Abstract
Objective: To explore factors associated with the difference in score between women's and doctors’ decisional conflict about hormone therapy (HT).
Design: Secondary analysis. Setting and participants: family doctors were randomized to prepare women for counselling about HT using either a decision aid or a pamphlet.
Main variables studied: After each counselling session, decisional conflict was assessed in women and doctors using the Decisional Conflict Scale (DCS) and the Provider Decision Process Assessment Instrument (PDPAI), respectively. The difference in score between the DCS and PDPAI was computed and entered as the dependent variable in a multilevel regression analysis.
Main outcome results: A total of 40 doctors and 167 women were included in the analysis. The intra‐doctor correlation coefficient was 0.25. Factors associated with women experiencing higher decisional conflict than their doctor were: age of doctor >45 years, women who were undecided about the best choice after the counselling session, women with a university degree and women who said that their doctor usually does not give them control over treatment decision. Factors associated with doctors experiencing more decisional conflict than women were: doctors who were undecided about the quality of the decision, length of visit <30 min and women who thought that the decision was shared with their doctor.
Conclusion: In order to reduce the disparities between women's and doctors’ decisional conflict about HT, interventions aimed at raising awareness of doctors about shared decision‐making should be encouraged.
Keywords: hormone replacement therapy, menopause, multilevel regression analysis, doctor–patient interaction, randomized controlled trial, shared decision‐making
Introduction
Effective doctor communication is highly valued by patients and middle‐aged women are no exception. 1 , 2 , 3 They have a desire to be informed and involved in decision‐making regarding hormone replacement therapy (HT). 4 , 5 They want to retain a sense of control over any decisions that may have an impact on their health. 6 Although menopausal women and doctors do not always share a common view about health‐related matters, 7 , 8 , 9 by the end of a clinical encounter they need to reach a mutual understanding (i.e. ‘common ground’) on the nature of the problem, the preferred course of action and their respective roles in decision‐making. 10 Women expect that HT will improve their sense of general well‐being and their capacity to fulfil their social roles. 11 , 12 In contrast, even with the existence of conflicting evidences about HT long‐term benefits, doctors are more likely to believe in its benefits 13 , 14 and to find that women use HT for a shorter period of time than they recommend. 15 It is in this context that menopausal women are said to be dissatisfied with the medical profession. 16
In recent years, new theoretical frameworks of shared decision‐making have emerged. They address gaps in mutual understanding of knowledge and values between doctors and their patients. 17 , 18 In the general context of care, results of studies of doctor–patient agreement on these issues point to a positive impact on clinical outcomes. 19 , 20 , 21 , 22 Thus in a previous study, we demonstrated the relevance of assessing the agreement between the level of a woman's self‐reported decisional conflict and her doctor's self‐reported decisional conflict towards HT. 23 This first study used a secondary analysis of a randomized controlled trial on decision support intervention towards HT. The decision support that was being tested was based on the Ottawa Decision Support Framework (ODSF). In the field of health‐related decision‐making, this conceptual framework stands out because of its inclusion and operationalization of decisional conflict. 18 Decisional conflict is recognized as a key determinant of decision‐making in health‐care. 24 It indicates the level of comfort when facing a health‐care decision. It can be expressed as a state of uncertainty about which course of action to take when choice among competing actions involves risk, loss, regret or challenge to personal life values. 25 It can also be used as an outcome to assess decision‐making support intervention. This framework also provides clinicians with tools to assess the relevance of a decision point, the stage in the decision‐making process, the desired role in decision‐making, the level of decisional conflict and the needs for tailored decision support intervention. Therefore, when a decision is made within a clinical encounter about the best course of action, assessing the doctor's and the patient's level of comfort with this decision will provide a better understanding of the process of care, help in the development of decision support tools in the future and facilitate evaluation and feedback when training doctors to communicate about complex decisions that involve trade‐offs between risks and benefits. 26 , 27
However, although we found that the agreement between the level of women's and their doctors’ decisional conflict enhanced our understanding of decision‐making processes in the context of clinical care, it did not provide us with a complete understanding of which combination of factors influenced the difference between both perspectives. Therefore, the aim of the present study was to explore factors that are associated with the difference in score between the levels of women's and their doctors’ decisional conflicts.
Methods
Data source
The database was created after a trial in which family doctors were randomized to prepare women for counselling about HT using one of two decision‐support interventions. Doctors were recruited from a random sample of family medicine practices in Ottawa, Canada. Eligibility criteria required for doctors were to: be involved in clinical care, have graduated from medical school at least 5 years before, be <60 years old and not be an academic. Doctors then identified five women aged 45–69 years in their practice who they knew were considering HT. Women needed to meet the following criteria: post‐menopausal for at least 1 year, able to read English, no previous use of HT, no history of osteoporosis‐associated fractures and no absolute contra‐indications to HT. Thus, all women recruited by a particular doctor received the same preparatory decision‐support strategy. The two interventions, used by women in their homes, were either a detailed self‐administered decision aid or a pamphlet produced by the American College of Doctors. The decision aid comprised an audio‐tape, a booklet and a worksheet that offered a structured process of decision‐making that included: outcome probabilities tailored to the women's clinical risk, a description of the pros and cons of different HT regimens and a value‐clarification process. The pamphlet included general information on risks, benefits and side‐effects. After reviewing the decision‐support strategy, women participated in a follow‐up counselling session with their doctor. More details about the design and recruitment strategy of this study can be found elsewhere. 23
Outcome measure
After each clinical encounter, decisional conflict was assessed in women with the Decisional Conflict Scale (DCS) and in doctors with the Provider Decision Process Assessment Instrument (PDPAI). The DCS is a self‐administered scale and is comprised of 16 items. 24 The development of the DCS was guided by the ODSF that was derived from the construct of decisional conflict. The DCS elicits: (i) heath‐care consumers’ uncertainty in making a health‐related decision; (ii) the factors contributing to the uncertainty; and (iii) health‐care consumers’ perceived effective decision‐making. It is useful to tailor decision‐supporting intervention to particular consumer needs (i.e. decisional conflict as a determinant), but also to evaluate health‐care consumer decision support intervention (i.e. decisional conflict as an outcome). The scale was first evaluated with 909 individuals deciding about influenza immunization or breast cancer screening. A sub‐sample of respondents was retested 2 weeks later. The test–retest reliability coefficient was 0.81. The DCS discriminated significantly (P < 0.0002) between those who had strong intentions either to accept or to decline invitations to receive influenza vaccine or breast cancer screening and those whose intentions were uncertain. The scale also discriminated significantly (P < 0.0002) between those who accepted or rejected immunization and those who delayed their decisions to be immunized. Each item is scored on a five‐point Likert scale (1 = strongly agree to 5 = strongly disagree). The DCS total score is obtained by summing up the 16‐item scores and dividing by 16, resulting in a score that ranges from 1 (i.e. low decisional conflict) to 5 (i.e. high decisional conflict). Internal consistency coefficients (i.e. Cronbach's alpha) ranged from 0.78 to 0.92.
