Abstract
Chronic stress accelerates metastasis - the main cause of death in cancer patients - through the activation of β-adrenoceptors (βARs). We have previously shown that β2AR signaling in MDA-MB-231HM breast cancer cells, facilitates invadopodia formation and invasion in vitro. However, in the tumor microenvironment where many stromal cells also express βAR, the role of β2AR signaling in tumor cells in metastasis is unclear. Therefore, to investigate the contribution of β2AR signaling in tumor cells to metastasis in vivo, we used RNA interference to generate MDA-MB-231HM breast cancer cells that are deficient in β2AR. β2AR knockdown in tumor cells reduced the proportion of cells with a mesenchymal-like morphology and, as expected, reduced tumor cell invasion in vitro. Conversely, overexpression of β2AR in low metastatic MCF-7 breast cancer cells induced an invasive phenotype. Importantly, we found that knockdown of β2AR in tumor cells significantly reduced the impact of stress on metastasis in vivo. These findings highlight a crucial role for β2AR tumor cell signaling in the adverse effects of stress on metastasis, and indicate that it may be necessary to block β2AR on tumor cells to fully control metastatic progression.
Keywords: triple negative breast cancer, β2-adrenoceptor, chronic stress, metastasis, invasion
INTRODUCTION
Despite advances in cancer treatment, metastasis remains the greatest clinical challenge in the management of cancer and contributes to the majority of deaths in cancer patients (Mehlen and Puisieux, 2006). Studies have revealed that chronic stress drives cancer progression by accelerating metastasis in in vivo mouse models of various cancer types (Ben-Eliyahu et al., 2000; Campbell et al., 2012; Kim-Fuchs et al., 2014; Lamkin et al., 2015; Le et al., 2016; Liu et al., 2015; Sloan et al., 2010; Thaker et al., 2006; Zhao et al., 2015). This raises the possibility that targeting stress responsive signaling may help slow cancer progression and metastatic dissemination (Sloan et al., 2010; Thaker et al., 2006), and provide a much needed additional therapy for the treatment of cancer.
The sympathetic nervous system (SNS) plays an important role in the response to stress, which triggers the release of catecholaminergic neurotransmitters from SNS nerve fibers (Elenkov et al., 2000). These neurotransmitters activate β-adrenoceptors (βAR), which induces downstream signaling in responsive cells and leads to transcriptional changes (Elenkov et al., 2000). A number of different cell types present within the tumor microenvironment express βARs, and thus are able to respond to stress signaling. These include immune cells and endothelial cells (Abrass et al., 1985; Graf et al., 1993; Sanders et al., 1997), which have a critical role in driving cancer progression (Condeelis and Pollard, 2006; Folkman, 2002; Le et al., 2016). In response to stress, stromal cells contribute to metastasis by remodeling tumor architecture in ways that favor dissemination of tumor cells. This includes macrophage recruitment into the primary tumor (Sloan et al., 2010; Zhao et al., 2015) and vascular remodeling to increase blood vessel (Sloan et al., 2010; Thaker et al., 2006) and lymph vessel (Le et al., 2016) routes of tumor cell dissemination. Experimental strategies that prevent either macrophage recruitment or vascular remodeling block stress-enhanced metastasis (Le et al., 2016; Sloan et al., 2010; Thaker et al., 2006), demonstrating that regulation of the tumor stroma plays an important role in the effects of stress on cancer progression.
Tumor cells also express βARs (Pon et al., 2016; Reeder et al., 2015), and activation of βAR signaling increases invasion of tumor cells, as measured by in vitro assays (Creed et al., 2015; Kim-Fuchs et al., 2014; Pon et al., 2016; Yamazaki et al., 2014) and in explant cultures (Creed et al., 2015). Previously, we discovered that the β2AR-selective agonist formoterol, but not the β1AR-selective agonist xamoterol, induced the formation of invadopodia in breast cancer cells (Creed et al., 2015). Invadopodia are actin-rich cellular structures that localize matrix metalloproteases (MMPs) and degrade the extracellular matrix for tumor cell invasion (Murphy and Courtneidge, 2011). However, the role of β2AR-regulated invasion in vivo is less clear. Unlike in simple in vitro assays, in the tumor microenvironment contextual cues provided by stromal cells influence whether tumor cells are able to escape the primary tumor and disseminate to distant tissues (Bissell and Labarge, 2005; Devaud et al., 2014). Therefore, in the complex tumor microenvironment where stromal cells also respond to βAR stress signaling, it is unclear whether β2AR signaling in tumor cells significantly contributes to metastasis. Previous studies that used systemic β-blockade to investigate βAR regulation of metastasis were unable to distinguish the contribution of βAR signaling in tumor cells, as systemic β-blockade indiscriminately targets both tumor cells and stromal cells (Campbell et al., 2012; Sloan et al., 2010; Thaker et al., 2006). While use of siRNA has shown that β2AR signaling affects the growth of tumor cells injected directly into metastatic target organs (Thaker et al., 2006), it is not known if signaling from β2ARs on tumor cells is required for the early stages of the metastatic cascade including tumor cell invasion and escape from the primary tumor.
To address this, we used an shRNA approach to generate breast cancer cells that were stably deficient in β2AR. Using MDA-MB-231HM cells, a human breast cancer cell line that is highly responsive to βAR signaling, we investigated the effect of tumor cell β2AR knockdown on metastasis from a primary orthotopic mammary tumor. Consistent with previous pharmacologic studies, genetic modulation of MDA-MB-231HM β2AR reduced cell invasion, and prevented a shift to mesenchymal cell morphology. Conversely, upregulating β2AR expression in MCF-7 tumor cells with low endogenous β2AR expression increased invadopodia formation, demonstrating a central role for β2AR in regulating tumor cell invasion. Furthermore, we show that β2AR knockdown in MDA-MB-231HM tumor cells attenuated stress-enhanced metastasis from primary mammary tumors. These findings show that in this model of breast cancer, β2AR-driven tumor cell invasion plays a significant role in the effects of stress on metastasis. These findings suggest that pharmacological strategies that block the effects of stress on metastasis may need to also target β2AR on tumor cells to fully control metastatic progression.
METHODS
Genetic manipulation of tumor cell β2AR expression
The human breast cancer cell line MDA-MB-231HM (a kind gift from Dr Zhou Ou, Fudan University Shanghai Cancer Center, China) (Chang et al., 2008) was transduced with a lentiviral vector containing codon optimized firefly luciferase under the control of the ubiquitin-C promoter as previously described (Le et al., 2016). The identity of the cell line was confirmed by karyotyping (CellBank Australia). Cells were maintained in DMEM (Invitrogen, USA) supplemented with 10% fetal bovine serum (FBS; Life Technologies, USA) at 37°C with 5% CO2. To silence ADRB2, cells were transduced with shRNA that specifically targets human ADRB2 (shADRB2a: 5’-TGCTGTGACTTCTTCACGA-3’; shADRB2b: 5’-GCCATCAACTGCTATGCCA-3’) or scramble control sequence (SCR): 5’-ATCTCGCTTGGGCGAGAGTAAG-3’ (Dharmacon, USA). MCF-7 cells were acquired from ATCC (Manassas, Virginia) and grown in MEM supplemented 10% FBS. The β2AR-GFP construct was made by sub-cloning the human β2AR coding sequence 5’ to a codon-humanized GFP gene derived from the vector pGFP2-N1 (Perkin Elmer), and sequence verified. Cells were stably transduced and GFP-positive cells sorted by FACS. All experiments used bulk cell populations to avoid clonal effects. Cells were confirmed negative for mycoplasma using MycoAlert™ Mycoplasma Detection Kit (Lonza, Australia).
