbook were generally favourable. Most patients were able to complete it on their own (78%), felt they had learned something (80%) and were willing to recommend it to someone else (98%). Similarly, 19 of 20 physicians found it helpful and would use it in practice with an average of 18% of their patients (range: 1–80%). The workbook helped to correct misperceptions patients had about their personal risk of a coronary event over the next 10 years (pre‐workbook (mean (SD) %): 35.2 (16.9) vs. post‐workbook: 17.3 (13.5), P < 0.0001; estimate according to algorithm: 10.6 (7.6)).
Conclusions Given a simple tool, patients can and will assess their own risk of CHD. Such tools could help inform otherwise healthy individuals that their risk is increased, allowing them to make more informed decisions about their behaviours and treatment.
Keywords: coronary heart disease, primary care, primary prevention
Introduction
Despite important gains in the fight against coronary heart disease (CHD) over the last few decades, CHD remains a major cause of premature death and disability in many developed countries. While many of the key risk factors for CHD, such as cigarette smoking and raised blood pressure and blood cholesterol, are readily detectable and can be modified to reduce patients' risk of premature disease, for many busy clinicians properly measuring and managing this risk can be challenging. Factors contributing to this challenge include the multifactorial nature of CHD, 1 recommendations that objective risk estimates be used to guide therapeutic decisions, 2 , 3 the need to actively involve patients in such decisions, 4 , 5, 6, 7, 8, –9 and constraints on time. 10 , 11 Unaided, many family doctors overestimate their patients' risk of CHD and the potential benefits of risk factor interventions, 12 suggesting that at least some patients are prescribed preventive therapies with only a marginal chance for short‐term benefit. Given that a third of patients might refuse such medications if they were fully informed of the potential risks and benefits of therapy, 13 , 14 the need for simple, feasible risk assessment methods seems clear.
Various tools have been developed to help clinicians estimate their patients' risk of CHD. 1 , 2 , 15 , 16, 17, 18, 19, 20, 21, 22, –23 However, while many of these aids have been available and promoted for some time, little is known about whether they are used or even feasible in primary care settings. 11 , 15 , 24 , 25, –26 In one survey of Ontario family doctors, a third of respondents cited lack of time or remuneration as key barriers to providing the kind of counselling these tools require. 11 One approach to this challenge in other contexts has been the development of self‐administered decision aids: interactive videodiscs or workbooks that patients review on their own, and then return to their doctor prepared for consultation. 27 , 28, –29 Other advantages of self‐administered decision aids have been their ability to correct misperceptions patients have about personal risks and benefits, and to facilitate patients' participation in treatment decisions. 27 , 28
Recently, we surveyed Ontario family doctors to assess whether there was need for `a workbook that would allow adults without symptoms of CHD to estimate their 10‐year risk of a coronary event'; 89% believed there was such a need. 11 Accordingly, we set out to develop a simple, inexpensive booklet using algorithms from the Framingham Heart Study. 1 Here we report on the feasibility and acceptability of the workbook as judged by 20 community‐based family doctors and 40 patients for whom they would have used the workbook under normal practice conditions. In addition, given evidence of how peoples' misperceptions can affect preventive health behaviour, 30 we assessed whether using the workbook had an effect on patients' self‐perceived risk of CHD.
Methods
The workbook
Scientific basis for Heartcheck
The workbook, which we tentatively called Heartcheck, was an adaptation of the American Heart Association's CHD Risk Factor Prediction Chart. 1 Like most other Framingham‐based aids, this chart uses a person's age, sex and risk factor values to estimate the individual's 10‐year risk of a coronary event (angina pectoris, myocardial infarction or coronary death) based on the experience of the original and offspring cohorts of the Framingham Heart Study. 1 Accordingly, patients were eligible to use Heartcheck if they met essentially the same criteria that governed entry into the Framingham cohorts:
(1) Age 30–74 years.
(2) Measurements for systolic (SBP) and diastolic blood pressure (DBP) (mmHg), cigarette smoking status (i.e. current smoker or quit within the last year), total and high density lipoprotein (HDL) cholesterol (mmol/L), and diagnoses (yes or no) of diabetes (i.e. on treatment with insulin or oral agents, or a fasting glucose of >7.8 mmol/L) and ECG‐detectable left ventricular hypertrophy (LVH) (when information about diabetes or LVH was not available, diagnoses were presumed negative).