The PDPAI is an adaptation of the DCS to be administered to doctors. As stated by the author, ‘… it is based on the construct of decisional conflict that was introduced into medical decision making by O'Connor’. 26 The instrument was created by adapting the 12 items contained in the DCS to reflect equivalent issues from the health‐care provider's perspective. It was first studied in two general internal medicine practices. Reliability, measured using Cronbach's alpha, was 0.90 (95% CI = 0.87–0.92). Construct validity was high with expected negative correlations ranging from −0.53 to −0.67. The instrument also satisfied standard criteria for item homogeneity. The tool includes 12 items, rated on Likert scale. The PDPAI total score is obtained by dividing the sum of the 12 items by 12, resulting in a score that ranges from 1 (i.e. low decisional conflict) to 5 (i.e. high decisional conflict). The difference in score between the DCS and PDPAI after each counselling session was computed and entered as the dependent variable in a multilevel regression analysis.
Explanatory variables
Woman level
All variables that could be attributed to a specific counselling session (i.e. one woman) were assessed as woman‐level variable even when they were determined by the doctor. Therefore, before the counselling session, women provided their age, education, employment status, medical and hysterectomy status history, and tendency towards adopting HT [scale: 1 (yes) to 15 (no) with 8 (unsure)]. They completed the four‐subscale domains of measure quality of life at menopause (MenQoL: vasomotor, psychological, sexual and physical). 28 Cronbach's alpha in each subscale range from 0.81 (i.e. psychosocial) to 0.89 (i.e. sexual). Test–retest reliability at 1 month is good with the intra‐class correlation coefficients in each subscale ranging from 0.60 to 0.85. Women also answered the following questions on roles in decision‐making:
-
(i)
Would your doctor ask you to help to make a decision? [scale: 1 (definitively no) to 5 (definitively yes)]
-
(ii)
How often does your doctor give you control over treatment? [scale: 1 (never) to 5 (very often)]
-
(iii)
How often does your doctor ask you to take some responsibility? [scale: 1 (never) to 5 (very often)]
-
(iv)
Who should make the decision? [scale: 1 (myself alone) to 5 (my doctor alone)].
These items are derived from studies in the field of health‐related decision‐making. 29 , 30 In addition, women were asked how long they had been seeing their doctor.
After a counselling session with her doctor, each woman completed a self‐administered questionnaire that included her thought about the best choice regarding HT (not using, using and unsure), and who made the decision about HT [scale: 1 (myself alone) to 5 (my doctor alone)]. Accordingly, for each woman, doctors completed a self‐administered questionnaire that included information on their preference regarding the decision about HT following the encounter (had been prescribed, had not been prescribed and decision deferred), and what decision they thought the woman had made regarding HT (accepted, leaning towards, did not accept and undecided). The doctor provided information on the estimated length of the counselling session (min) and compared it with his/her typical HT counselling visit time (longer than usual or same/shorter). The doctor also reported [scale: 0 (i.e. low) to 10 (i.e. high)] the quality of the decision.
Doctor level
At baseline, doctors provided their age, gender, year of graduation, and indicated whether they were certified by the College of Family Doctors of Canada (CFPC) or not. They were asked about their usual HT prescribing pattern when providing care for middle‐aged women (most of the time, some of the time or rarely), and their level of satisfaction with professional autonomy [scale: 1 (very satisfied) to 5 (very dissatisfied)].
Analysis
First, we dichotomized explanatory variables to ease the interpretation of results. The ODSF defines a good decision as one that is informed by the best evidence, congruent with one's personal values and acted upon. Therefore, the decision‐making process should result in reduced decisional conflict and not in a decision set by the expert. This grouping was not decided on the knowledge of the data but on the ODSF. For example, we focused on a perception that a shared decision‐making process had occurred vs. an unshared decision‐making process. We believe this focus was more coherent with the core conceptual aspects of the ODSF than the focus on the extreme of each response scale (i.e. the woman took the decision alone vs. the doctor took the decision alone). We also wanted to be consistent throughout the study and kept the same grouping for variables with the same categories. Therefore, in keeping with the ODSF, variables were regrouped on the following basis:
-
(i)
Leaning towards HT [unsure (8) compared with sure (<8 and >8)]
-
(ii)
Would your doctor ask you to help to make a decision? [no (1–4) compared with yes (5)]
-
(iii)
How often does your doctor give you control over treatment? [never (1–3) compared with often (4 and 5)]
-
(iv)
How often does your doctor ask you to take some responsibility? [never (1–3) compared with often (4 and 5)]
-
(v)
Who should make the decision? [shared (3) compared with unilateral (<3 and >3)]
-
(vi)
Thought about best choice regarding HT after a clinical encounter [sure (not using/using) compared with unsure]
-
(vii)
Who made the decision about HT [shared (3) compared with unilateral (<3 and >3)]
-
(viii)
Preference of doctor regarding his/her preference about the decision following the encounter [undecided (decision deferred) compared with decided (had been prescribed/had not been prescribed)]
-
(ix)
What decision the doctor thought the woman had made regarding HT [undecided compared with decided (accepted/leaning towards HT/did not accept)].