Functional analysis of β2AR knockdown in tumor cells by cAMP accumulation assay
Tumor cells (4 × 104) were seeded into a 96-well plate and serum-starved overnight. Cells were then washed and incubated in pre-warmed stimulation buffer (140 mM NaCl, 5 mM KCl, 800 nM MgSO4, 200 nM Na2HPO4, 440 nM KH2PO4, 5 mM HEPES, 1.3 mM CaCl2, 5.6 mM glucose, 0.1% w/v BSA, 500 µM 3-isobutyl-1-methylxanthine, pH 7.4) at 37°C in 0% CO2 for 30 minutes. Formoterol hemifumarate (β2AR-selective agonist; Tocris, UK) at indicated concentrations was then added for 10 minutes before cells were lysed with ice-cold 100% ethanol, evaporated and rehydrated with 50 µL detection buffer (0.1% BSA, 5 mM HEPES, 0.3% Tween20, pH 7.4). cAMP in cell lysates (5 µL) was then incubated with 1 unit of AlphaScreen™ acceptor beads (Perkin Elmer, USA) diluted in detection buffer, followed by incubation with 1 unit of donor beads. Fluorescence signal was measured using a Fusion plate reader (Perkin Elmer, USA). cAMP accumulation was expressed as pmol/well.
Tumor cell proliferation
Tumor cells (5 × 104) were seeded into a 12 well plate and serum-starved overnight. Cells were then treated with 1 µM isoproterenol (non-selective β-agonist Sigma, USA) in vehicle (DMEM supplemented with 2% FBS). Isoproterenol was replenished every 24 hours. Cell numbers were quantified using the Tali™ Image-Based Cytometer (Invitrogen, USA) at indicated time points.
Cell morphology assay
Tumor cells (5 × 103) were seeded into a 96 well plate and serum-starved overnight. Cells were then treated with or without 1 µM isoproterenol in the absence or presence of ICI-118,551 (β2AR-selective antagonist, 1 µM, Tocris, UK) in DMEM supplemented with 2% FBS. Isoproterenol and ICI-118,551 were replaced every 24 hr to minimize possible effects of auto-oxidation. Cells were imaged using a 20× long WD objective every 24 hr for 3 days using the Operetta High-Content Imaging System (Perkin Elmer, USA). At each time point, nine random fields of view from each well were captured and analyzed using Harmony® High Content Imaging and Analysis Software (version 3.5, Perkin Elmer). The roundness of individual cells was assessed using the proprietary analytical tools provided in the Harmony Analysis software. The cell roundness parameter ranged between 0 and 1 where a value of 1 is equivalent to perfect roundness. The parameter threshold to define a mesenchymal-like cell was set at 0.5 (Ren et al., 2015; Yang and Weinberg, 2008). The proportion of mesenchymal-like tumor cells in the population meeting this criterion was then quantified.
Invadopodia assay
Gelatin was labelled with Alexa Fluor-568 protein labeling kit according to the manufacturer instructions. Culture vessels were coated with an 8:1 ratio of unlabeled to labeled 0.2% gelatin and topped with a thin layer of unlabeled 10 µg/mL human fibronectin to promote cell adherence. MCF7-WT or MCF7-β2AR cells were serum-starved overnight. Cells were then seeded onto the prepared cultured vessels containing 10% serum, ± βAR agonists (0.5 µM isoproterenol or 0.5 µM formoterol hemifumarate) for 5 hours. Cells were then fixed in 4 % paraformaldehyde, actin stained with phalloidin and nuclei counterstained with 1 µg/ml Hoechst 33242. Invadopodia were imaged on a Leica SP8 Confocal using a 63× PL APO CS2 1.4NA objective. Images were captured through LAS AF software version 3.2 (Leica Microsystems, North Ryde Australia). Images were prepared as Tiff stacks for actin and gelatin using ImageJ and submitted to the Invadopodia Analysis Server (IAS) and analysed as previously described (Creed et al., 2015).
Gene expression analysis
Tumor cells (7 × 105) were seeded into a 6 well plate and serum-starved overnight before treatment with vehicle, isoproterenol (1 µM), and/or ICI-118,551 (β2AR-selective antagonist, 1 µM) in DMEM supplemented with 2% FBS for the time indicated, prior to analysis. Total RNA was isolated using RNeasy Mini Kit (Qiagen, Germany). qRT-PCR was used to quantify gene expression in 100 ng of total RNA using Taqman probes (Applied Biosystems, USA) targeting human βAR subtypes (ADRB1 Hs02330048_s1, ADRB2 Hs00240532_s1, ADRB3 Hs00609046_m1) and MMP2 (MMP2 Hs01548727_m1) and iScript One-Step RT-PCR kit (Biorad, USA) with 50 PCR amplification cycles of 15 seconds of strand separation at 95°C and 30 seconds of annealing and extension at 60°C. Transcript levels for genes of interest were normalized relative to scramble control cells.
Tumor cell invasion assay
Serum-starved tumor cells (1.5 × 105) were suspended in 300 µL DMEM supplemented with 2% FBS with or without 1 µM isoproterenol and seeded into Transwell® chambers (8 µm pores, PET membrane, Sigma, USA) that were pre-coated with 100 µL Matrigel (2mg/mL, BD, USA). For 24 hr, cells were allowed to invade toward the bottom chamber, which was filled with media supplemented with 10% FBS with or without 1 µM isoproterenol. Membranes were then fixed with 4% paraformaldehyde (Sigma, USA) and stained with Hoechst 33342 (1:1000, Sigma, USA) and imaged using a Nikon Ti-E microscope, fitted with 10× Plan Apo 0.3NA objective and a Photometrics CoolSNAP Myo camera. Fluorescent excitation and emission was provided by a Nikon UV-A filter cube (excitation 355/50 and emission 420LP). Images were captured using NIS Elements software (version 4.3, Nikon Instruments). Invaded cells were counted using the Fiji distribution of ImageJ (Schindelin et al., 2012).
Breast cancer model with restraint stress
To investigate spontaneous metastasis from an orthotopic primary tumor, 2 × 105 tumor cells in 20 µL PBS (Invitrogen, USA) were injected into the left 4th mammary fat pad of anesthetized (3% isoflurane) 8 week-old female BALB/c nu/nu mice (University of Adelaide, Australia). Mice were housed under PC2 barrier conditions (temperature and humidity controlled, 12 h dark/light cycle) and acclimatized in a home cage for a week prior to tumor cell inoculation. Primary tumors were monitored by digital caliper twice a week and volume calculated using the formula: (length × width2)/2. Mice were randomly assigned to either stress or non-stress group (n = 5 per group) and acclimatized in a home cage for a week prior to tumor cell inoculation. To induce chronic stress, mice were subjected to a well-characterized restraint stress paradigm (Sloan et al., 2010{Le, 2016 #3; Thaker et al., 2006). Mice were placed in a ventilated Perspex chamber that restricted movement for 2 hours per day for a total of 21 days, commencing 7 days before tumor cell injection. Mice in the non-stress group remained in their home cage throughout the 2 hour period. Distant metastasis to lymph nodes and lung was monitored longitudinally twice weekly using bioluminescence imaging, which allows repeated measure analysis of metastatic burden in the same mouse throughout the experiment. Mice were injected with d-luciferin (150 mg/kg, CHOICE Analytical) via tail vein and imaged using an IVIS Lumina II (Perkin Elmer) (Le et al., 2016; Sloan et al., 2010). Mice were euthanized 28 days after tumor cell injection and tissue-specific metastasis was confirmed in lung and axillary lymph node by ex vivo bioluminescence imaging. In vivo experiments were conducted in duplicate. All procedures involving mice were carried out under protocols approved by the Institutional Animal Ethics Committee and in accordance with National Health and Medical Research Council animal ethics guidelines.
