Abstract
Purpose To study the cognitive processes of early‐stage prostate cancer patients as they determined which treatment they preferred, using our cognitively based decision aid.
Method The aid was a one‐to‐one interview that included the structured presentation of information, listing exercises in which the patient identified attributes important to his decision, and trade‐off exercises to help him weigh and integrate those attributes together. At various points of the interview, patients identified the attributes they felt were important to their decision, rated their treatment options and completed standardized assessments relating to their decision. In addition, patients participated in a follow‐up interview at the time they made their actual treatment decision and again 3 months later.
Results Sixty of 70 (86%) of the invited patients participated in the study. Participating patients identified a median of four important attributes (range 1–10); 36 different attributes were identified at some point in the interview by the group. During the interview, 78% of patients changed which attributes they considered important, and 72% changed their treatment ratings. Stability of treatment choice after the interview and lack of regret after the decision were each positively associated with increasing differentiation between treatment options over time.
Conclusions The decision process appears to be dynamic for the patients with great variability across patients in what is important to the decision. Increasing stability of choice and lack of regret appear to be related positively to increasing difference over time in how attractive the preferred option is over its closest competitor, rather than to the size of the difference at any one point in time.
Keywords: decision aid, patient support, prostate cancer, shared decision‐making
Introduction
While there is general agreement that decision aids in medical settings are interventions designed to help patients participate in decisions about their health care, there is less agreement on what the aids should include or what they should accomplish. Reviews of decision aids have noted that the aids often focus exclusively on information provision. 1 , 2 Unlike the educational programmes that provide information, decision aids are intended to help the patient through the decision process, but only a few aim to do so explicitly. 1 , 2
What decision aids should be designed to accomplish is controlled, to some extent, by our views of the limitations faced by decision makers. Herbert Simon 3 viewed humans as fundamentally rational decision makers but hampered by limited resources such as memory and energy. The resource limitations lead to the use of resource‐reducing short‐cut strategies, or heuristics, but at a cost of being less thorough. 4 Because being thorough is integral to rationality, one approach for decision aids is to help patients by augmenting their limited processing resources; 5 the augmentation would in turn limit the need for heuristics that would compromise the decision. The potential impact of such decision aids is illustrated by experimental evidence that they can be designed to encourage the use of the more comprehensive, and therefore more resource demanding, ‘compensatory’ decision rules. 6 , 7 , 8
To build a decision aid that could augment patients’ cognitive resources, one needs to identify the cognitive processes that people typically use when making decisions and then identify the likely resource limitations. Svenson's ‘Differentiation and Consolidation’ (Diff Con) theory, a descriptive ‘‘process theory’’ of decision making, provides such a framework. Diff Con is one of the more comprehensive process theories (see also 9 , 10 , 11 ) and it explicitly describes processes that people typically engage in when making complex decisions in novel situations. 12 , 13
According to Diff Con, people make decisions to achieve particular goals. The decision processes aim to make one alternative (of those offered) sufficiently superior over its competitor(s) that it should be able to withstand both internal (e.g. a change of own values) and external (e.g. a poor outcome) threats to its selection as the preferred alternative. The superiority of the preferred alternative is created through the application of decision rules and restructuring processes, called ‘‘differentiation’’; ‘‘consolidation’’ describes the same processes but they occur after the decision has been made. Central to Diff Con is the assumption that sufficient restructuring (differentiation and consolidation) protects the decision maker from threats, and those threats can manifest themselves in our experiencing cognitive dissonance and/or regret. A decision aid based on Diff Con, therefore, would aim to help patients increase the difference in attractiveness between the preferred option and the other alternatives by encouraging differentiation and consolidation. We have designed a decision aid, guided by Diff Con, that allows us to study patients’ cognitive processes as they figure out the treatment option they prefer.
The lack of a gold standard for determining a ‘good’ decision 14 makes evaluating decision aids a challenge and it has been recognized that appropriate evaluations of decision aids would be driven to a large extent by the theory underlying the aid. 15 Svenson (see also Baron 16 ) suggests that we naturally make decisions to achieve particular goals; thus, the ideal assessment would be to determine if the decision aid helped the patient achieve his goals. Many goals differ from one decision to the next; however, Svenson and others 17 , 18 , 19 suggest that a goal common to all decisions is to limit the possibility of regretting the decision at a later date. In spite of the fact that regret can only be assessed after the decision is made and its ultimate assessment can only happen after all outcomes related to the decision are fully experienced, our natural tendency to consider regret when we make decisions means that it is a natural outcome to evaluate. Thus, assessing regret before all the decision's consequences are experienced may help us to determine the potential of regret as an outcome measure. Our decision aid allows us to investigate the potential for assessing regret and to investigate how it relates to cognitive processes that occur.