(3) Freedom from cardiovascular disease (i.e. stroke, transient ischemia, CHD (angina pectoris, myocardial infarction), congestive heart failure and intermittent claudication). For patients who did not know their blood cholesterol values, age/sex‐specific population values were provided in the workbook. 31
Comparisons of Framingham‐based risk estimates with the results of other cohort studies and clinical trials show the prediction models to be reasonably accurate and valid. 23 , 25 , 32 However, the models do have some limitations, particularly when used for estimating the benefits of specific risk factor interventions. First, projections based on observational data represent the average benefit expected among a group of similar individuals; in reality, individual gains will vary. Secondly, the predictions assume immediate intervention effects on CHD risk, when in fact the effects are delayed by periods that vary by risk factor. Previously reported lag periods for smoking cessation, cholesterol‐lowering and antihypertensive therapy are 2–4 years, 2–3 years and 1 year, respectively. 32 Thirdly, sufficiently large randomized trials against which CHD benefit projections can be validated are few and have only rarely included women or the elderly. Projections for smoking cessation programmes, for example, presently cannot be validated, and those for antihypertensive therapy and cholesterol‐lowering therapy with statins may over‐ and under‐estimate benefits, respectively. 33 , 34
Developing Heartcheck
The study protocol, which was approved by an Ethics Review Board at Sunnybrook Health Science Centre in Toronto, Ontario, involved convening two sequential groups of family physicians: the first attended focus group meetings to help develop Heartcheck and an accompanying practitioners' guide; the second used the workbook and guide in practice.
The focus groups were two convenience samples of five family physicians in community practice recruited by mail from Sunnybrook Health Science Centre's Department of Family and Community Medicine. Prior to meetings, participants' initial reactions to draft versions of the workbook and guide were captured on an evaluation form and summarized for presentation during the meetings. The objectives of the meetings were to: (a) learn about participants' overall impressions of the workbook and guide; (b) discuss their responses to the more detailed questions on the evaluation form; and (c) provide a forum for participants to share their ideas for revisions, further evaluation and implementation. Subsequently, participants were supplied with revised versions of both documents and were contacted by telephone to confirm whether the prototypes were suitable for field testing. This call also was used to obtain advice regarding the evaluative questions to be asked of physicians and patients involved in the field test.
Format and content of Heartcheck
Heartcheck was a 15‐page, 14.0 × 21.6 cm booklet written in English at a grade 8 reading level. It had three main sections. Section one defined CHD and the concepts of risk and risk factor, identified who was eligible to use the workbook, and described three `other risk factors' excluded from the formal risk calculation (obesity, physical inactivity and family history of premature CHD), indicating why they were excluded. Section two used a separate page to described each major risk factor and present a table for translating the patient's risk factor value into a corresponding point score. 1 For patients who did not know their blood cholesterol values, population‐based values were supplied. 31 A fold‐out page at the end of section two provided space for recording each risk factor point score and tallying the total point score. This page also included a table for translating the total point score into the patient's risk estimate. We defined the estimate as representing `… the number of people, out of 100 people just like you, who are likely to have angina or a heart attack (which may or may not be fatal) sometime over the next 10 years'. Finally, section three included a table for assessing the patient's relative risk, a page listing the names and telephone numbers of organizations providing further information about CHD, and a fold‐out `Risk Factor Log' which provided space for patients to record their risk factor values and corresponding risk estimates for future reference.
The field test: subjects and methods
The field test, which took place between September 1996 and January 1997, involved a new sample of 20 family physicians in community practice recruited by mail from the Department of Family and Community Medicine, Sunnybrook Health Science Centre, Toronto (n=10) and the Teaching Practices Network (Network) of the University of Toronto's Department of Family Medicine (n=10). All Network members within 2‐hours' driving distance from Toronto were approached. The first 20 doctors who responded to our mailing were enrolled.
For physicians, participation involved: (a) meeting with a project coordinator to review the study protocol; (b) using Heartcheck in consultations with two patients; and (c) discussing their experiences with Heartcheck during a 30‐minute follow‐up interview with the coordinator. Basic demographic and practice information also was collected during the interview. For their participation, physicians received an honorarium (at a rate recommended by the Ontario Medical Association) for the time they spent preparing for and meeting with the coordinator, and for study‐related consultations with patients. Patients' travel expenses also were paid.
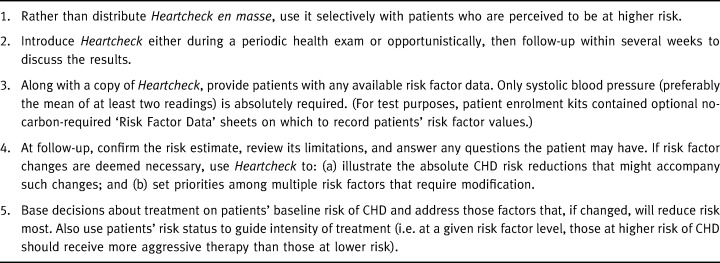
Physicians were instructed to enrol two patients who met the study entry criteria (30–74 years of age and no symptoms of CHD), and for whom they would be likely to use Heartcheck under normal practice conditions. In addition, they were asked to consider some basic procedural guidelines recommended by the focus groups (Table 1).
Table 1.