Statistical analysis
Longitudinal mixed-effect linear models were used to determine the effect of stress on the trajectory of metastasis, and to evaluate if those effects were modified by tumor β2AR knockdown (Verbeke and Molenberghs, 2009). We examined the stress×treatment (tumor cell β2AR knockdown) interaction in a 2 (non-stress vs. stress) × 3 (scramble control vs. shADRB2a vs. shADRB2b) experimental design. Data were analyzed according to the model Yij = β1di1tij + β2di2tij + β3di3tij + β4di4tij + β5di5tij + β6di6tij + εij, where: Yij are mouse-specific and day-specific primary tumor volume or luciferase signals (Fig. 4a) for the ith mouse on the logarithmic scale; tij is the time (days of follow up), dij (j=1,…,6) are binary variables so that dij = 1 if the ith mouse belongs to the jth group and 0 otherwise; εij is the random error term. Linear models were fitted using the routine lm while multiple comparisons used the routine glht in the package multicomp (Bretz et al., 2010). The effect of stress on the trajectory of metastasis or primary tumor volume was calculated using the difference in gradient of fitted lines between stress and non-stress conditions for each tumor cell type. Pearson’s correlation analysis was performed to assess the relationship between the effect of stress on the trajectory of primary tumor volume and the effect of stress on the trajectory of metastasis. For ADRB expression in MCF7-WT and MCF7-β2AR cells, unpaired t-tests were used to compared each βAR subtype between the different cell lines. For cell proliferation and cell morphology assays, the effect of isoproterenol on cell proliferation or mesenchymal-like phenotype of each cell lines were compared using one-way ANOVA repeated measures followed post hoc Tukey’s planned comparison tests when applicable. For all other experiments, a two-way ANOVA test was first performed to identify the presence of interaction between treatments. Post hoc Tukey’s planned comparison tests were performed when applicable, to test the simultaneous differences between pairs of treatment effects on experimental responses (MMP2 gene expression, in vitro invasion, number of invadopodia, ex vivo bioluminescence signals and primary tumor mass). Experiments were conducted 2–4 times. Treatment effects showing p-values smaller than 0.05 were regarded as statistically significant. Statistical analysis was carried out in both R computing environment (Team, 2014) and Prism 6 software (GraphPad Software, La Jolla, CA, USA).
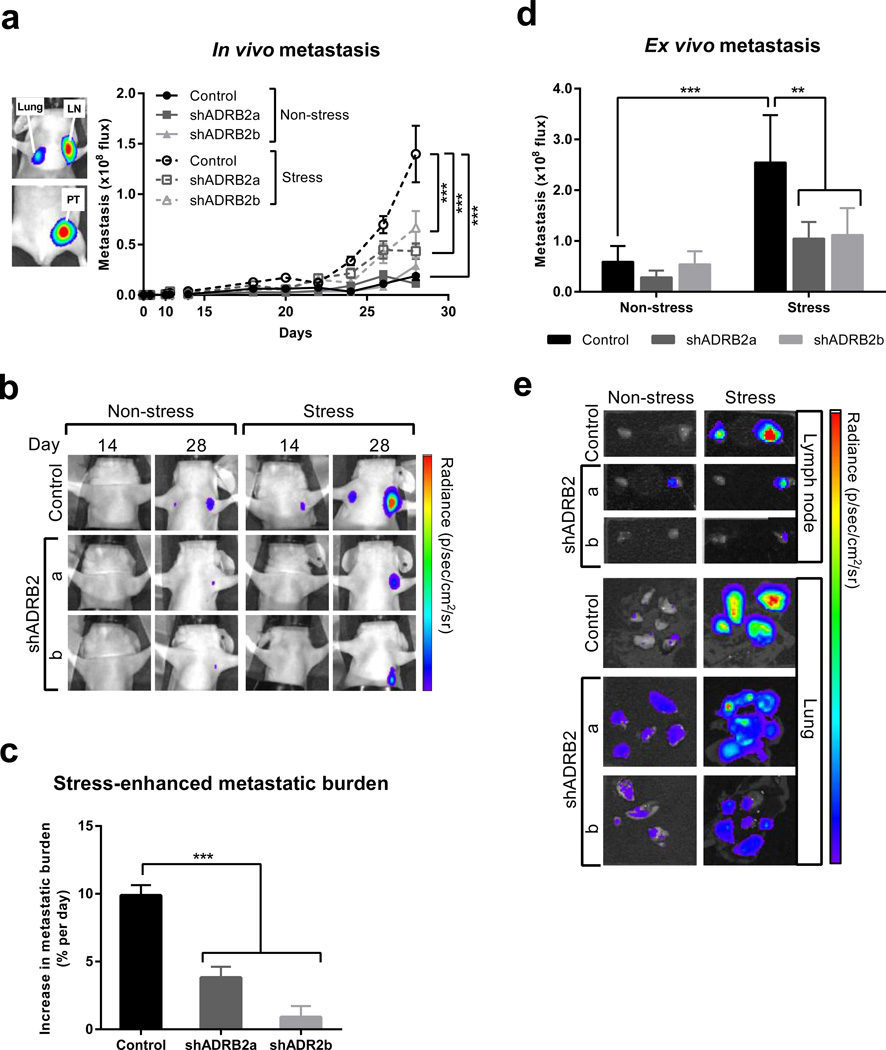
Fig. 4. β2AR knockdown in MDA-MB-231HM breast cancer cells impairs metastasis in vivo.
(a) Left panel: Representative in vivo bioluminescence image of the orthotopic MDAMB-231 breast cancer model showing primary tumor (PT) and spontaneously disseminated metastasis in lymph node (LN) and lung 28 days after tumor cell injection. Right panel: Quantification of distant metastasis by bioluminescence imaging in mice with tumors derived from scramble control or β2AR-deficient cells (shADRB2a and shADRB2b). Mice were exposed to non-stress or stress conditions. (n = 5 at each time point). (b) Representative images of metastasis in vivo at day 14 and 28 after tumor cell injection. Note: the primary tumor is not visible. Scale: min. 3 × 106 photons/sec; max. 1 × 108 photons/sec. (c) The magnitude of the effect of stress on metastatic burden was computed as the difference in the rate of metastatic progression under non-stress and stress conditions. (d) Ex vivo quantification of distant metastasis in target organs that were harvested from mice 28 days after tumor cell injection (n = 5). (e) Representative images of metastasis in lung and lymph node ex vivo at 28 days post tumor cell inoculation. Scale: lymph node: min. 5 × 106; max. 1 × 108 and lung: min. 2 × 105; max. 8 × 106 photons/sec. Data represent mean ± standard error. *p < 0.05 and ***p < 0.001 by (c) one-way ANOVA or two-way ANOVA (a) with or (d) without repeated measures followed by post hoc Tukey’s planned comparison tests.
RESULTS
Generation of MDA-MB-231HM cells deficient in β2AR
We have previously shown that β2AR is the only functional βAR subtype in MDA-MB-231HM cells (Pon et al., 2016). Therefore, we used two different hairpin sequences that target the gene encoding β2AR (ADRB2) to knock down expression of β2AR in MDA-MB-231HM breast cancer cells, and generated shADRB2a and shADRB2b cell lines. To examine the efficiency of ADRB2 silencing, we used qRT-PCR to quantify the expression of ADRB2 in shADRB2a and shADRB2b cells relative to control cells transduced with a scrambled shRNA sequence (scramble control). ADRB2 expression was reduced by > 50% in both shADRB2a and shADRB2b cells compared to scramble control cells (p < 0.01) (Fig. 1a). ADRB1 expression was not affected by shRNA transduction, and ADRB3 expression was not detected in these cell lines (Fig. 1a), consistent with our previous findings (Creed et al., 2015; Pon et al., 2016). Stable knockdown was confirmed with reduction of the ADRB2 transcript maintained even after 6 weeks in culture (data not shown).
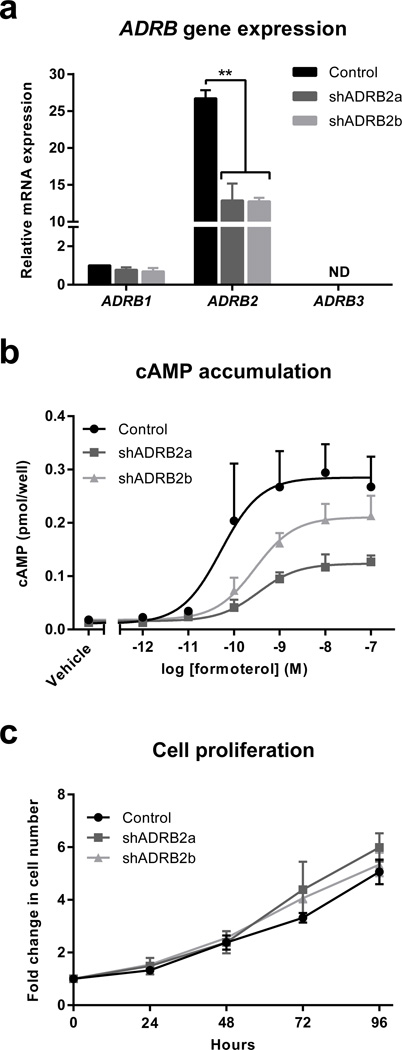
Fig. 1. Generation of MDA-MB-231HM breast cancer cells deficient in β2AR.