The doctor's perception about the quality of the decision was regrouped as follows: undecided (5) compared with decided (<5 and >5). Secondly, we performed descriptive analysis to assess the distribution of the difference in score between the DCS and PDPAI, and the explanatory variables. Some variables had missing information. In case of a missing difference in score between the DCS and PDPAI, the whole observation was removed from the analysis. Otherwise, a dummy variable was constructed to assess if those who did not respond to this particular question were not different with those who answered. If there was no impact from the missing information, we imputed the mean for continuous variables and for categorical variables, the mode.
Using Spearman correlation coefficient, univariate analyses were performed to assess the association between each pair of explanatory variable. Again to ease the interpretation, given some variables were ordinal and other continuous, we decided to use a non‐parametric method to evaluate the association between studied variables. Non‐parametric methods do not assume any underlying distribution of the data. Therefore, we identified pairs of explanatory variables with a Spearman correlation coefficient >0.40. Among these pairs, the variable that was the most associated with the difference in score measure was kept for subsequent steps in the analysis.
A multilevel model approach was considered in order to explore factors that were associated with the difference in score between the levels of a woman's and her doctor's decisional conflicts, because of the nested structure of the data (i.e. women within doctors). The multilevel model package HLM 5 (Scientific Software International, Inc., Lincolnwood, IL, USA) was used to fit the models of the difference in decisional‐conflict score between women and their doctors. 31 We developed three two‐level models that offered simultaneous consideration of i women nested within j doctors. The first model, usually called the ‘empty’ or ‘null’ model, was estimated with no explanatory variables. This is similar to a random‐effect analysis of variance (anova). 32 The empty model measured the relative importance of women and doctor effects by accounting for variation in the difference in score between the DCS and PDPAI. Therefore, it provided the information required to compute an intra‐doctor correlation coefficient. This coefficient provided information about the average correlation among the difference in score between the DCS and PDPAI within doctors. This intra‐doctor correlation coefficient also helps to quantify the variation in the outcome measure that lies between doctors. The second model was estimated with only the woman‐level variables. It provided information about how much the variation is reduced with these variables in the model. It also provided information about the importance of using a multilevel approach to analyse the data. Based on the conceptual framework, a ‘full’ or ‘final’ model was constructed. It included those explanatory variables, at the woman and doctor levels, that were significantly (P < 0.05) associated with the outcome measure. The Ottawa Hospital Research Ethics Board approved the study.
Results
Descriptive analysis
A total of 40 family doctors (n = 20 in each group) and 167 women (n = 87 in the decision aid group and n = 80 in the pamphlet group) provided data. There were 11 male doctors in the decision aid group and 10 in the pamphlet group. Overall, doctors recruited a mean of four women each. However, four doctors only recruited one woman each. Descriptive statistics for woman‐ and doctor‐level explanatory variables are presented in Table 1. Although not the main aim of this study, the dependant variable did not change between the trial arms.
Table 1.
Descriptive statistics for the main outcome and explanatory variables
| Decision aid group (n = 87 completed dyads) | Pamphlet group (n = 80 completed dyads) | |
|---|---|---|
| Woman‐level variables | Mean (SD) | |
| Outcome: difference in score between DCS and PDPAI | −0.06 (0.57) | 0.02 (0.61) |
| Age | 55.5 (6.2) | 54.5 (5.4) |
| Number of years since the last menstrual period | 8.8 (7.7) | 6.6 (5.3) |
| MenQoL sexual | 2.5 (2.0) | 2.7 (1.8) |
| MenQoL vasomotor | 2.9 (1.9) | 3.0 (2.0) |
| MenQoL psychological | 3.1 (1.6) | 3.1 (1.6) |
| MenQoL physical | 3.2 (1.4) | 3.3 (1.3) |
| Baseline opinion about HT [1 (no) to 15 (yes)] | 10.1 (4.2) | 10.1 (4.1) |
| Doctor's perception of the quality of the decision the woman made (0 = low to 10 = high) | 8.2 (1.4) | 7.3 (1.8) |
| Length of the counselling session (min) | 34.1 (19.2) | 27.8 (9.1) |
| Frequency (%)* | ||
| Education | ||
| Less than high school diploma | 10 (10) | 5 (6) |
| High school diploma | 23 (26) | 13 (16) |
| Some post‐secondary | 30 (34) | 34 (42) |
| University degree | 24 (28) | 28 (35) |
| Women currently employed | 51 (59) | 57 (71) |
| Women who had an hysterectomy | 23 (26) | 16 (24) |
| Women who reported having seen the same doctor for more than 5 years | 48 (55) | 52 (65) |
| Would your doctor ask you to help to make a decision? | ||