Procedural guidelines for using Heartcheck
For patients, participation involved: (a) making an office visit (or extending one previously arranged) to discuss study participation, provide informed consent and estimate their perceived 10‐year risk of CHD; (b) completing Heartcheck and meeting with their doctors to discuss the results; and (c) participating in a 30‐minute follow‐up interview with the project coordinator. Perceived risk was assessed during the patient's initial physician visit and again at the beginning of the follow‐up interview with the project coordinator using a standardized, self‐administered questionnaire (Box 8).Copies of the questions used in the physician and patient interviews are available on request.
Table 2.
Box 1 Question used to assess patients' self‐perceived coronary risk

Sample size and data analysis
To help judge the acceptability of the workbook, we predefined some performance criteria based on discussions with the focus groups. Their key concern was whether the workbook would be truly self‐administered. We assumed that most physicians would be willing to try the workbook if at least 70% of patients could complete it unassisted. Thus, we aimed for a sample size that would allow us to detect an 80% rate of self‐administration within 10% and with 90% confidence. 35
Proportions and means (with standard deviations (SD)) were used to summarize the study results. Comments on the workbook were grouped into categories and reported as frequency counts and proportions. Finally, a paired t‐test was used to test for a difference between patients' mean pre‐ and post‐Heartcheck risk perceptions, setting Type I error (alpha) at 0.05. Data were managed and analyzed using Foxpro® and SAS® software.
Results
Characteristics of participants
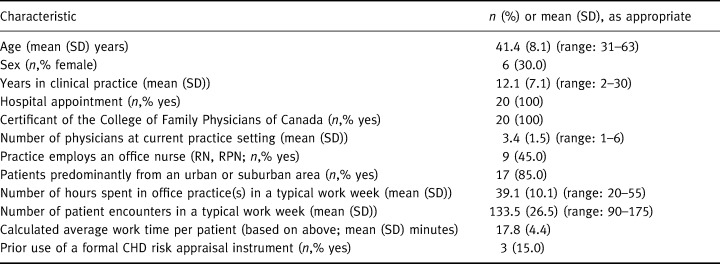
Table 2 summarizes the characteristics of the study physicians and practices. The age and sex distributions of the doctors were similar to those of a random sample of primary care physicians drawn from the College of Physicians and Surgeons of Ontario's Registrant Database for an earlier survey. 11 They also were similar to survey respondents in terms of the number of hours they worked and the number of patients they saw during a typical work week. 11 However, given our sampling frame, it is not surprising that our subjects were more likely to both be certified family physicians (as opposed to general practitioners) and practice in an urban or suburban area.
Table 2.
Characteristics of physicians and their practices. n=20
Patients' risk factor data and CHD risk estimates are presented in Table 3, along with other information we collected during our follow‐up interviews (conducted an average of 12.8 (SD: 13.1) days after patients' second physician visit). Because we did not have access to patients' charts, we did not confirm the accuracy of the risk factor data that patients reported or whether they had clinical evidence of CHD that would have made them ineligible for study.
Table 3.
Characteristics of patients. n = 40, unless otherwise indicated
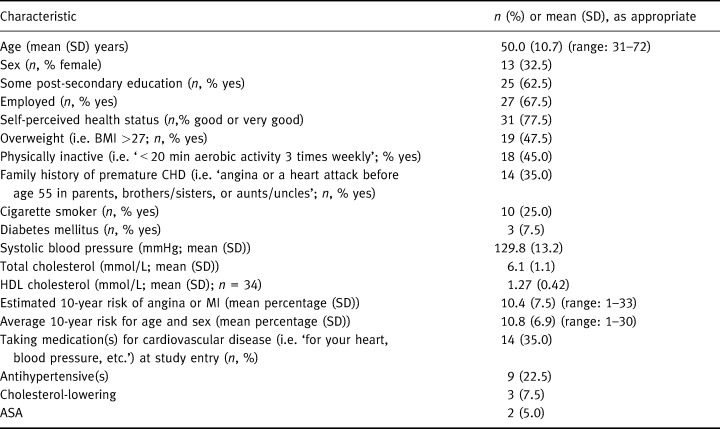
The focus groups recommended using Heartcheck mainly with high‐risk patients. However, as shown in Table 3, patients' risk estimates varied considerably, ranging from 1% to 33%, half with 10‐year CHD risks of less than 10%. Subjects were well educated, perceived themselves to be in good health compared with others their age, and were described by study physicians as being representative of patients for whom they would use Heartcheck under normal practice conditions. Physicians estimated the size of the target population to be about 18% of their patients on average (median: 10%; range: 79%).
Feasibility and perceived helpfulness of Heartcheck
Table 4 presents some measures of feasibility and helpfulness. Excluding trial‐related discussion, it took study physicians an average of 7.6 (SD: 4.4) minutes to introduce Heartcheck and a further 11.3 (SD: 5.8) minutes to discuss the results, for an average overall time commitment of 18.9 (SD: 9.1) minutes: roughly equivalent to physicians' average consultation time during a typical work week (Table 2). Thirty‐one (78%) patients reported completing the workbook without assistance.