Treatment decisions for early‐stage prostate cancer are among those decisions that patients find particularly challenging (e.g. see Grove 20 ). At the time of the study, standard treatments included three options: surgery, radiotherapy, and ‘no treatment for now’ (watchful waiting), although not all patients are offered all three options. The decision typically requires processing a large amount of information from which pertinent risks and benefits must be identified and integrated, which clearly makes it demanding of cognitive resources. It is even more challenging because facing such a ‘‘high‐stakes’’ decision is typically a new experience for the patient, and he often feels some urgency to make a decision quickly but frequently is in an emotional state that reduces his processing resources. 21 , 22 Thus, this treatment decision as faced by these patients is particularly at risk of being compromised by limited cognitive resources.
The purpose of this report is to describe the insights we have gained into patients’ cognitive processing as they made their treatment decisions using our process‐oriented decision aid. The insights help us better understand both similarities and differences among the patients within the group. Objectives related to studying the cognitive processes were:
-
1
to identify attributes that the patients considered important to their decision,
-
2
to determine what patients identify as particular challenges as they make their decisions,
-
3
to describe the proportion of patients that appear to show differentiation and consolidation through:
-
(a)
changing which attributes were important to their decisions, and
-
(b)
changing their ratings of how attractive the various treatment options are,
-
(a)
-
4
to identify aspects of cognitive processing that are associated with:
-
(a)
the stability of the preferred treatment option; i.e. the likelihood that the patients’ actual treatment decision was the treatment they preferred at the end of the interview, and
-
(b)
regret as scored after they had completed their treatment.
-
(a)
Method
Participants
The participants were consecutive patients diagnosed with low or medium‐risk early‐stage prostate cancer, i.e. patients with stage T1N0/T2N0 disease, prostate specific antigen (PSA) < 20, Gleason score <8. They also had to be able to understand English and be able to tolerate the interview in the opinion of the attending radiation oncologist. The study had ethics approval from the Queen's University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board.
Procedure – overview
The decision aid is a one‐to‐one interview with a research assistant, that occurs between an initial consultation when the doctor presents the treatment options to the patient and a second consultation that occurs about 1 week later when the treatment decision is made. Thus, the aid is intended to help the patient become clearer about which treatment option he prefers in order to make the decision with his doctor at his next visit.
This study involved the decision‐aid interview, a first follow‐up interview (follow‐up 1) that occurred after the patient made his actual treatment choice with his doctor, and a second follow‐up interview (follow‐up 2) that occurred about 3 months after the treatment decision, when the acute side‐effects of the active treatments would have resolved. Figure 1 shows all interviews, with all of the activities down the centre of the figure (in temporal order from top to bottom), the interview outputs listed on the left, and study assessments on the right. Activities related to each of the three components of the decision‐aid interview are identified with the appropriate letter: (A) the structured presentation of the information, (B) listing exercises (the focus of this report; they are highlighted in grey), and (C) treatment trade‐off exercises.
Figure 1.
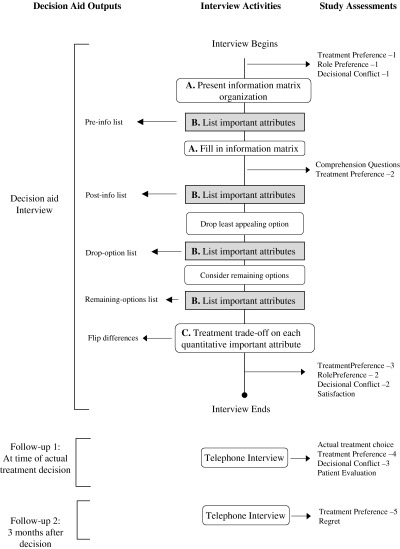
Order of activities in decision‐aid interview. Activities are identified in the rounded boxes down the central column of the figure; they are listed from the top of the figure down, as they occur chronologically in the interview. Arrows from particular activity boxes to the left identify outputs of the activity. Arrows from the central column to the right identify additional assessments included in the interview in order to evaluate the impact of particular aspects of the interview.
Procedure – details
Decision‐aid interview
After the patient consented to participate in the study, his physician identified which of the three treatment options were being offered, and some patients considered only two options while others considered three. Because the study was run in a cancer centre, all patients were offered radiation treatment, but not all offered either surgery (because of co‐morbidities) or offered no treatment for now (often because the doctor felt the patient was too young). The physician also provided probability estimates for each of seven quantitative outcomes relevant for each of offered treatments that were specific to that patient (identified with an ‘*’ in Table 1), taking into account the patient's age and associated co‐morbidities, if any, in addition to his disease which we incorporated into the information we presented to the patient (the physicians were provided with outcome ranges found in the literature to anchor their estimates 4 ).
Table 1.