| Definitively no | 1 (1) | 1 (1) |
| Probably no | 0 (0) | 1 (1) |
| Maybe | 3 (3) | 5 (6) |
| Probably yes | 31 (36) | 24 (30) |
| Definitively yes | 21 (24) | 46 (58) |
| How often does your doctor give you control over treatment? | ||
| Never | 1 (1) | 1 (1) |
| Seldom | 4 (5) | 3 (4) |
| Sometimes | 11 (13) | 7 (9) |
| Often | 28 (32) | 28 (35) |
| Very often | 40 (46) | 31 (39) |
| How often does your doctor ask you to take some responsibility? | ||
| Never | 4 (5) | 1 (1) |
| Seldom | 3 (3) | 3 (4) |
| Sometimes | 11 (13) | 11 (14) |
| Often | 35 (40) | 27 (34) |
| Very often | 29 (33) | 27 (34) |
| Who should make the decision? | ||
| Myself alone | 8 (9) | 16 (20) |
| Myself after considering the opinion of my doctor | 54 (62) | 46 (58) |
| Myself and my doctor | 22 (25) | 15 (19) |
| My doctor after considering my opinion | 3 (3) | 2 (3) |
| My doctor alone | 0 (0) | 1 (1) |
| Who made the decision? | ||
| Myself alone | 10 (11) | 20 (25) |
| Myself after considering the opinion of my doctor | 48 (55) | 37 (46) |
| Myself and my doctor | 25 (29) | 17 (21) |
| My doctor after considering my opinion | 2 (2) | 4 (5) |
| My doctor alone | 0 (0) | 1 (1) |
| Thoughts on the best choice for me after counselling session with doctor | ||
| Not using HT | 41 (47) | 36 (45) |
| Using HT | 24 (28) | 26 (33) |
| Unsure | 22 (25) | 18 (22) |
| Decision regarding HT after counselling session with doctor | ||
| Not using HT | 44 (51) | 50 (63) |
| Using HT | 23 (26) | 21 (26) |
| Unsure | 18 (21) | 15 (19) |
| Doctor's perception of the patient's decision | ||
| Accepted | 20 (23) | 16 (20) |
| Leaning towards HT but no prescription | 11 (13) | 7 (9) |
| Did not accept HT | 44 (51) | 43 (54) |
| Undecided | 8 (9) | 11 (14) |
| Other | 4 (5) | 3 (4) |
| Doctor's preference | ||
| HT | 44 (51) | 45 (56) |
| No HT | 21 (24) | 16 (20) |
| Decision deferred | 22 (25) | 19 (24) |
| Doctor's perception of the length of the counselling session | ||
| Shorter as usual | 5 (6) | 2 (3) |
| The same as usual | 28 (32) | 26 (33) |
| Longer than usual | 30 (34) | 30 (34) |
| Mean (SD) | ||
| Doctor‐level variables (n = 20 doctors) | ||
| Age | 44.7 (8.4) | 45.6 (6.8) |
| Year of graduation | 1979 | 1979 |
| Satisfaction with autonomy | 1.6 (0.6) | 2.0 (1.2) |
| Frequency (%)* | ||
| Intervention: decision aid | 20 (100) | 20 (100) |
| Male | 11 (55) | 10 (50) |
| Certified by CFPC | 15 (75) | 14 (70) |
| Usual prescribing pattern of HT | ||
| Most of the time | 16 (80) | 18 (90) |
| Sometimes | 4 (20) | 1 (5) |
| Rarely | 0 (0) | 1 (5) |
CFPC, College of Family Doctors of Canada; DCS, Decisional Conflict Scale; HT, hormone therapy; MenQoL, measure quality of life at menopause; PDPAI, Provider Decision Process Assessment Instrument.
*Total might be different from 100% because of missing data.
Multilevel regression analysis
The difference between the DCS score (Cronbach's alpha = 0.82) and the PDPAI score (Cronbach's alpha = 0.78; 95% CI = 0.77–0.79) had approximately a normal distribution with mean of −0.02 (range = −1.42 to 1.50; SD = 0.59) (Fig. 1). This suggested that there was a similar proportion of consultations in which a woman presented more discomfort with the decision than the doctor (Fig. 1, right‐hand side of the graph) and of those in which a doctor presented more discomfort with the decision than the woman (Fig. 1, left‐hand side of the graph).
Figure 1.
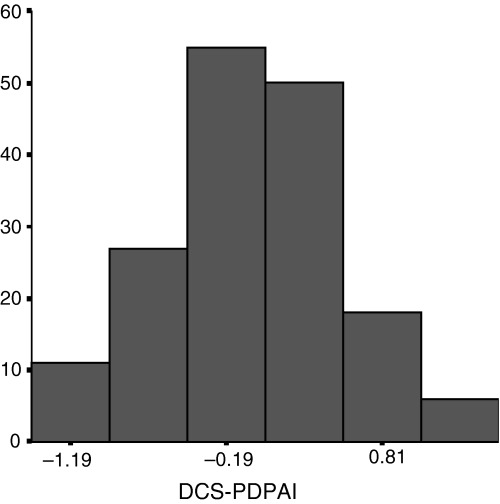
Distribution of the outcome measure: the difference in score between the Decisional Conflict Scale (DCS) and the Provider Decision Process Assessment Instrument (PDPAI).
Therefore, the difference in score between the DCS and PDPAI fitted the necessary statistical assumptions to be entered as a dependent variable in a multilevel regression analysis. There was no missing data for the following variables: woman's level of education, employment status, hysterectomy status and perception about who should make the decision. Missing data were <10% for the other variable that were used. Only if the impact was found non‐significant between missing and non‐missing observations, the missing observations were imputed to the reference category. Otherwise, a flag for missing information was kept in the model to adjust for missing information.
Female doctors were found to be more likely to prefer HT for their patient after the counselling session than male doctors. Thus, we kept the variable ‘preference of doctor after the counselling session’ in the model and removed the variable ‘gender of the doctor’ from the model, because the former variable showed higher correlation with the dependent variable than the latter. Education level and age of women were kept in the model, because they showed higher association with the dependent variable than the employment status, which was easily predicted by women's age. Number of years since the last menstrual period was highly correlated with hysterectomy. Thus, we kept the number of years since the last menstrual period in the model, because it was a slightly better predictor of the outcome measure than the status of hysterectomy.
