Abstract
Objectives To assess (1) the clinical issues addressed during the medical encounter; (2) the feasibility of the process of shared decision‐making in clinical practice and (3) patients’ desires concerning the question of ‘who should take the decision in breast cancer treatments?’
Design Qualitative pilot study based on clinical encounters using decision boards and information booklets.
Setting Centre Léon Bérard, a comprehensive cancer centre in the Rhône‐Alpes region of France.
Participants One breast cancer surgeon and 22 breast cancer patients.
Main outcome measures Analysis of patients’ reactions to a shared decision‐making process concerning surgery and chemotherapy, and analysis of its practical feasibility (i.e. duration of the consultations).
Results (1) Twenty‐one patients participated in the decision regarding surgery; all chose conservative treatment; 15 patients had their own say about chemotherapy (nine chose no chemotherapy, six chose chemotherapy). (2) Participating in treatment choice generated anxiety for a majority of patients. Some were dissatisfied because they had to make a choice and consequently to give up the other option. Finally, some were uncertain about making the right choice. Nevertheless, most were satisfied with the information given and the possibility of participating to the treatment decision‐making process. (3) The total duration of the entire process of shared decision‐making is consistent with the time spent with patients with such a severe disease.
Discussion/conclusion Most of the patients expressed their satisfaction regarding the possibility to participate in treatment decision‐making, knowing that offering treatment choice is very unusual in France. From this pilot study it appears that shared decision‐making is feasible in clinical practice. Nevertheless, a quantitative study based on a large sample of patients is necessary to: (1) confirm this hypothesis, (2) ensure that patients are willing to participate in their treatment decision‐making, and (3) measure the potential benefits related to this participation.
Keywords: breast cancer, chemotherapy, information, shared medical decision, surgery
Introduction
Everyone agrees that there is a growing demand for patient information and participation in decision‐making, 1 either in France or in other western countries. Although the concern of health‐care users with medical information is obviously growing, 2 this paper will investigate whether breast cancer patients are actually willing to participate in the process of assessing their preferences when confronted with this stressful disease.
Due to the workload of medical teams and the lack of doctors specialized in the treatment of cancer, time is potentially a major limiting factor in doctor–patient communication. Besides, clinical activities are granted little economic weight in the medical community, as compared with high‐technology medical care. In such a context, the feasibility of shared decision‐making remains a major matter of debate.
The goals of the present study were to assess (1) the clinical issues addressed during the medical encounter, (2) patients’ desires concerning the question ‘who should take the decision in breast cancer treatments?’ and (3) the feasibility of the process of shared decision‐making in clinical practice.
Patients and method
This qualitative pilot study, conducted on a 4‐month period, included 22 consecutive female patients. All had operable breast cancer that could be treated either with mastectomy or lumpectomy plus axillary dissection or sentinel node biopsy. All patients were kept under study, whatever their nodal status. The duration of the consultation with the surgeon was unlimited and was measured as an outcome.
Decision aids consisted of two leaflets (a paper version of two decision boards), one for surgery and one for adjuvant therapy, that were used as guides for delivering oral information to the patient (Box 1). This included a values clarification exercise (Box 2). Moreover, a booklet describing in more details the techniques, their limitations, benefits and potential side‐effects was given by the surgeon at the end of the first consultation. All documents were built with the help of patients and healthy volunteers gathered in a focus group, then evaluated. 3 , 4 , 5 Benefit was measured as a function of prognostic factors evaluated in each patient.
Table Box 1 .
Decision boards
| Surgery leaflet: | |||
| Technique | Consequences | Benefits | Drawbacks |
| Chemotherapy leaflet: | |||
| Mandatory treatments: Radiotherapy Hormone therapy | Chemotherapy or no chemotherapy | Benefits | Drawbacks |
Table Box 2 .
Values clarification exercises
| Chemotherapy | Surgery |
| How important for me are: | |
| Loosing my hair | Loosing my breast |
| Nausea and vomiting | Scars |
| Fatigue | Radiation |
| Treatment duration | Treatment duration |
| Treatment consequences on: | |
| My sexual life | |
| My family life, my children | |
| My professional life | |
| Relations with others (spouse, partner) | |
| My social life and leisure activities | |
The options presented to the patients concerned surgery and chemotherapy. Hormone therapy and radiotherapy were also presented but not considered as options in this preliminary study.
For surgery, the choice was between lumpectomy or mastectomy and axillary dissection or sentinel node biopsy. Ten days after surgery the patients were fully informed of prognostic factors. Chemotherapy was presented as an option.
Schematically, each encounter was divided into three different steps:
1. The patient was informed by the surgeon using the decision boards on the treatments, their benefits and possible side‐effects, including recurrence rates observed with each treatment method in similar clinical situations. At the end of the presentation, the surgeon asked the patient whether she could identify that there were options. If not, more explanations were given about the meaning of each option and emphasis was put on the differences.