Table 4.
Feasibility and perceived helpfulness of Heartcheck
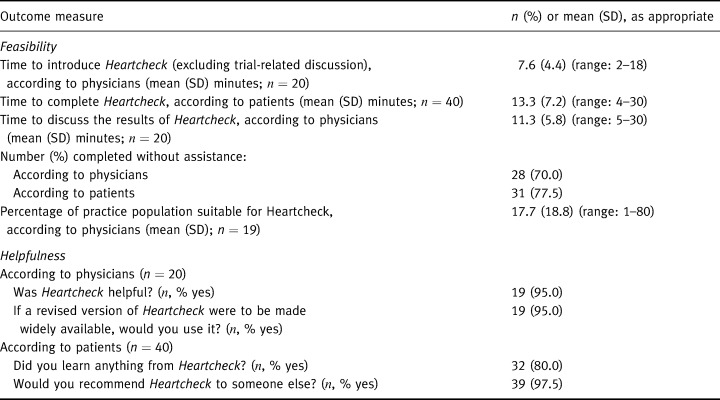
Heartcheck's reviews were generally favourable. Thirty‐two (80%) patients felt they had learned something from the workbook and all but one indicated they would recommend it to someone else. Similarly, only one physician did not consider the workbook helpful. Most said they would use it essentially as recommended by the focus groups (Table 1). Physicians' specific concerns are listed in Table 5. Key issues were the absence of `family history of premature CHD' from the algorithm, and the perceived usefulness of Heartcheck for younger patients (e.g. those under 45 years of age) and those with low reading or numeracy levels. Concerns raised by at least two patients were: difficulties with the arithmetic; difficulty understanding the concept of `risk'; confusion about the meaning of SBP and DBP (e.g. `definition of `high' BP differs from my physician's'); and difficulty extracting appropriate age/sex‐specific risk data from the table provided in the workbook.
Table 5.
Physicians' concerns about and perceived barriers to using Heartcheck. n= 16 respondents; multiple responses permitted
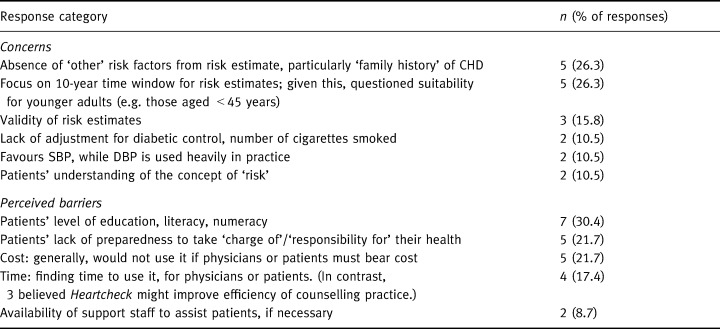
Patients' perceived vs. objective risk of CHD
Thirty‐seven patients gave an assessment of their perceived coronary risk both before and after using Heartcheck. As shown in Fig. 1, prior to using the workbook patients almost universally overestimated their risk, some by a considerable margin. A mean of 12.8 (SD: 13.1) days after using Heartcheck, this gap was significantly reduced (t=6.886; P < 0.0001). Self‐perceived risk fell in 28 subjects, remained unchanged in seven subjects and rose slightly in two.
Figure 1.
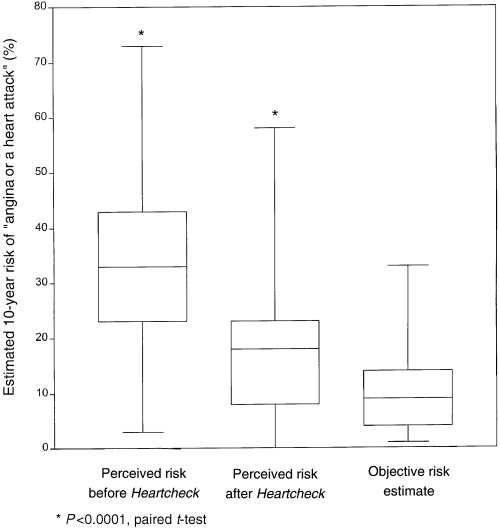
Patients' objective vs. perceived coronary risk before and after using Heartcheck.
When asked what their immediate reaction was on learning their risk estimate, given their perceptions, not surprisingly, most patients were relieved. The full range of their reactions is listed in Table 6.
Table 6.