(a) Quantification of ADRB mRNA transcript levels using qRT-PCR in MDA-MB-231HM cells transduced with shRNA scramble sequence (control) or shRNA targeting ADRB2 (shADRB2a and shADRB2b) (n = 4). ND: not detected. (b) Quantification of cAMP accumulation in scramble control, shADRB2a and shADRB2b tumor cells in response to increasing concentrations of the β2AR selective-agonist formoterol (n = 3). (c) Proliferation of control, shADRB2a and shADRB2b tumor cells (n = 3). Data represent mean ± standard error. **p < 0.01 by one-way ANOVA with post hoc Tukey’s planned comparison tests.
To investigate the effect of ADRB2 knockdown on receptor function, we evaluated cAMP accumulation as an indicator of receptor signaling. Cells were stimulated with the β2AR-selective agonist formoterol, which induced cAMP accumulation in scramble control cells (Emax = 0.29 ± 0.06 pmol/well) (Fig. 1b). However, the cAMP response was blunted in β2AR-deficient cell lines (shADRB2a: Emax = 0.13 ± 0.02 pmol/well; shADRB2b: Emax = 0.21 ± 0.04 pmol/well) (Fig. 1b). Reduction of the cAMP response in β2AR-deficient cell lines was also demonstrated by a need for a greater concentration of formoterol to reach the EC50 (50% of the maximal response, control cells: 13.30 ± 9.25 nM vs. shADRB2a: 63.94 ± 38.67 nM; shADRB2b: 32.58 ± 13.21 nM) (Fig. 1b).
To determine if ADRB2 knockdown affected tumor cell proliferation, we compared the in vitro proliferative activity of β2AR-deficient cells to scramble control cells. β2AR knockdown did not modulate the proliferation of tumor cells, either under baseline conditions (Fig. 1c), or after treatment with the non-selective βAR agonist isoproterenol (Supplementary Fig. 1). Collectively, these findings confirmed the generation of MDA-MB-231HM tumor cell lines with reduced levels of ADRB2 transcription and blunted β2AR signaling, and without compromised tumor cell proliferative capability.
Characterization of tumor cell invasiveness in MDA-MB-231HM cells deficient in β2AR
Our previous studies using pharmacological modulation of β2AR found a crucial role for tumor cell β2AR signaling in promoting invadopodia formation and tumor cell invasion (Creed et al., 2015; Pon et al., 2016). To examine if genetic knockdown of β2AR similarly affected tumor cell invasive capacity, we first assessed the effect on MMP2 expression, as a transcriptional readout of tumor cell invasion. Treatment with the βAR agonist isoproterenol increased MMP2 expression in scramble control tumor cells (black bars, Fig. 2a), consistent with previous findings that βAR signaling regulates MMP expression (Yang et al., 2006), but the effect was attenuated in β2AR knockdown cells (grey bars, Fig. 2a). Pharmacological blockade of β2AR signaling using the β2AR-selective antagonist ICI-118,551 abolished the effect of isoproterenol on MMP2 expression in scramble control cells, providing pharmacological confirmation that MMP2 expression is sensitive to β2AR signaling (Fig. 2a). Of note, ICI-118,551 further reduced MMP2 expression in isoproterenol-treated β2AR-deficient cells to baseline levels (Fig. 2a), indicating that the incomplete knockdown of β2AR (Fig. 1a) may be responsible for the residual MMP2 expression observed in isoproterenol-treated knockdown cell lines (Fig. 2a).
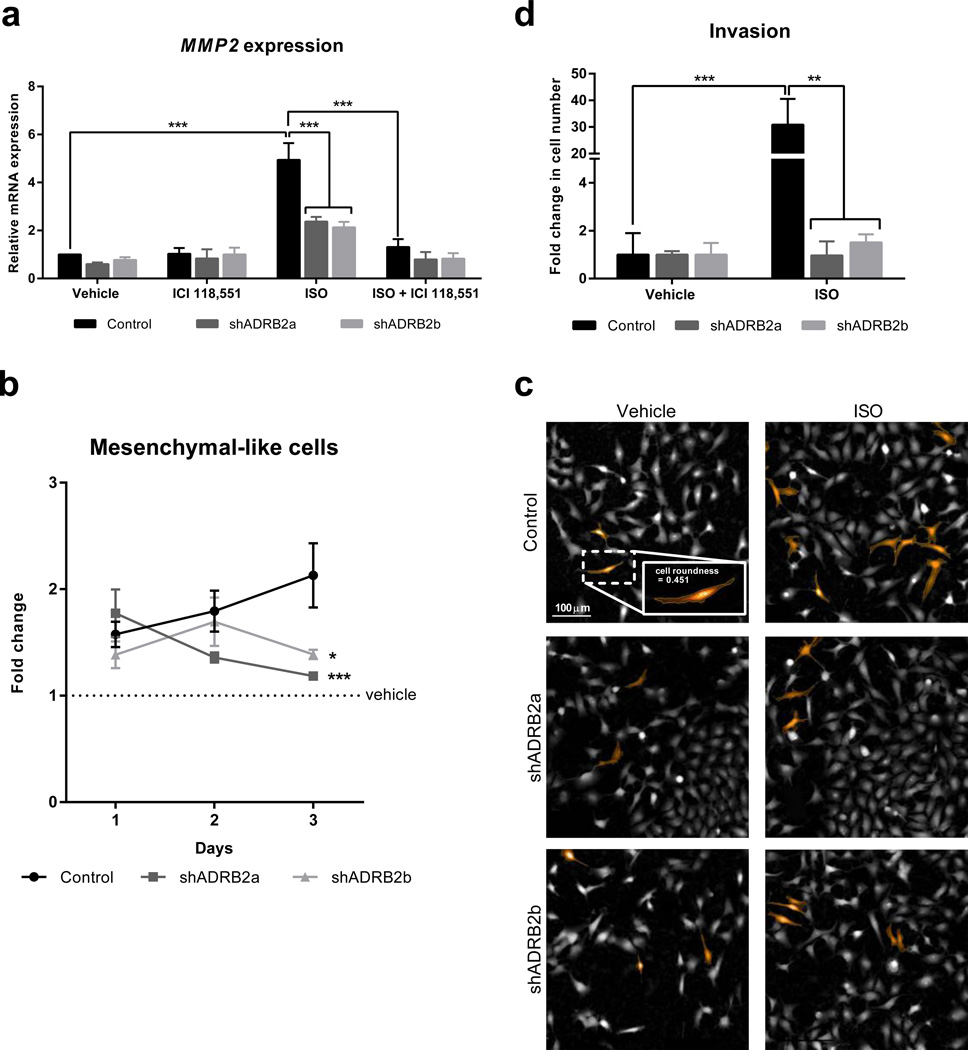
Fig. 2. Characterization of tumor cell invasiveness in β2AR-deficient MDA-MB-231HM breast cancer cells.
(a) Quantification of MMP2 mRNA transcript levels using qRT-PCR in scramble control, shADRB2a and shADRB2b tumor cells treated with vehicle, isoproterenol (non-selective βAR agonist, ISO, 1 µM) and/or ICI-118,551 (β2AR-selective antagonist, 1 µM) (n = 3 – 5). (b) Fold change in proportion of tumor cells that exhibit mesenchymal-like morphology (defined by cell roundness < 0.5) in response to ISO (1 µM, normalized to vehicle) (n = 5 – 6). (c) Representative images of mesenchymal-like cells (roundness < 0.5) highlighted in orange, in cultures of scramble control, shADRB2a, shADRB2b cells after treatment with vehicle or 1 µM ISO. Inset: a magnified view of a mesenchymal-like tumor cell. Scale bar: 100 µm). (d) Quantification of invasion through transwells by scramble control, shADRB2a and shADRB2b tumor cells in response to ISO (1 µM) treatment (n = 4). Data represent mean ± standard error. *p < 0.05, **p < 0.01 and ***p < 0.001 by (b) one-way ANOVA with repeated measures or (a and d) two-way ANOVA with post hoc Tukey’s planned comparison tests. # p < 0.05, ### p < 0.001 for ISO vs. ISO + ICI-118551 within each cell line.