The first model (empty model) is presented in Table 2. The intercept, −0.013 (SD = 0.062) informs us on the estimated average in the difference in score between the DCS and PDPAI. The estimates for the random‐effects portion of the model suggest that doctors do differ in their average difference in score between the DCS and PDPAI. They also suggest that there is even more variation among women within doctors. The intra‐doctor correlation coefficient, also known as the intra‐cluster correlation coefficient, was 0.25 (i.e. 0.088/0.088 + 0.265). This means that 25% of the total variation in the outcome measure can be explained by doctors’ characteristics only. In other words, only by knowing which doctor the woman visited, it can inform us up to 25% of the total variability observed in the difference between her own decisional‐conflict score and of her doctor. This also suggests that there is a significant clustering effect within doctors of the difference in score between the DCS and PDPAI, and that an Ordinary Least Square analysis of these data would not be appropriate. 32
Table 2.
Results of multilevel modelling of the difference in score between DCS and PDPAI
| Empty model | Women‐level model | Final model | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fixed effects | |||||||||
| Woman level | |||||||||
| Intercept | −0.013 (0.062) | 0.141 (0.136) | 0.157 (0.178) | ||||||
| Age (centred to 55 years) | 0.004 (0.007) | 0.005 (0.007) | |||||||
| Number of years since the last menstrual period (centred to 6 years) | −0.011 (0.007) | −0.009 (0.007) | |||||||
| University degree | 0.152 (0.090) | 0.151 (0.087) | |||||||
| MenQoL vasomotor | 0.156 (0.104) | 0.160 (0.103) | |||||||
| Doctor does not give control over treatment | 0.282 (0.080)** | 0.286 (0.078)** | |||||||
| Myself and my doctor made the decision | −0.245 (0.095)* | −0.256 (0.096)** | |||||||
| Undecided about the best choice for me after counselling session with doctor | 0.275 (0.099)** | 0.257 (0.098)** | |||||||
| Doctor's undecided about the quality of the decision the woman made | −0.210 (0.097)* | −0.198 (0.098)* | |||||||
| Visit's length <30 min | −0.132 (0.092) | −0.186 (0.083)* | |||||||
| Visit's length >30 min | 0.009 (0.106) | −0.016 (0.097) | |||||||
| Doctor level | |||||||||
| Age (centred to 45 years) | 0.015 (0.006)* | ||||||||
| Certified by CFPC | 0.158 (0.129) | ||||||||
| Intervention decision aid | −0.116 (0.107) | ||||||||
| Usual prescribing pattern of HT | −0.194 (0.125) | ||||||||
| Random effects | |||||||||
| Variation | Est. | d.f. | χ 2 | Est. | d.f. | χ 2 | Est. | d.f. | χ 2 |
| Between doctors parameter variance | 0.088 | 39 | 92.6 | 0.084 | 39 | 94.2 | 0.075 | 35 | 79.0 |
| Within doctors parameter variance | 0.265 | 0.227 | 0.228 | ||||||
| Total | 0.354 | 0.311 | 0.304 | ||||||
| Reliability | 0.561 | 0.585 | 0.560 | ||||||
CFPC, College of Family Doctors of Canada; DCS, Decisional Conflict Scale; Est., estimate; HT, hormone therapy; MenQoL, measure quality of life at menopause; PDPAI, Provider Decision Process Assessment Instrument.
*P < 0.05; **P < 0.01.
The second model (the woman‐level model) indicates that four variables were significantly associated with the difference in score between the DCS and PDPAI: (i) doctor's perception of the quality of the decision that was made; (ii) women's thought about the best choice after the counselling session; (iii) usual role given by doctor in control over treatment decision; and (iv) the role given to woman during the counselling session (Table 2). The random effect variance portion of the model indicates that these variables can explain 14% of the variability in the outcome measure within doctor (i.e. 0.265 − 0.227/0.265). However, these variables do not have a significant impact on the variance component between doctors. This means that characteristics from the woman level do not explain the variability observed between doctors in the outcome measure. Fig. 2 shows the breakdown of the total variation of the outcome measure.
Figure 2.
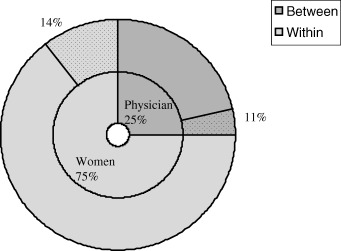
Breakdown of the total variance of the difference in score between the Decisional Conflict Scale (DCS) and the Provider Decision Process Assessment Instrument (PDPAI), and percentage of the explained variation per level.
The last model (full model) is based on a triangulation of what we know from a conceptual point of view, statistical significance and consensus between researchers (Table 2). In order of importance, at the woman level, the following factors were significantly and positively associated with the difference in score between the DCS and PDPAI (i.e. difference getting larger which means that the woman is more likely to experience higher decisional conflict than her doctor): women who reported that they usually are not given control over the treatment decision by their doctor, women who were unsure about the best choice for themselves after the counselling session and women who had a university degree. At the doctor level, only age was significantly associated with the outcome measure. This means that the older the doctor was, the more likely the woman was experiencing higher decisional conflict than her doctor.
In order of importance, the following factors were significantly and negatively associated with the difference in score between the DCS and PDPAI (i.e. difference getting smaller which means that the doctor is more likely to experience higher decisional conflict than the woman): women who believed that a shared decision‐making process had occurred during the counselling session, doctors being undecided about the quality of the decision and length of visit <30 min. We tested relevant interaction terms in the final model. However, none of these interaction terms made a significant contribution (data not shown). Finally, the reliability coefficients for each of these three models were shown to be above 0.50. This means there was an acceptable fit of each one of these models.