Patients' immediate reactions to learning their estimated risk of CHD. n= 40 respondents; multiple responses permitted

Patients' intentions on using Heartcheck
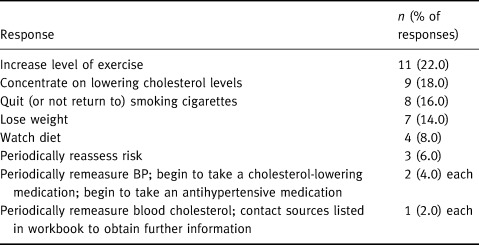
Twenty‐seven patients (68%) identified a total of 50 actions they would take as a result of using the workbook. These are summarized in Table 7. The most prevalent intentions were to increase activity levels, lower blood cholesterol levels, quit smoking and lose weight. Importantly, two subjects reported they were less inclined to change risky behaviours; both were male and less than 45 years of age.
Table 7.
Patients' intensions on using Heartcheck n=27 respondents; multiple responses permitted
Discussion
Although various risk assessment tools are now widely available and promoted, 1 , 2 , 15 , 16, 17, 18, 19, 20, 21, 22, –23 few studies have assessed whether they are used or even feasible in primary care settings. 15 , 24 , 26 , 36 , 37 In this study of 20 largely urban teaching practices, only three physicians (15%) had used a coronary risk prediction chart before. Might doctors make better use of risk/benefit data if they were easier to access? To test this hypothesis, Lowensteyn and colleagues offered 455 Ontario and Quebec family doctors an opportunity to use a free, centralized CHD risk appraisal service. 26 To obtain a risk estimate, physicians were asked to record a patient's risk factor data on a standardized form and submit it to the study centre at least 1 week prior to the patient's office visit. In return, they received a one‐page printout that displayed the patient's estimated 8‐year risk of CHD and the amount by which this risk would be reduced if risk factors were modified. By the end of the 6‐month study, only half of the physicians who were offered the service had used it. Among the authors' recommendations was a call to get patients more involved. 26
Here we provide some preliminary evidence for the feasibility and acceptability of one approach to involving patients in the risk assessment process. Most of the patients who were given Heartcheck completed it on their own, claimed they had learned something, and were willing to recommend it to someone else. Using Heartcheck also largely corrected misperceptions patients had about their personal risk. Similarly, all but one physician found Heartcheck helpful, and the remainder were willing to use it with an average of 18% of their patients. While our subjects were a select group, even half this proportion represents an important opportunity for education.
That said, physicians believed that Heartcheck had some practical limitations. Most important was a lack of formal recognition of the contribution of family history of premature CHD to coronary risk. Although Framingham researchers have not identified `family history' as an independent risk factor, 38 other groups, such as the US National Cholesterol Education Program, have recommended that physicians consider it when appraising risk in individual patients. 39 This led reviewers to conclude that Heartcheck was at odds with current practice and teaching. One possible solution is to simply add `family history' to the formal risk calculation by assigning it a `token' weight and appropriately highlighting the caveats. This change would somewhat compromise the accuracy of the risk estimates, but the trade‐off in terms of improved utilization may be worthwhile. Larger studies would be needed to determine whether such revisions are justified. A second criticism of the workbook was its reliance on systolic rather than diastolic blood pressure. This also was seen as contrary to current practice, despite the fact that SBP is a better predictor of vascular events. 38 Finally, several physicians were concerned about the validity of the risk estimates given the workbook's failure to discriminate between light and heavy smokers, or between well‐controlled and uncontrolled diabetics. As a group, these concerns, some of which could be mitigated through better physician education materials, apply equally well to most other risk assessment tools based on the Framingham equations. 1 , 2 , 16 , 17, 18, 19, 20, 21, 22, –23 Although both physicians and patients identified literacy and numeracy as potential barriers to Heartcheck's broader application, we did achieve our target self‐administration rate of 70%. Whether and how we can improve upon this rate remains to be explored.
One issue that deserves further study is the potential for adverse effects on patients who have multiple risk factors but who, by virtue of their age, are at low absolute risk of a coronary event in the short term. Experience using the workbook with these individuals led physicians to believe that Heartcheck might reassure rather than motivate, a phenomenon independently confirmed by our patient interviews (Table 6), and reported by others. 40 One suggestion was to restrict the workbook to patients over 45 years of age, unless they are particularly anxious about their risk. Since high levels of anxiety can interfere with health‐seeking behaviour, 30 this could be an unintended but potentially useful role for the workbook.