To examine if β2AR knockdown also affected tumor cell morphology, we used high-content screening to investigate the effect of β2AR knockdown on MDA-MB-231HM cell morphology. Treatment of scramble control cells with the βAR agonist isoproterenol (vs. vehicle) in vitro, steadily increased the proportion of mesenchymal-like cells over 3 days of treatment (black line, Fig. 2b). In contrast, β2AR knockdown attenuated the effect of isoproterenol on mesenchymal morphology at day 3 (p < 0.001 for shADRB2a, p < 0.05 for shADRB2b; grey lines, Fig. 2b and 2c). Additionally, pharmacological blockade of β2AR signaling using the β2AR-selective antagonist ICI-118,551 abolished the effect of isoproterenol on mesenchymal morphology in scramble control cells (Supplementary Fig. 2a and 2b), pharmacologically confirming a role for β2AR in tumor cell mesenchymal phenotype.
Morphological transition from epithelial-like to a mesenchymal-like phenotype has been linked with increased tumor cell invasiveness. To examine whether β2AR knockdown also impaired the functional invasiveness of tumor cells, we treated MDA-MB-231HM cells with the non-selective βAR agonist isoproterenol and examined tumor cell invasion through a basement membrane using a transwell invasion assay. Isoproterenol increased invasion in scramble control cells by 30-fold (p < 0.05) (Fig. 2d). However, this effect of isoproterenol was abrogated in tumor cells deficient for β2ARs (Fig. 2d), confirming that β2AR signaling in MDA-MB-231HM tumor cells drives invasion. Collectively, these findings confirm that genetic knockdown of β2AR modulates the tumor cell invasive phenotype in a way that replicates pharmacological β2AR blockade (Creed et al., 2015; Pon et al., 2016).
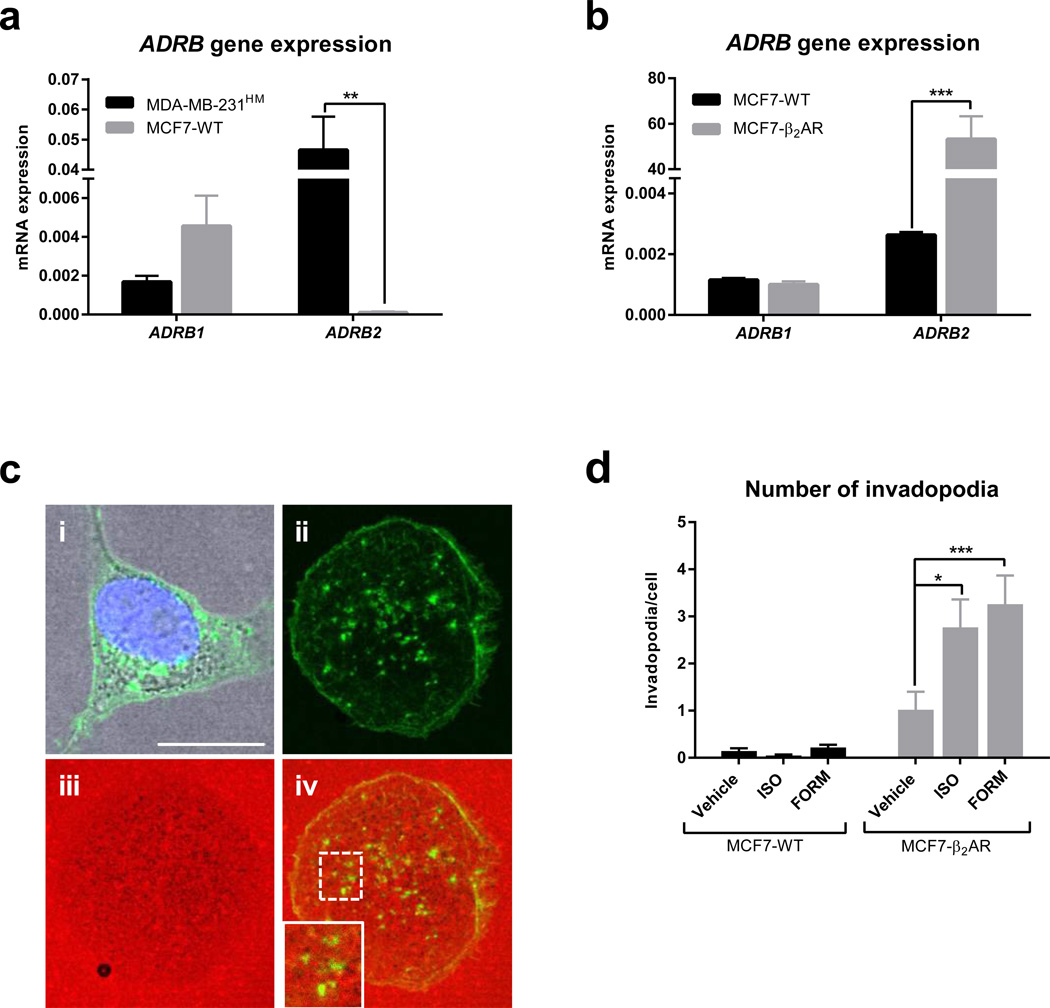
MMPs are localized at subcellular structures called invadopodia that regulate invasion of tumor cells (Murphy and Courtneidge, 2011). We found previously that β2AR regulation of invasion is mediated by formation of invadopodia (Creed et al., 2015). To independently validate the findings that genetic modulation of β2AR affects tumor cell invasion, we overexpressed β2AR in MCF-7 breast cancer cells which have low endogenous levels of β2AR, compared to MDA-MB-231HM cells (Fig. 3a), and investigated the effect on formation of invadopodia. MCF-7 cells were transduced with a construct encoding a β2AR-GFP fusion protein and β2AR overexpression was confirmed at both the transcript and protein level using qRT-PCR and fluorescence microscopy, respectively (Fig. 3b and 3c). Tumor cell invasive capacity was assessed by formation of invasive invadopodia structures that degrade underlying extracellular matrix (Fig. 3c). Treatment with the β2AR-selective agonist formoterol or the non-selective agonist isoproterenol induced formation of invadopodia in cells that overexpressed β2AR, but not in wildtype MCF-7 cells (Fig. 3d). Collectively, these in vitro findings confirm that genetically modulating β2AR by either over-expression or knockdown, affects isoproterenol-induced tumor cell invasiveness, as demonstrated by transcriptional, morphological and functional readouts (Fig. 2 and Fig. 3).
Fig. 3. Overexpression of β2AR in MCF-7 cells increases tumor cell invasiveness.
(a and b) Quantification of ADRB mRNA transcript levels using qRT-PCR in (a) MDA-MB-231HM and MCF-7 wildtype (MCF7-WT) cells (n = 3) and (b) MCF7-WT and MCF-7 overexpressing β2AR (MCF7-β2AR) cells (n = 3). Normalized to ACTB levels. (c) Representative images of invadopodia formation assay. Panel i. A cell showing β2AR-GFP (green). Panels ii–iv. A cell showing localization of actin foci (green, ii), matrix degradation (grey patches of degradation on red-fluorescent gelatin, iii), and merged image with inset panel showing active invadopodia that have degraded matrix (iv) Scale bar: 10 µm. (d) Quantification of invadopodia per cell in MCF7-WT cells and MC7-β2AR cells treated with vehicle, isoproterenol (non-selective βAR agonist, ISO, 0.5 µM) or formoterol (β2AR-selective agonist, FORM, 0.5 µM) (n > 80 cells per condition). Data represent mean ± standard error. *p < 0.05 and ***p < 0.001 by (a and b) unpaired t-tests or (d) one-way ANOVA with post hoc Tukey’s planned comparison tests.