Discussion
To our knowledge, this is the first study that explores factors associated with the difference in score between the decisional conflict of women and the decisional conflict of their doctor in the context of decision‐making about HT. Agreement and mutual understanding between patients and their doctors are highly valued outcomes in health‐care. 10 , 33 , 34 In the past, women have clearly expressed their desire for mutual discussion with their doctors when facing complex health‐related decision in order to reach a common understanding of the problem and available options. 35 However, it is not always clear how women and their health‐care providers can assess that this has occurred. Although we can not guarantee that each scale assesses identical constructs, we believe that assessing the agreement between the DCS and PDPAI, 23 and the difference in score between the DCS and PDPAI can provide complementary insight about factors related to mutual understanding by women and their doctors of the decision‐making process.
The operationalization of both the agreement measure and the difference in score between the DCS and PDPAI was possible because of the conceptual framework on which it is based. 18 This framework has been shown to be effective in understanding the decision‐making processes of women in the past. 36 To our knowledge, the ODSF is one of the few conceptual frameworks in the field of shared decision‐making and communication that provides validated and easy to use self‐administered instruments to assess the concomitant perspective of patients and doctors about a specific clinical encounter. 1
Results of the multilevel regression analysis provided interesting findings when taken in the context of shared decision‐making. The ‘empty’ model showed a relatively high intra‐doctor correlation coefficient of 0.25. In the past, estimates of intra‐cluster correlation coefficients for process variables among group practice in primary care settings were shown to be in the order of 0.05–0.15 whilst those in secondary care were in the order of 0.3. 37 In our study, the higher level of the intra‐cluster correlation coefficient for a primary care process variable could be explained by three reasons. First, our analysis used the doctor level, a lower level than group practice level. Secondly, care of the menopausal woman is a more specialized type of care than usual primary care. 38 Thirdly, it is possible that intra‐doctor correlation coefficients for process variables specific to patient–doctor interaction are higher than those associated with other process variables. For example, Elwyn and his colleagues assess the skills of family doctors in shared decision‐making and found an intra‐doctor correlation coefficient of 0.22. 39
Other interesting findings have emerged from this exploratory study. We found that the main factor associated with a doctor experiencing more decisional conflict than his/her patient was when women reported, after the counselling session, that the decision was shared with their doctor in contrast with women who said it was not. This is interesting given the growing emphasis on doctors for them to engage into shared decision‐making. 40 , 41 Again, it could be that for these women, attempts to win their doctor into a more shared decision‐making process was interpreted as menacing by their doctor. Indeed, there is a large body of literature that deplores the lack of shared decision‐making process experienced in the context of clinical care in comparison with more ‘paternalistic’ models of decision‐making. 42 , 43 , 44 This finding supports the need for further assessing doctors’ attitudes and willingness to engage in new models of shared decision‐making. It also emphasizes the need to encourage the education and support of health‐care providers in order to gain their acceptance of shared decision‐making processes. Doctors who were neutral about the quality of the decision after the counselling session were also more likely to experience less comfort with the decision than women. It is intuitively appealing to observe that there would be such a relationship between this type of indecisiveness and being more uncomfortable with the decision than the patient.
In contrast, the main factor associated with a woman more likely to experience higher decisional conflict than her doctor was when she reported, before the counselling session, that her doctor usually does not give her control over treatment decision. Women put a lot of emphasis on the egalitarian nature of the interaction with their doctor when making a decision about HT. 45 , 46 Again, this finding emphasizes the need for educating and supporting doctors into shared decision‐making processes. Other factors associated with a woman experiencing more decisional conflict than her doctor included women who were undecided about the best choice for them after the counselling session. This suggests that doctors might not be competent at identifying that these women are experiencing decisional conflict at the end of the clinical encounter. Thus, it would be necessary for doctors to integrate in their practice the construct and scale of decisional conflict in order to identify patients in need of more decision support expertise. 23 Women with university degree were also more likely to experience more decisional conflict that their doctor. It could be that as well‐educated women are more at ease to interact with doctors, they might be confronting with what the doctor says and asking for more information about alternatives to HT. At the doctor‐level variables, only doctor's age was found associated to the outcome measure. This result is congruent with the existing literature, which suggests that younger doctors are known to have a more egalitarian pattern of communication with patients. 47 A recent survey of residents in an American medicine programme indicated that they were even more willing than both their patients and their teachers to engage into shared decision‐making. 48
However, despite these interesting findings, limitations of the present analysis need to be addressed. First, this is a secondary analysis of a randomized controlled trial whose primary outcome of interest was women's decisional‐conflict scores. Thus, the data that it provided was not intended primarily for the analysis we performed. Secondly, one of the major limitation in this study was the relatively small sample size (<200 patients within 40 doctors) combined with a relatively high intra‐doctor correlation coefficient. The power of regression analysis for such multilevel models can be affected. In order to minimize this limitation, the number of covariates was reduced, the probability of rejecting the null hypothesis (P‐value) was set up at 0.05 and the multilevel model used for the analysis was limited to a random intercept model only. Although some doctors only recruited one woman each, the impact of single unit usually has minor influence on such models. The model will assign average estimates for these clusters based on the other clusters. Thirdly, our exploration of factors was restricted to those included in the existing database. Among the factors included in the analysis, we based our choice of variables on the following reasons: conceptual framework, the high intra‐doctor correlation coefficient, results of the correlation between each variable, and consensus among the researchers. More research in this field will need to be carried out to reproduce our results and to further understand the complex variables we were working with. Nonetheless, given the originality of this exploratory study and the foremost importance of improving mutual understanding between menopausal women and their doctors of the decision‐making process with respect to HT, we believe our results can help design future studies.
In summary, implications of this study for clinicians are as follows. If, indeed, agreement on the nature of the problem and available options is desirable when practising medicine, further interventions would need to address factors that were identified. Factors that could be addressed in the short‐term are: education of doctors about shared decision‐making processes, identification by doctors of the role that women desire in decision‐making and implementation of the DCS in the process of care. If doctors cannot afford to take more time with menopausal women, then new models of collaborative care need to be designed. This study also has larger implications for future research in the field of shared decision‐making. Multilevel regression analysis allowed us to better understand the impact of doctor‐level variables on the disparities between women's and doctors’ comfort with the decision. Given the size of the intra‐doctor correlation coefficient we found, future randomized controlled trials using a cluster design should adjust their sample size accordingly. Finally, this study has given the strong interest to understand practice variation and its contribution to population health 49 and provides some evidence that the gap in understanding between patients and their doctors is an important area to pursue.