Ours is not the first study to show that people commonly misperceive their risk of CHD, 41 , 42, 43, –44 or that these misperceptions can be corrected. 41 , 45 On the other hand, it may be the first to report such high levels of overestimation. In studies of population samples 41 , 42, –43 and people screened for CHD risk factors, 44 subjects have been more likely to underestimate than overestimate their risk. Possible reasons for this discrepancy include the fact that prior studies assessed relative risk rather than absolute risk and none involved patients approached by their physicians specifically for the purpose of risk factor counselling. Although we know that providing personalized risk information can help correct people's misperceptions, surprisingly little is known about its effects on subsequent health behaviour. This is due largely to a shortage of well‐designed, well‐conducted trials. 40 However, other potential problems include the typically weak messages imparted by most health risk appraisal (HRA) instruments (e.g. `If you adhere to an exercise program prescribed by your doctor, your risk of dying from heart disease will be reduced and you will extend your useful life expectancy by 0.1 years') and a trend towards using HRAs in non‐clinical settings, without the support of risk factor counselling. 40
In the trial by Lowensteyn and colleagues, patients whose counselling sessions were accompanied by coronary risk profiles showed significantly greater 3‐month reductions in both blood cholesterol levels and estimated coronary risk, relative to controls. 26 In another trial, Kreuter and Strecher found that, among smokers who received no information about their risk, those who had accurate stroke risk perceptions at baseline were more likely to have quit smoking 6 months later than smokers whose risk perceptions were overly optimistic. 45 Similarly, those who had pessimistic perceptions of cancer risk were more likely at follow‐up to have seen their doctor at least twice in the preceding 2 months than those who had accurate risk perceptions. In this particular study, personal health risk information reduced optimistic bias for perceived stroke risk and pessimistic bias for perceived cancer risk, but had no effect on pessimistic perceptions of stroke risk, optimistic perceptions of cancer risk, or any risk perception biases related to heart attacks or motor vehicle accidents. 45 Taken together, these findings suggest that although individualized risk data may increase the likelihood of certain positive behaviours in some people, perceptions of risk for different diseases also may interact in complex ways. More work is needed to help identify those most likely to benefit from Heartcheck and tools like it.
For physicians, one potential benefit of Heartcheck may be more efficient use of resources for counselling and follow‐up. In the trial by Lowensteyn et al. relative to control physicians, those who had access to risk profiles reassessed a larger proportion of higher‐ risk patients within 6 months of study entry, implying that the profiles improved doctors' ability to target their counselling efforts and/or selectively motivated the higher‐risk patients to return. 26 This finding has important implications for practice. Related questions are whether using the workbook affects physicians' actual contact time with patients and what effect, if any, it has on patients' decisions about and adherence to preventive medications. Here we paid physicians to introduce the workbook and to obtain patients' consent to participate. Excluding trial‐related discussion, this took an average of about 7 minutes or roughly 40% of the total time physicians spent with subjects. While this kind of commitment would seriously limit the usefulness of Heartcheck in actual practice, we believe that strategies to reduce or eliminate this burden could be found, particularly with the help of office staff. Identifying these strategies would be another important objective for future research. If absolute risk thresholds for cholesterol testing or preventive drug therapy were to accompany Heartcheck, the resource implications of such thresholds would have to be explored. 46 , 47
While this is one of few studies to assess the feasibility of coronary risk appraisal in primary care practice, the study's size and sampling strategy prevent us from drawing firm conclusions about the workbook's broader application. Nevertheless, our results are encouraging. We have shown that, given a simple tool, patients can and will assess their own risk of CHD. At the very least, such tools could help inform otherwise healthy individuals that their risk is increased, allowing them to make more informed decisions about their behaviours and treatment. More work is needed to help identify those most likely to benefit from these tools.
Acknowledgements
We would like to thank the physicians and patients who participated in this study. We also thank Michele Melady and Donna Polyak for their help in conducting interviews and preparing the report. This study was funded by the Institute for Clinical Evaluative Sciences.
The results and conclusions are those of the authors. No endorsement by the Ontario Ministry of Health or the Institute for Clinical Evaluative Sciences is intended or should be inferred.