2. The second step was an exploration of the patient's willingness to decide. The surgeon and the patient had a discussion about who would decide: the patient alone, the doctor alone on request of the patient (and only after presentation of the options), or both after a deliberation process. If her option was to decide for herself, the surgeon and the patient had a discussion about values. If not, the deliberation phase was started.
3. In the third step, the decision was taken. If the patient asked the surgeon to make the decision, her decision was respected. The surgeon then presented her the treatment modalities chosen by the clinical committee for each clinical situation. The decision could also be taken by the patient herself after a discussion about her values with the surgeon. Finally, the decision could be based on a mutual agreement after the deliberation process. In all cases, the patient had a 8‐day period of reflection before taking her treatment decision.
At the end of each consultation the surgeon (AB) fulfilled a questionnaire concerning first, all the items included in both leaflets and in the values clarification exercises and secondly, patient's anxiety, patient's decision‐making process, patient's choice, patient's remarks concerning the overall information and decision‐making process and consultation times.
The surgeon analysed all these items.
There were four consultations in total. At the first one the different surgical procedures were presented. Surgery was performed after 10–15 days. She was encouraged to discuss with other people such as relatives, friends or doctors. Until the very day before surgery, during the second consultation, she had the opportunity to change her mind. The third consultation was scheduled 12 days after surgery. At that time all the results concerning prognostic factors were given and explained, and adjuvant chemotherapy was considered. The last consultation occurred with a medical oncologist or a radiotherapist just before the beginning of adjuvant treatment.
Results
About patient preferences on decision‐making
Only one of 22 patients declined participation in the decision regarding surgery (Fig. 1). Concerning chemotherapy, three patients were not offered a choice because they were referred to another doctor, not participating in the study, during a holiday period. Fifteen patients made their own choice. Among them, five gave their decision immediately after the presentation of the options, whereas 10 needed more time; they decided only after a deliberation process during which they could express their fears about possible side‐effects (mainly hair loss) and their wish to minimize the risk of recurrence. Four patients did not want to express a preference even after the deliberation process. They asked for the doctor to make the decision.
Figure 1.
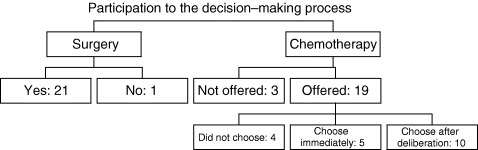
Patient decision process.
What was the patients’ choice?
Surgery
All 21 patients who decide to participate in the decision regarding surgery chose conservative treatment with sentinel node biopsy. One patient considered mastectomy as ‘I'm worried conserving a breast with cancer inside’. Ultimately she decided to undergo lumpectomy but she was happy to have had the opportunity to discuss the choice of mastectomy.
Chemotherapy
Among the 15 patients who expressed their preferences, six decided not to have chemotherapy (Fig. 2). All the patients of this group could expect <5% reduction in the recurrence risk at 10 years with chemotherapy and belonged to a good prognosis group.
Figure 2.
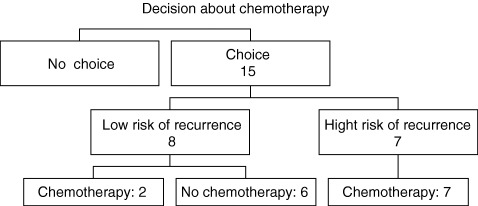
Patient preferences concerning chemotherapy.
Nine patients actually chose chemotherapy. Among these, two could only expect a 3% reduction in the risk of recurrence with chemotherapy, with a disease‐free survival of 92% without chemotherapy. Seven had a potential benefit evaluated from 5 to 12%.
Patient's anxiety, dissatisfaction, decisional conflict
At the end of the process, 14 patients (63.6%) spoke of their anxiety at participating in the choice of treatment. Some of them clearly asked: ‘What will happen to me if cancer recurs, will I feel guilty?’. Some patients were dissatisfied because being involved in the decision process made them realize that choosing an option always implies that the benefit of the other option is lost. For instance, one said: ‘I would like to have chemotherapy, but I'm afraid of losing my hair.’ A third concern was about the uncertainty of making the right choice because some patients thought that only the surgeon could take the treatment decision. Some said: ‘I think that you have more information than the ones you gave me today, so you're the only who can take the decision.’
Patient's general comments on the overall decision‐making process
All the patients involved in the study were surprised that a doctor would present the different options and ask them for their preferences regarding treatments. Nevertheless, most were satisfied with the information and the possibility of expressing their desires.
Duration of the encounters
The mean duration of the two major encounters (information and choice of surgery, information and choice of chemotherapy) was 35 (25–45) min. Combined with the other consultations, the total duration of the process was about 1 h and a half (for doctors only). Nevertheless, the procedure did not take into account preferences about hormone therapy and radiotherapy. The total duration of the entire process of shared decision‐making is therefore not so longer than the usual medical encounter (1 h).