β2AR-driven tumor cell invasion contributes to stress-enhanced metastasis in vivo
Having established that knockdown of β2AR in MDA-MB-231HM cells limits βAR-regulated invasion in vitro (Fig. 2d) without compromising proliferation (Fig. 1c), we investigated whether knockdown of β2AR in tumor cells also modulates the effect of stress on metastatic dissemination from a primary tumor in vivo. In the complex environment of the tumor, many stromal cell populations can also respond to β2AR signaling, which plausibly may offset the effect of β2AR-knockdown in tumor cells on metastasis. To model breast cancer, scramble control tumor cells or β2AR knockdown tumor cells were injected into the mammary fat pad of mice and bioluminescence imaging was used to non-invasively quantify the onset and kinetics of metastasis to distant organs (Fig. 4a). Longitudinal analysis of spontaneous dissemination of metastatic tumor cells from the primary tumor found that under baseline (non-stress) conditions, knockdown of β2AR had no effect on metastasis (Fig. 4a solid lines, and 4b). By day 28 of tumor progression, in mice with tumors derived from scramble control tumor cells, chronic restraint stress increased metastasis 10-fold, compared to non-stress conditions (Fig. 4a), consistent with previous findings (Lamkin et al., 2015; Le et al., 2016; Sloan et al., 2010). This overall increase in metastasis occurred as a consequence of a daily 9.9% ± 0.77% increase in metastatic burden for the duration of the experiment (Fig. 4c). Significantly, knockdown of ADRB2 in tumor cells reduced this cumulative effect of stress on metastasis, with mice bearing shADRB2a or shADRB2b tumors displaying a reduction in stress-enhanced metastasis by 61.41% ± 12.25% (p < 0.001) and 85.86% ± 13.30% (p < 0.001) respectively, compared to mice with scramble control tumors (Fig. 4a–c).
To evaluate the effect of β2AR knockdown on tumor burden in metastatic target organs, we used ex vivo bioluminescence imaging to quantify metastatic tumor cells that had spread from the primary mammary tumor to lymph node and lung. Ex vivo analysis confirmed that β2AR knockdown in MDA-MB-231HM tumor cells blocked stress from increasing metastasis to both lymph node and lung (p = 0.001, versus scramble control tumor cells) (Fig. 4d and 4e).
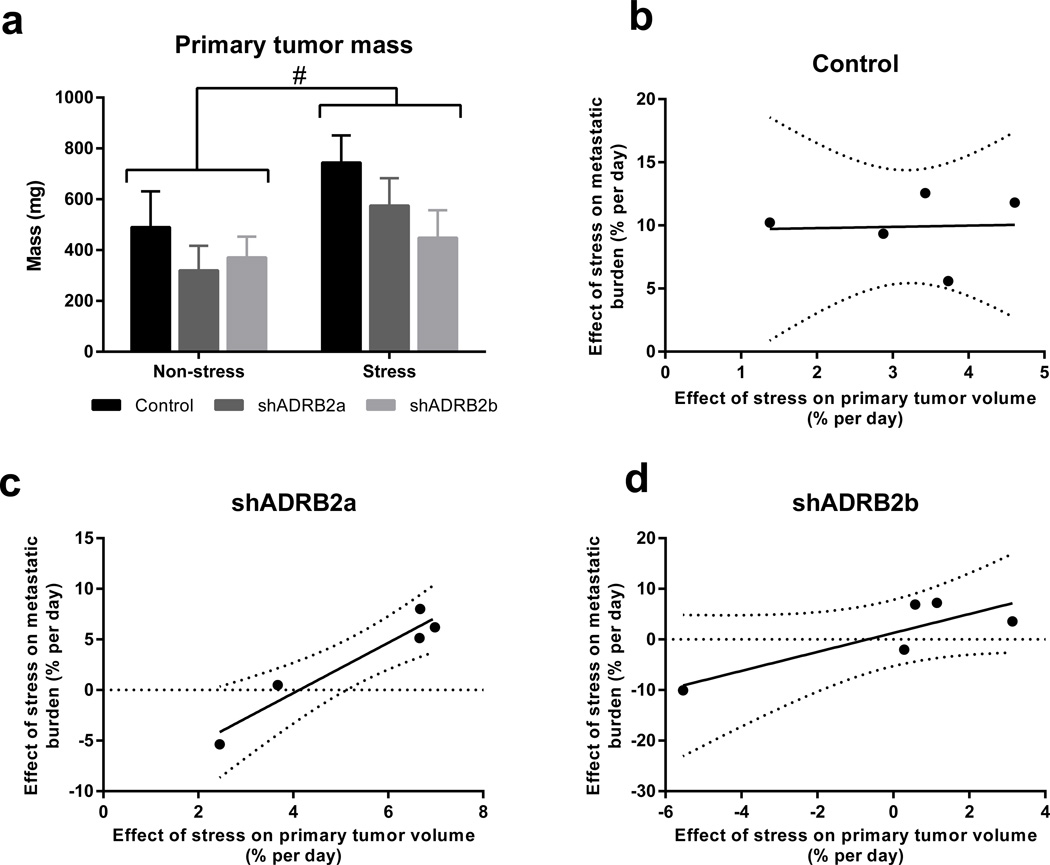
The rate of primary tumor growth is highly prognostic for the extent of tumor cell spread (Weigelt et al., 2005). Therefore, the effects of β2AR signaling on metastasis may have occurred secondary to the effects on the primary tumor. In fact, assessment of primary tumor mass on day 28 of tumor growth revealed that stress produced a small, but significant, increase in primary tumor growth regardless of the β2AR status of tumors (p < 0.05; Fig. 5a). We used regression analysis to evaluate the relationship between the effect of stress on primary tumor growth rate and the effect of stress on metastasis. Interestingly, the effect of stress on the primary tumor did not predict the effect of stress on metastasis in control tumor cells with intact β2AR signaling (Fig. 5b). However, knockdown of β2AR resulted in the expected positive correlation between primary tumor growth rate and trajectory of metastatic burden (Fig. 5c and 5d). Therefore, when β2AR is present on tumor cells, stress drives metastasis independently of primary tumor growth. Collectively, these findings demonstrate that even in the complex tumor microenvironment where multiple cell populations may respond to βAR signaling, β2AR-driven tumor cell invasion significantly contributes to the effects of stress on accelerating metastasis. Furthermore, these findings demonstrate the feasibility of targeting β2AR-driven tumor cell invasion to inhibit the adverse effects of stress on breast cancer metastasis.
Fig. 5. Stress-enhanced metastasis is independent of primary tumor size.
(a) Mass of tumors derived from control or β2AR-deficient cells, from mice exposed to non-stress or stress conditions (n = 5). (b – d) Pearson correlation analysis of the relationship between the effect of stress on the trajectory of metastasis and the effect of stress on the trajectory of primary tumor growth in mice bearing (b) control (r2 = 0.001938, p = 0.944), (c) shADRB2a (r2 = 0.9224, p = 0.0094), and (d) shADRB2b (r2 = 0.6995, p = 0.0775) tumors. #p < 0.05 for main stress effect by two-way ANOVA.
DISCUSSION
Despite major advances in cancer treatment, metastasis remains a significant clinical challenge in the breast cancer clinic. We and others have shown that chronic stress acts through βAR signaling to drive metastasis (Kim-Fuchs et al., 2014; Le et al., 2016; Liu et al., 2015; Sloan et al., 2010; Thaker et al., 2006; Zhao et al., 2015), and our previous in vitro findings have demonstrated that β2AR signaling in cancer cells enhances invadopodia formation and promotes tumor cell invasion in vitro (Creed et al., 2015; Pon et al., 2016). However, the contribution of direct activation of β2AR signaling in tumor cells (rather than through effects on the tumor stroma) to metastatic dissemination from a primary tumor is unclear. Here, we extend our understanding by showing that β2AR signaling in human MDA-MB-231HM breast cancer cells contributes to the deleterious effects of stress on metastatic dissemination from the complex microenvironment of a primary mammary tumor. We found that silencing β2AR expression in MDA-MB-231HM breast cancer cells blocked the effect of stress on metastasis in vivo, which identifies tumor cells as an additional in vivo target of stress-induced βAR signaling. These results expand on earlier findings that demonstrated a role for stromal cells in the tumor microenvironment in the adverse effects of stress on metastasis, and suggests that βAR signaling in both tumor cells and stromal cells contributes to the effect of stress on metastasis. This raises the possibility that inhibiting stress signaling to either tumor cells (as shown here) or to the tumor stroma (Le et al., 2016; Sloan et al., 2010) may be sufficient to modulate metastasis.