Acknowledgements
We thank the participating doctors and women, Mrs Lucile Turcot‐Lemay for statistical support, M. Michael Carlson for reviewing the manuscript and M. Roger Beaudoin for administrative support. Dr Légaré is supported by a scholarship from the ‘Fonds de la Recherche en Santé du Québec’ and Dr O'Connor has support from an Ontario Ministry of Health Career Scientist Award. This research was supported through grants from the Canadian Arthritis Society, research grant #96056 and the doctor data were collected as part of the Medical Research Council of Canada, research grant #13304, ‘Physicians’ Attitudes toward Decision Support Technologies’.
References
- 1. Cegala DJ, McNeilis KS, McGee DS, Jonas AP. A study of doctors’ and patients’ perceptions of information processing and communication competence during the medical interview. Health Communication, 1995; 7: 179–203. [Google Scholar]
- 2. Grol R, Wensing M, Mainz J et al. Patients’ priorities with respect to general practice care: an international comparison. Family Practice, 1999; 16: 4–11. [DOI] [PubMed] [Google Scholar]
- 3. Patterson F, Ferguson E, Lane P et al. A competency model for general practice: implications for selection, training, and development. British Journal of General Practice, 2000; 50: 188–193. [PMC free article] [PubMed] [Google Scholar]
- 4. Hampson SE, Hibbard JH. Cross‐talk about the menopause: enhancing provider‐patient interactions about the menopause and hormone therapy. Patient Education and Counseling, 1996; 27: 177–184. [DOI] [PubMed] [Google Scholar]
- 5. Rothert ML, Holmes‐Rovner M, Rovner D et al. An educational intervention as decision support for menopausal women. Research in Nursing and Health, 1997; 20: 377–387. [DOI] [PubMed] [Google Scholar]
- 6. Logothetis ML. Women's decisions about estrogen replacement therapy. Western Journal of Nursing Research, 1991; 13: 458–474. [DOI] [PubMed] [Google Scholar]
- 7. DeLorey C. Women at midlife: women's perceptions, physicians’ perceptions. Journal of Women & Aging, 1989; 1: 57–69. [Google Scholar]
- 8. Defey D, Storch E, Cardozo S, Diaz O, Fernandez G. The menopause: women's psychology and health care. Social Science & Medicine, 1996; 42: 1447–1456. [DOI] [PubMed] [Google Scholar]
- 9. Ghali WA, Freund KM, Boss RD, Ryan CA, Moskowitz MA. Menopausal hormone therapy: physician awareness of patient attitudes. American Journal of Medicine, 1997; 103: 3–10. [DOI] [PubMed] [Google Scholar]
- 10. Brown JB, Weston WW, Stewart MA. Patient‐centred interviewing part II: finding common ground. Canadian Family Physician, 1989; 35: 153–157. [PMC free article] [PubMed] [Google Scholar]
- 11. Hunter MS, Liao KL. Intentions to use hormone replacement therapy in a community sample of 45‐year‐old women. Maturitas, 1994; 20: 13–23. [DOI] [PubMed] [Google Scholar]
- 12. Fox‐Young S, Sheehan M, O'Connor V, Cragg C, Del Mar C. Women's knowledge about the physical and emotional changes associated with menopause. Women & Health, 1999; 29: 37–51. [DOI] [PubMed] [Google Scholar]
- 13. Hemminki E, Topo P, Malin M, Kangas I. Physicians’ views on hormone therapy around and after menopause. Maturitas, 1993; 16: 163–173. [DOI] [PubMed] [Google Scholar]
- 14. Saver B, Taylor T, Woods N, Stevens N. Physician policies on the use of preventive hormone therapy. American Journal of Preventive Medicine, 1997; 13: 358–365. [PubMed] [Google Scholar]
- 15. Hokkanen T, Hemminki E, Aalto P et al. Patient managed clinical trial. Controlled Clinical Trials, 1997; 18: 140–150. [DOI] [PubMed] [Google Scholar]
- 16. McVeigh C. Menopause and healthy aging: a pilot project. Australian and New Zealand Journal of Public Health, 1996; 20: 95–96. [DOI] [PubMed] [Google Scholar]
- 17. Towle A, Godolphin W. Framework for teaching and learning informed shared decision making. British Medical Journal, 1999; 319: 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Connor AM, Tugwell P, Wells GA et al. A decision aid for women considering hormone therapy after menopause: decision support framework and evaluation. Patient Education and Counseling, 1998; 33: 267–279. [DOI] [PubMed] [Google Scholar]
- 19. Stewart M, Brown JB, Donner A et al. The impact of patient‐centered care on outcomes. Journal of Family Practice, 2000; 49: 796–804. [PubMed] [Google Scholar]
- 20. Stevenson FA, Barry CA, Britten N, Barber N, Bradley CP. Doctor–patient communication about drugs: the evidence for shared decision making. Social Science & Medicine, 2000; 50: 829–840. [DOI] [PubMed] [Google Scholar]
- 21. Stevenson FA, Gerrett D, Rivers P, Wallace G. GPs’ recognition of, and response to, influences on patients’ medicine taking: the implications for communication. Family Practice, 2000; 17: 119–123. [DOI] [PubMed] [Google Scholar]
- 22. Krupat E, Rosenkranz SL, Yeager CM et al. The practice orientations of physicians and patients: the effect of doctor–patient congruence on satisfaction. Patient Education and Counseling, 2000; 39: 49–59. [DOI] [PubMed] [Google Scholar]
- 23. Légaré F, O'Connor A, Graham I et al. The effect of decision aids on the agreement between women's and physician's decisional conflict about hormone replacement therapy. Patient Education and Counseling, 2003; 50: 211–221. [DOI] [PubMed] [Google Scholar]