Discussion
Complete information about surgery and decision aids was previously evaluated in a randomized study by Goel et al. 6 and in a large study by Whelan et al. 7 Moreover, other studies also investigated chemotherapy for node‐negative breast cancers. O'Connor et al. 8 studied the opinion of clinicians. Levine et al. 9 made up a decision board to elicit preferences regarding chemotherapy.
The originality of our study lies in the application of the process of shared decision‐making to the entire treatment process, from surgery to adjuvant treatment. For the latter, the decision board was designed to be applied to all patients, whether node‐negative or node‐positive. So each patient could participate in the decision at each phase of treatment.
In contrast with results from Whelan et al., 7 no patients actually chose mastectomy. This may be due to the relatively limited series of patients included in our study. Besides, contrary to other institutions, almost all patients treated for breast cancer in our hospital have radiotherapy, even after mastectomy, except when they have small (<3 cm) node‐negative, external tumours. As a consequence, the interest of mastectomy as compared with other surgical options may appear limited to our patients. But presenting mastectomy as an option gave each woman an opportunity of considering and possibly deciding to undergo this surgical procedure.
In good prognosis cancers, the patients are not generally offered chemotherapy. Nevertheless, two of eight patients from our cohort considered that the reduction in the recurrence risk, regarded as low by the doctors, was important for them, thus demonstrating that patients’ values are different from those of doctors. 10
Patients who had chosen to express their personal preferences were more often anxious at the end of the decision process. Most of them had not been encouraged by their family to make a choice. However, a randomized study 6 demonstrated that anxiety was not more prevalent in the group of patients using the decision aid. Moreover, 6 months after enrolment there was no more decision regret in that group than in women who had not used this opportunity.
Conclusion
This type of medical‐patient encounter is really not usual in France. Nevertheless, learning from our experience, it seems that shared decision‐making is feasible in clinical practice. The duration of the necessary medical encounters is consistent with the time that should be spent with patients in such a severe disease population. However, a quantitative study based on a large sample of patients is necessary to: (1) confirm that shared decision‐making is feasible in routine clinical practice, (2) ensure that patients are willing to participate to their treatment decision‐making, and (3) measure the benefits and potential drawbacks (anxiety, dissatisfaction, regret) for patients from taking part in decisions regarding their treatments.
References
- 1. Les français et la réforme du système médical . IPSOS, Paris, 2001. [Google Scholar]
- 2. Coulter A. Shared decision‐making: a summary and future issues In: Maslin AM, Powles TJ. (eds) Breast Cancer. Sharing the Decision. Oxford: Oxford University Press, 1999: 99–108. [Google Scholar]
- 3. Carrère MO, Moumjid‐Ferdjaoui N, Charavel M, Brémond A. Eliciting patients’ preferences for adjuvant chemotherapy in breast cancer: development and validation of a bedside decision‐making instrument in a French Regional Cancer Centre. Health Expectations, 2000; 3: 97–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morelle M, Moumjid‐Ferdjaoui N, Brémond A, Charavel M, Carrère MO. Comment évaluer la qualité du transfert d'information du médecin au patient? Choix des tests psychométriques pour un arbre de décision dans un Centre Anticancéreux régional. Revue d'Epidemiologie et de Santé Publique, 2001; 49: 299–313. [PubMed] [Google Scholar]
- 5. Moumjid N, Morelle M, Carrère MOC, Bachelot T, Mignotte H, Brémond A. Elaborating patient information with patients themselves: lessons from a cancer treatment focus group. Health Expectations, 2003; 6: 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goel V, Sawka CA, Thiel EC, Gort EH, O'Connor AM. Randomized trial of a patient decision aid for choice of surgical treatment for breast cancer. Medical Decision-Making, 2001; 21: 1–6. [DOI] [PubMed] [Google Scholar]
- 7. Whelan T, Levine M, Gafni A et al. Mastectomy or lumpectomy? Helping women make informed choices. Journal of Clinical Oncology, 1999; 17: 1727–1735. [DOI] [PubMed] [Google Scholar]
- 8. O'Connor AM, Llewellyn‐Thomas HA, Sawka C, Pinfold SP, To T, Harrison DE. Physician's opinion about decision aids for patient considering systemic adjuvant therapy for axillary‐node negative breast cancer. Patient Education and Counselling, 1997; 30: 143–153. [DOI] [PubMed] [Google Scholar]
- 9. Levine MN, Gafni A, Markham B, MacFarlane D. A bedside decision instrument to elicit a patient's preference concerning adjuvant chemotherapy for breast cancer. Annals of Internal Medicine, 1992; 117: 53–58. [DOI] [PubMed] [Google Scholar]
- 10. Macquart‐Moulin G, Viens P, Bouscary ML et al. Discordance between physician's estimations and breast cancer patients’ assessment of side effects of chemotherapy: an issue for quality of care. British Journal of Cancer, 1997; 76: 1640–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]