Invasion is one of the critical steps along the metastatic cascade that determines successful metastatic outgrowth. Notably, incomplete knockdown of β2AR in MDA-MB-231HM tumor cells (Fig. 1a) was sufficient to diminish stress-enhanced metastasis (Fig. 4a and 4c), suggesting that even partial modulation of tumor cell β2AR may be sufficient to slow cancer progression. We also showed that the effects of stress-induced tumor β2AR signaling on metastasis was independent of the effect on primary tumor size (Fig. 5b–d). These results extend our previous findings that pharmacological modulation of tumor cell β2AR regulates tumor cell invasion in vitro (Creed et al., 2015; Pon et al., 2016), by showing that β2AR signaling in tumor cells also plays a key role in driving metastasis in vivo.
It is possible that β2AR signaling may regulate an epithelial-to-mesenchymal transition in tumor cells to increase metastatic invasion and dissemination. Here, we observed that isoproterenol treatment shifted MDA-MB-231HM cells towards a mesenchymal morphology (Fig 2a), an effect that was attenuated by β2AR knockdown (Fig. 2a). It will be important to characterize if β2AR agonism induces similar behavior in other tumor cell lines and in vivo. Notably, several recent in vitro studies suggest that βAR signaling induces an epithelial-mesenchymal transition in gastric, colon and lung cancer cell lines (Lu et al., 2015; Shan et al., 2014; Zhang et al., 2016). To better understand βAR regulation of epithelial-mesenchymal transition it will be necessary to clarify the molecular mechanisms. While we found no evidence that βAR signaling regulates expression of the mesenchymal marker vimentin in MDA-MB-231HM breast cancer cells (data not shown), it will be important to evaluate other molecules that are known to influence epithelial-mesenchymal transition, including SNAIL and TWIST1. Such studies should control for catecholamine-induced auto-oxidation, which may also induce morphological changes.
While the in vivo findings described here are limited to the contribution of tumor cell β2AR in metastatic dissemination in the MDA-MB-231HM model of breast cancer, findings that stress also increases metastasis in models of ovarian cancer (Thaker et al., 2006), pancreatic cancer (Kim-Fuchs et al., 2014), and colorectal cancer (Liu et al., 2015; Zhao et al., 2015), suggest that it will be important to examine the role of tumor cell β2AR in metastatic dissemination of other tumor types. Additionally, while these results highlight βAR-regulated tumor cell invasion as a significant contributor to cancer progression, they do not exclude a role for βAR signaling to stromal cells in the adverse effects of stress on metastasis (Le et al., 2016; Sloan et al., 2010; Thaker et al., 2006). It is likely that the beneficial effects of βAR-targeted therapies (e.g., β-blockers) on slowing metastasis are due to effects on both tumor cells and stromal cells. Myeloid cells are sensitive to βAR signaling (Le et al., 2016; Powell et al., 2013), which leads to their accumulation in primary tumors where they remodel blood and lymphatic vasculature to provide additional routes for tumor cell dissemination (Le et al., 2016; Sloan et al., 2010; Thaker et al., 2006). To fully characterize the interactions between tumor cells and stromal cells that mediate the effects of stress on metastasis, it will be valuable to use additional strategies including ADRB2 knockout mice and cell-specific ADRB2 deletion mutants to probe the contributions of various cell types in the tumor microenvironment to stress-induced cancer progression.
There are three subtypes of βAR that are expressed on both stroma cells and tumor cells (Badino et al., 1996; Du et al., 2014; Reeder et al., 2015; Vandewalle et al., 1990). While β2AR is the only functional βAR subtype in MDA-MB-231HM cells (Creed et al., 2015; Pon et al., 2016), other cancer cells have been shown to express β1AR and β3AR subtypes (Dal Monte et al., 2013; Kim-Fuchs et al., 2014; Magnon et al., 2013). However, the role of these receptor subtypes in cancer progression is only starting to be elucidated (Magnon et al., 2013). As the use of β-blockers to slow metastasis is clinically translated, it will be important to investigate the role of other βAR subtypes in cancer progression. Second generation β-blockers were developed for increased cardio-specificity by increasing their selectivity for β1ARs, and are now more commonly used than older generation non-selective β-blockers (Salpeter, 2003). Our findings that β2ARs on MDA-MB-231HM tumor cells also contribute to metastasis suggest that some breast cancers may be more effectively targeted with antagonists that also inhibit β2ARs (e.g., propranolol). This is supported by a recent retrospective epidemiological study that found the use of non-selective β-blockers that target both β1AR and β2AR, but not β1AR-selective blockers, was associated with improved cancer outcome (Barron et al., 2011). These findings provide insights into the contribution of tumor cell β2AR signaling in stress-enhanced cancer progression and further support the use of β-blockers to modulate metastasis.
Supplementary Material
Highlights.
β2-adrenoceptors on MDA-MB-231HM breast cancer cells contribute to stress-enhanced metastasis
β2-adrenoceptor signaling regulates a shift to mesenchymal morphology in MDA-MB-231HM breast cancer cells
Genetic modulation of β2-adrenoceptor on breast cancer cells regulates invasion
Acknowledgments
The authors thank Dr Kaylene Simpson from the Victorian Centre for Functional Genomics at Peter MacCallum Cancer Center for assistance with shRNA techniques, Dr Bronwyn Evans from Monash University for the β2AR-GFP construct, and the Imaging, Flow Cytometry and Analysis Core at Monash Institute of Pharmaceutical Sciences for imaging and data analysis support. This research was supported by the Australian National Health and Medical Research Council (1008865), the Australian Research Council (LE110100125), the National Cancer Institute (CA160890), a National Breast Cancer Foundation fellowship to Adam K. Walker (PF-15-014), a National Health and Medical Research Council RD Wright Fellowship to Michelle L. Halls (1061687), Monash Institute of Pharmaceutical Sciences-Drug Discovery Biology (MIPS-DDB) Strategic Funding, the David Skewes Foundation and the Peter Mac Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
CONTRIBUTIONS
Aeson Chang and Caroline P. Le designed and conducted experiments, analyzed data, and wrote the manuscript. Adam K. Walker interpreted data and wrote the manuscript. Sarah J. Creed conducted in vitro experiments. Cindy K. Pon conducted cAMP experiments. Sabine Albold generated knockdown cell lines. Dominic Carroll conducted in vivo experiments. Michelle L. Halls, J. Robert Lane, Bernhard Riedel and Davide Ferrari analyzed data. Erica K. Sloan designed the study, conducted experiments, analyzed data and wrote the manuscript. All authors reviewed the manuscript.