- 24. O'Connor AM. Validation of a decisional conflict scale. Medical Decision Making, 1995; 15: 25–30. [DOI] [PubMed] [Google Scholar]
- 25. Carpenito LJ. Nursing Diagnosis: Application to Clinical Practice. Philadelphia: Lippincott, 2000: 312–321. [Google Scholar]
- 26. Dolan JG. A method for evaluating health care providers’ decision making: the Provider Decision Process Assessment Instrument. Medical Decision Making, 1999; 19: 38–41. [DOI] [PubMed] [Google Scholar]
- 27. Mead N, Bower P. Patient‐centredness: a conceptual framework and review of the empirical literature. Social Science & Medicine, 2000; 51: 1087–1110. [DOI] [PubMed] [Google Scholar]
- 28. Hilditch JR, Lewis J, Peter A et al. A menopause‐specific quality of life questionnaire: development and psychometric properties. Maturitas, 1996; 24: 161–175. [DOI] [PubMed] [Google Scholar]
- 29. Strull WM, Lo B, Charles G. Do patients want to participate in medical decision making? Journal of the American Medical Association, 1984; 252: 2990–2994. [PubMed] [Google Scholar]
- 30. Kaplan SH, Greenfield S, Gandek B, Rogers WH, Ware JE, Jr . Characteristics of physicians with participatory decision‐making styles. Annals of Internal Medicine, 1996; 124: 497–504. [DOI] [PubMed] [Google Scholar]
- 31. Bryk AS, Raudenbush SW. Hierarchical Linear Models: Applications and Data Analysis Methods. Newbury Park: Sage Publications, 1992. [Google Scholar]
- 32. Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. Journal of Educational and Behavioral Statistics, 1998; 24: 323–355. [Google Scholar]
- 33. Starfield B, Wray C, Hess K et al. The influence of patient–practitioner agreement on outcome of care. American Journal of Public Health, 1981; 71: 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stott N, Kinnersley P, Elwyn GJ. Measuring general practice‐based primary care: generic outcomes. Welsh Primary Care Outcomes Group. Family Practice, 1997; 14: 486–491. [DOI] [PubMed] [Google Scholar]
- 35. Brown JB, Carroll J, Boon H, Marmoreo J. Women's decision‐making about their health care: views over the life cycle. Patient Education and Counseling, 2002; 48: 225–231. [DOI] [PubMed] [Google Scholar]
- 36. O'Connor AM, Jacobsen MJ, Stacey D. An evidence‐based approach to managing women's decisional conflict. Journal of Obstetrical Gynaecological Neonatal Nursing, 2002; 31: 570–581. [DOI] [PubMed] [Google Scholar]
- 37. Campbell M, Grimshaw J, Steen N. Sample size calculations for cluster randomised trials. Changing Professional Practice in Europe Group (EU BIOMED II Concerted Action). Journal of Health Service Research Policy, 2000; 5: 12–16. [DOI] [PubMed] [Google Scholar]
- 38. Ringa V, Légaré F, Dodin S, Godin G, Norton J. Prescription of hormone replacement therapy among French and Canadian physicians. Menopause, 2003; 11 (in press). [Google Scholar]
- 39. Elwyn G, Edwards A, Wensing M et al. Shared decision making: developing the OPTION scale for measuring patient involvement. Quality and Safety in Health Care, 2003; 12: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Elwyn G, Edwards A, Kinnersley P. Shared decision‐making in primary care: the neglected second half of the consultation. British Journal of General Practice, 1999; 49: 477–482. [PMC free article] [PubMed] [Google Scholar]
- 41. Godolphin W, Towle A, McKendry R. Challenges in family practice related to informed and shared decision‐making: a survey of preceptors of medical students. Canadian Medical Association Journal, 2001; 165: 434–435. [PMC free article] [PubMed] [Google Scholar]
- 42. Charles C, Gafni A, Whelan T. Shared decision‐making in the medical encounter: what does it mean? (or it takes at least two to tango). Social Science & Medicine, 1997; 44: 681–692. [DOI] [PubMed] [Google Scholar]
- 43. Charles C, Gafni A, Whelan T. Decision‐making in the physician‐patient encounter: revisiting the shared treatment decision‐making model. Social Science & Medicine, 1999; 49: 651–661. [DOI] [PubMed] [Google Scholar]
- 44. Falkum E, Forde R. Paternalism, patient autonomy, and moral deliberation in the physician–patient relationship. Attitudes among Norwegian physicians. Social Science & Medicine, 2001; 52: 239–248. [DOI] [PubMed] [Google Scholar]
- 45. Marmoreo J, Belle Brown J, Batty HR, Cummings S, Powell M. Hormone replacement therapy: determinants of women's decisions. Patient Education and Counseling, 1998; 33: 289–298. [DOI] [PubMed] [Google Scholar]
- 46. Kittell LA, Mansfield PK. What perimenopausal women think about using hormones during menopause. Women & Health, 2000; 30: 77–91. [DOI] [PubMed] [Google Scholar]
- 47. Roter DL, Hall JA. Doctors Talking with Patients/Patients Talking with Doctors: Improving Communication in Medical Visits. Westport: Auburn House, 1992. [Google Scholar]
- 48. Keefe CW, Thompson ME, Noel MM. Medical students, clinical preventive services, and shared decision‐making. Academic Medicine, 2002; 77: 1160–1161. [DOI] [PubMed] [Google Scholar]
- 49. Folmer Andersen T, Mooney G. The Challenges of Medical Practice Variations. London: The Macmillan Press Ltd, 1990. [Google Scholar]