References
- 1. Anderson KM, Wilson PWF, Odell PM, Kannel WB. An updated coronary risk profile: a statement for health professionals. Circulation, 1991; 83 : 356–362. [DOI] [PubMed] [Google Scholar]
- 2. Wood D, De Backer G, Faergeman O, Graham I, Mancia G, Pyorala K. Prevention of coronary heart disease in clinical practice: recommendations of the Second Joint Task Force of European and other Societies on Coronary Prevention. Atherosclerosis, 1998; 140 : 199–270. [DOI] [PubMed] [Google Scholar]
- 3. Grundy SM, Balady GJ, Criqui MH et al. Primary prevention of coronary heart disease: guidance from Framingham: a statement for healthcare professionals from the AHA Task Force on Risk Reduction. American Heart Association. Circulation, 1998; 97 : 1876–1887. [DOI] [PubMed] [Google Scholar]
- 4. Brett AS. Ethical issues in risk factor modification. American Journal of Medicine, 1984; 76 : 557–561. [DOI] [PubMed] [Google Scholar]
- 5. Farrow L, Wartman SA, Brock DW. Science, ethics, and the making of clinical decisions: implications for risk factor modification. Journal of the American Medical Association, 1988; 259 : 3161–3167. [PubMed] [Google Scholar]
- 6. Marshall KG. Prevention. How much harm? How much benefit? 4. The ethics of informed consent for preventive screening programs. Canadian Medical Association Journal, 1996; 155 : 377–383. [PMC free article] [PubMed] [Google Scholar]
- 7. Donovan J. Patient decision making: the missing ingredient in compliance research. International Journal of Technology Assessment in Health Care, 1995; 11 : 443–455. [DOI] [PubMed] [Google Scholar]
- 8. Britten N. Communication: the key to improved compliance. Prescriber, 1998; 9 : 27–31. [Google Scholar]
- 9. Rosser W. Application of evidence from randomized controlled trials to general practice. Lancet, 1999; 353 : 661–664. [DOI] [PubMed] [Google Scholar]
- 10. Fix KN, Oberman A. Barriers to following National Cholesterol Education Program guidelines. An appraisal of poor physician performace. Archives of Internal Medicine, 1991; 152 : 2385–2387. [PubMed] [Google Scholar]
- 11. Llewellyn‐Thomas HA, Norton PG, Melady M, Paterson JM. Cholesterol‐lowering medication in primary prevention: physicians' opinions about patients' decision aids. Medical Decision Making, 1996; 16 : 448 448(Abstract). [Google Scholar]
- 12. Grover SA, Lowensteyn I, Esrey KL et al. Do doctors accurately assess coronary risk in their patients? Preliminary results of the coronary health assessment study. British Medical Journal, 1995; 310 : 975–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Llewellyn‐Thomas HA, Paterson JM, Carter JA et al. Cholesterol‐lowering medication in primary prevention: can physicians meet patients' `demands' for reduced coronary risk? Medical Decision Making, 1994; 14 : 436 436(Abstract). [Google Scholar]
- 14. Llewellyn‐Thomas HA, Carter JA, Paterson JM, Naylor CD. Antihypertensive medication for primary prevention: are patients' risk reduction requirements realistic? Medical Decision Making, 1995; 15 : 431 431(Abstract). [Google Scholar]
- 15. Tunstall‐Pedoe. The Dundee coronary risk‐disk for management of change in risk factors. British Medical Journal, 1991; 303 : 744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jackson R, Barham P, Bills J et al Management of raised blood pressure in New Zealand: a discussion document. British Medical Journal, 1993; 307 : 107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pyorala K, De Backer G, Graham I, Poole‐Wilson P, Wood D. Prevention of coronary heart disease in clinical practice: recommendations of the Task Force of the European Society of Cardiology, European Atherosclerosis Society and European Society of Hypertension. Atherosclerosis, 1994; 110 : 121–161. [DOI] [PubMed] [Google Scholar]
- 18. Jackson R, Beaglehole R. Evidence‐based management of dyslipidaemia. Lancet, 1995; 344 : 1440–1441. [DOI] [PubMed] [Google Scholar]
- 19. Haq IU, Jackson PR, Yeo YY, Ramsey LE. Sheffield risk and treatment table for cholesterol lowering for primary prevention of coronary heart disease. Lancet, 1995; 346 : 1467–1471. [DOI] [PubMed] [Google Scholar]
- 20. Ramsey LE, Haq IU, Jackson PR et al. Targeting lipid‐lowering drug therapy for primary prevention of coronary heart disease: an updated Sheffield table. Lancet, 1996; 348 : 387–388. [DOI] [PubMed] [Google Scholar]
- 21. McCormack JP, Levine M, Rangno RE. Primary prevention of heart disease and stroke: a simplified approach to estimating risk of events and making drug treatment decisions. Canadian Medical Association Journal 1997; 157 : 422–428. [PMC free article] [PubMed] [Google Scholar]
- 22. Wilson PWF, D'Agostino RB, Levy D et al. Prediction of coronary heart disease using risk factor categories. Circulation, 1998; 97 : 1837–1847. [DOI] [PubMed] [Google Scholar]
- 23. Hingorani AD, Vallence P. A simple computer program for guiding management of cardiovascular risk factors and prescribing. British Medical Journal, 1999; 318 : 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hutchison B, Birch S, Evans CE et al. Screening for hypercholesterolaemia in primary care: randomised controlled trial of postal questionnaire appraising risk of coronary heart disease. British Medical Journal, 1998; 316 : 1208–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Durrington PN, Prais H, Bhatnagar D et al. Indications for cholesterol‐lowering medication: comparison of risk‐assessment methods. Lancet, 1999; 353 : 278–281. [DOI] [PubMed] [Google Scholar]