REFERENCES
- Abrass CK, O'Connor SW, Scarpace PJ, Abrass IB. Characterization of the beta-adrenergic receptor of the rat peritoneal macrophage. Journal of immunology. 1985;135:1338–1341. [PubMed] [Google Scholar]
- Badino GR, Novelli A, Girardi C, Di Carlo F. Evidence for functional beta-adrenoceptor subtypes in CG-5 breast cancer cell. Pharmacological research. 1996;33:255–260. doi: 10.1006/phrs.1996.0036. [DOI] [PubMed] [Google Scholar]
- Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population- based study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:2635–2644. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- Ben-Eliyahu S, Shakhar G, Page GG, Stefanski V, Shakhar K. Suppression of NK cell activity and of resistance to metastasis by stress: a role for adrenal catecholamines and beta-adrenoceptors. Neuroimmunomodulation. 2000;8:154–164. doi: 10.1159/000054276. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Labarge MA. Context, tissue plasticity, and cancer: are tumor stem cells also regulated by the microenvironment? Cancer cell. 2005;7:17–23. doi: 10.1016/j.ccr.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JP, Karolak MR, Ma Y, Perrien DS, Masood-Campbell SK, Penner NL, Munoz SA, Zijlstra A, Yang X, Sterling JA, Elefteriou F. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS biology. 2012;10:e1001363. doi: 10.1371/journal.pbio.1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang XZ, Li DQ, Hou YF, Wu J, Lu JS, Di GH, Jin W, Ou ZL, Shen ZZ, Shao ZM. Identification of the functional role of AF1Q in the progression of breast cancer. Breast cancer research and treatment. 2008;111:65–78. doi: 10.1007/s10549-007-9761-y. [DOI] [PubMed] [Google Scholar]
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Creed SJ, Le CP, Hassan M, Pon CK, Albold S, Chan KT, Berginski ME, Huang Z, Bear JE, Lane JR, Halls ML, Ferrari D, Nowell CJ, Sloan EK. beta2-adrenoceptor signaling regulates invadopodia formation to enhance tumor cell invasion. Breast cancer research : BCR. 2015;17:145. doi: 10.1186/s13058-015-0655-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte M, Casini G, Filippi L, Nicchia GP, Svelto M, Bagnoli P. Functional involvement of beta3-adrenergic receptors in melanoma growth and vascularization. Journal of molecular medicine. 2013;91:1407–1419. doi: 10.1007/s00109-013-1073-6. [DOI] [PubMed] [Google Scholar]
- Devaud C, Westwood JA, John LB, Flynn JK, Paquet-Fifield S, Duong CP, Yong CS, Pegram HJ, Stacker SA, Achen MG, Stewart TJ, Snyder LA, Teng MW, Smyth MJ, Darcy PK, Kershaw MH. Tissues in different anatomical sites can sculpt and vary the tumor microenvironment to affect responses to therapy. Molecular therapy : the journal of the American Society of Gene Therapy. 2014;22:18–27. doi: 10.1038/mt.2013.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Zhou L, Wang Y, Yan T, Jiang Y, Shao Z, Yin W, Lu J. Association of alpha2a and beta2 adrenoceptor expression with clinical outcome in breast cancer. Current medical research and opinion. 2014;30:1337–1344. doi: 10.1185/03007995.2014.890928. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacological reviews. 2000;52:595–638. [PubMed] [Google Scholar]
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Seminars in oncology. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- Graf K, Grafe M, Dummler U, O'Connor A, Regitz-Zagrosek V, Kunkel G, Auch-Schwelk W, Fleck E. Regulation of beta-adrenergic receptors on endothelial cells in culture. European heart journal. 1993;14(Suppl I):173–176. [PubMed] [Google Scholar]
- Kim-Fuchs C, Le CP, Pimentel MA, Shackleford D, Ferrari D, Angst E, Hollande F, Sloan EK. Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain, behavior, and immunity. 2014;40:40–47. doi: 10.1016/j.bbi.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkin D, Sung H, Yang G, David J, Ma J, Cole S. alpha2-Adrenergic blockade mimics the enhancing effect of chronic stress on breast cancer progression. Psychoneuroendocrinology. 2015;51:262–270. doi: 10.1016/j.psyneuen.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le CP, Nowell CJ, Kim-Fuchs C, Botteri E, Hiller JG, Ismail H, Pimentel MA, Chai MG, Karnezis T, Rotmensz N, Renne G, Gandini S, Pouton CW, Ferrari D, Moller A, Stacker SA, Sloan EK. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nature communications. 2016;7:10634. doi: 10.1038/ncomms10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Deng GH, Zhang J, Wang Y, Xia XY, Luo XM, Deng YT, He SS, Mao YY, Peng XC, Wei YQ, Jiang Y. The effect of chronic stress on anti-angiogenesis of sunitinib in colorectal cancer models. Psychoneuroendocrinology. 2015;52:130–142. doi: 10.1016/j.psyneuen.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Lu YJ, Geng ZJ, Sun XY, Li YH, Fu XB, Zhao XY, Wei B. Isoprenaline induces epithelial-mesenchymal transition in gastric cancer cells. Mol Cell Biochem. 2015;408:1–13. doi: 10.1007/s11010-015-2477-0. [DOI] [PubMed] [Google Scholar]
- Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- Murphy DA, Courtneidge SA. The 'ins' and 'outs' of podosomes and invadopodia: characteristics, formation and function. Nature reviews. Molecular cell biology. 2011;12:413–426. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pon CK, Lane JR, Sloan EK, Halls ML. The beta2-adrenoceptor activates a positive cAMP-calcium feedforward loop to drive breast cancer cell invasion. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016;30:1144–1154. doi: 10.1096/fj.15-277798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:16574–16579. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder A, Attar M, Nazario L, Bathula C, Zhang A, Hochbaum D, Roy E, Cooper KL, Oesterreich S, Davidson NE, Neumann CA, Flint MS. Stress hormones reduce the efficacy of paclitaxel in triple negative breast cancer through induction of DNA damage. British journal of cancer. 2015;112:1461–1470. doi: 10.1038/bjc.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren ZX, Yu HB, Li JS, Shen JL, Du WS. Suitable parameter choice on quantitative morphology of A549 cell in epithelial-mesenchymal transition. Bioscience reports. 2015;35 doi: 10.1042/BSR20150070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter SR. Cardioselective Beta Blocker Use in Patients With Asthma and Chronic Obstructive Pulmonary Disease: An Evidence-Based Approach to Standards of Care. Cardiovascular Reviews & Reports. 2003;24:1. [Google Scholar]
- Sanders VM, Baker RA, Ramer-Quinn DS, Kasprowicz DJ, Fuchs BA, Street NE. Differential expression of the beta2-adrenergic receptor by Th1 and Th2 clones: implications for cytokine production and B cell help. Journal of immunology. 1997;158:4200–4210. [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nature methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan T, Cui X, Li W, Lin W, Li Y, Chen X, Wu T. Novel regulatory program for norepinephrine-induced epithelial-mesenchymal transition in gastric adenocarcinoma cell lines. Cancer Sci. 2014;105:847–856. doi: 10.1111/cas.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas BD, Wu L, Sood AK, Cole SW. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer research. 2010;70:7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, Merritt WM, Lin YG, Mangala LS, Kim TJ, Coleman RL, Landen CN, Li Y, Felix E, Sanguino AM, Newman RA, Lloyd M, Gershenson DM, Kundra V, Lopez-Berestein G, Lutgendorf SK, Cole SW, Sood AK. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nature medicine. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- Vandewalle B, Revillion F, Lefebvre J. Functional beta-adrenergic receptors in breast cancer cells. J Cancer Res Clin Oncol. 1990;116:303–306. doi: 10.1007/BF01612908. [DOI] [PubMed] [Google Scholar]
- Weigelt B, Peterse JL, van 't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Miyoshi N, Kawabata K, Yasuda M, Shimoi K. Quercetin-3-O-glucuronide inhibits noradrenaline-promoted invasion of MDA-MB-231 human breast cancer cells by blocking beta(2)-adrenergic signaling. Archives of biochemistry and biophysics. 2014;557:18–27. doi: 10.1016/j.abb.2014.05.030. [DOI] [PubMed] [Google Scholar]
- Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB, Jewell S, Flavahan NA, Morrison C, Yeh PE, Lemeshow S, Glaser R. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer research. 2006;66:10357–10364. doi: 10.1158/0008-5472.CAN-06-2496. [DOI] [PubMed] [Google Scholar]
- Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Developmental cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Zhang J, Deng YT, Liu J, Wang YQ, Yi TW, Huang BY, He SS, Zheng B, Jiang Y. Norepinephrine induced epithelial-mesenchymal transition in HT-29 and A549 cells in vitro. J Cancer Res Clin Oncol. 2016;142:423–435. doi: 10.1007/s00432-015-2044-9. [DOI] [PubMed] [Google Scholar]
- Zhao L, Xu J, Liang F, Li A, Zhang Y, Sun J. Effect of Chronic Psychological Stress on Liver Metastasis of Colon Cancer in Mice. PloS one. 2015;10:e0139978. doi: 10.1371/journal.pone.0139978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.