- 26. Lowensteyn I, Joseph L, Levinton C et al. Can computerized risk profiles help patients improve their coronary risk? The results of the Coronary Health Assessment Study (CHAS). Preventive Medicine, 1998; 27 : 730–737.DOI: 10.1006/pmed.1998.0351 [DOI] [PubMed] [Google Scholar]
- 27. O'Connor AM, Tugwell P, Wells GA et al. A decision aid for women considering hormone therapy after menopause: decision support framework and evaluation. Patient Education and Counseling, 1998; 33 : 267–279.DOI: 10.1016/s0738-3991(98)00026-3 [DOI] [PubMed] [Google Scholar]
- 28. Barry MJ, Fowler FJ Jr, Mulley AG Jr, Henderson JV Jr, Wennberg JE. Patient reactions to a program designed to facilitate patient participation in treatment decisions for benign prostatic hyperplasia. Medical Care, 1995; 33 : 771–782. [DOI] [PubMed] [Google Scholar]
- 29. Shepperd S, Coulter A, Farmer A. Using interactive videos in general practice to inform patients about treatment choices: a pilot study. Family Practice, 1995; 12 : 443–447. [DOI] [PubMed] [Google Scholar]
- 30. Lerman C, Schwartz M. Adherence and psychological adjustment among women at high risk for breast cancer. Breast Cancer Research and Treatment, 1993; 28 : 145–155. [DOI] [PubMed] [Google Scholar]
- 31. Connelly PW, MacLean DR, Horlick L et al. Plasma lipids and lipoproteins and the prevalence of risk for coronary heart disease in Canadian adults. Canadian Heart Health Surveys Research Group. Canadian Medical Association Journal, 1992; 146 : 1977–1987. [PMC free article] [PubMed] [Google Scholar]
- 32. Grover SA, Coupal L. Risk‐benefit assessment of drug treatment to prevent coronary heart disease. Estimating the benefits of risk factor modification. Drug Safety, 1994; 10 : 301–309. [DOI] [PubMed] [Google Scholar]
- 33. Collins R, Peto R, MacMahon S et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short‐term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet, 1990; 335 : 827–838. [DOI] [PubMed] [Google Scholar]
- 34. West of Scotland Coronary Prevention Study Group. Influence of pravastatin and plasma lipids on clinical events in the West of Scotland Coronary Prevention Study (WOSCOPS). Circulation, 1998; 97 : 1440–1445. [DOI] [PubMed] [Google Scholar]
- 35. Lwanga SK, Lemeshow S. Sample Size Determination in Health Studies Geneva: World Health Organization, 1991.
- 36. Isles CG, Ritchie LD, Murchie P, Norrie J. Risk assessment in primary prevention of coronary heart disease: randomized comparison of three scoring methods. British Medical Journal, 2000; 320 : 690–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Montgomery AA, Fahey T, Peters TJ, MacIntosh C, Sharp DJ. Evaluation of computer‐based clinical decision support and risk chart for management of hypertension in primary care: randomized controlled trial. British Medical Journal, 2000; 320 : 686–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kannel WB, Larson M. Long‐term epidemiologic prediction of coronary disease. The Framingham experience. Cardiology, 1993; 82 : 137–152. [DOI] [PubMed] [Google Scholar]
- 39. Summary of the Second Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, Treatment of High Blood Cholesterol in Adults. Journal of the American Medical Associaiton, 1993; 269 : 3015–3023. [PubMed] [Google Scholar]
- 40. Schoenbach V, Wagner EH, Beery WL. Health risk appraisal: review of evidence for effectiveness. Health Services Research, 1987; 22 : 553–580. [PMC free article] [PubMed] [Google Scholar]
- 41. Avis NE, Smith KW, McKinlay JB. Accuracy of perceptions of heart attack risk: what influences perceptions and can they be changed? American Journal of Public Health, 1989; 79 : 1608–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Niknian M, McKinlay SM, Rakowski W, Carleton RA. A comparision of perceived and objective CVD risk in a general population. American Journal of Public Health, 1989; 79 : 1653–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ayanian JZ, Cleary PD. Perceived risks of heart disease and cancer among cigarette smokers. Journal of the American Medical Association, 1999; 281 : 1019–1021. [DOI] [PubMed] [Google Scholar]
- 44. Marteau TM, Kinmonth AL, Pyke S, Thompson SG. Readiness of lifestyle advice: self‐assessments of coronary risk prior to screening in the British family heart study. Family Heart Study Group. British Journal of General Practice, 1995; 45 : 5–8. [PMC free article] [PubMed] [Google Scholar]
- 45. Kreuter MW, Strecher VJ. Changing inaccurate perceptions of health risk: results from a randomized trial. Health Psychology, 1995; 14 : 56–63. [DOI] [PubMed] [Google Scholar]
- 46. Robson J, Boomla K, Hart B, Feder G. Estimating cardiovascular risk for primary prevention: outstanding questions for primary care. British Medical Journal, 2000; 320 : 702–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baker S, Priest P, Jackson R. Using thresholds based on risk of cardiovascular disease to target treatment for hypertension: modelling events and number treated. British Medical Journal, 2000; 320 : 680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]