Information board organization
| Category | Issue | No treatment for now | Radiotherapy | Surgery |
|---|---|---|---|---|
| Background | What cancer is How cancer progresses Where cancer spreads to Procedure for treatment Length of time to decide Seeking a second opinion | |||
| Treatment details | How the treatment works When can the treatment start How to know if the treatment is working | |||
| Possible benefits of treatment | Chances of PSA rising* Chances of cancer causing symptoms* Chances of dying from the cancer* Effect on how long I will live | |||
| Possible harms of treatment | Chances of dying from treatment* Effect on sexual functioning* Effect on bladder functioning* Effect on bowel functioning* | |||
| Options if treatment does not work | Options if cancer gets worse Options if cancer does not disappear after treatment Options if cancer comes reappears | |||
| What others choose | Choices other patients make Choices doctors make |
As shown in Fig. 1, the decision‐aid interview with a research associate began with collecting the baseline assessments. Of the baseline assessments collected, this report focusses on the treatment preference assessment (TPA). The assessment requested that the patient rate each of the treatment options he was offered on a five‐point ordinal scale: 0, ‘Do not want this option’ to 4, ‘Definitely want this option’.
After the assessments, the information matrix was presented by first outlining how the matrix was organized and then filling in the information, a strategy intended to facilitate patient understanding. 23 The information addressed the issues identified empirically as that which patients consider important to their decisions, 24 with the content to address each issue developed from the literature as best as could be; physicians then approved of what we presented, they did not influence the content at all. Table 1 shows the items included in the presentation and Table 2 shows the information provided for one item to show the extent of detail provided. The patient‐specific outcome estimates provided by the physician were incorporated into the presentation (in the blanks of Table 2) and were provided as frequencies out of 100 and presented in numeric format. Further details of the presentation procedure can be found elsewhere. 25
Table 2.
An example of the information provided in the decision aid
| Issue | No treatment for now | Radiotherapy | Surgery |
|---|---|---|---|
| Effect on bladder functioning | If you choose no treatment for now, growth of the prostate cancer can sometimes affect your ability to pass urine. This happens in about four of 100 men in your situation within 10 years. The problem can usually be corrected with a day‐surgery procedure. | Temporary: You will probably need to empty your bladder more frequently starting in the third or fourth week of radiation treatment. This happens to about 60 of 100 men who have radiation. Also, you may have some discomfort when you empty your bladder. These symptoms last until 2–3 weeks after treatment. Months or years after radiation treatment, scar tissue may develop and interfere with the flow of urine. This happens to about two of 100 men receiving radiation and can usually be corrected with a day‐surgery procedure. Permanent: A few men have to empty their bladder more frequently after radiation. In addition, some men who have had radiation develop problems with bladder control: of these men, __ of 100 have dribbling that requires a pad to keep their clothes dry; and __lose total control, needing either an adult diaper or a tube inserted into their bladder to drain the urine into a bag. | Temporary: You will lose bladder control and need a catheter (a tube in your penis) right after the surgery. Most men regain complete control of their bladder within a few months. Also, months or years after surgery, scar tissue may develop and interfere with the flow of urine. This happens to about two of 100 men having surgery. It can usually be corrected with a day‐surgery procedure. Permanent: About __ of the 100 men who have had surgery do not regain complete control of their bladder. __ of 100 men regain some control but have dribbling that requires a pad to keep their clothes dry. __ of 100 men do not regain any control, needing either an adult diaper or a tube inserted into their bladder to drain the urine into a bag. |
After the matrix labels were introduced, the patient identified the attributes important to his decision (Pre‐info list). He listed the attributes in their order of importance and was encouraged to list all important attributes, even if they were not included in the information board. After the information was presented, the patient was asked standardized comprehension questions, and was encouraged to look at the information board for the answer when he could not recall it. Following confirmation of his understanding the information, the patient revised his list of important attributes if needed (Post‐info list) and completed a second TPA. If he was offered three treatment options, he then identified his least‐preferred option to drop from further consideration, and listed the attributes important to that drop (Drop‐option list). After the drop, he identified the attributes important to the decision between the remaining two options (Remaining‐option list).
Once considering only two options, each patient completed the treatment trade‐off exercises: one exercise focussed on each quantitative attribute important to his choice. The quantities were presented as vertical bars in the exercises. 26 We do not focus on the treatment trade‐off exercises in this report.
After the trade‐off exercises, final assessments were completed and they included four assessments: (1) TPA, (2) a role preference assessed with Degner & Sloan's Control Preference Scale, 27 (3) decisional conflict assessed with O'Connor's Decisional Conflict Scale, 28 and (4) satisfaction assessed with Graham's Satisfaction with Preparation for Decision Making Scale. 29 We focus primarily on the TPA and on three of the 13 items in the Decisional Conflict Scale in this report. The three items are: (i) I know how important the benefits of each option are to me in this decision, (ii) I know how important the risks and side‐effects of each option are to me in this decision, and (iii) It is hard to decide that the benefits are more important to me than the risks, or if the risks are more important. Responses to each item used a five‐point ordinal scale from ‘strongly agree’ to ‘strongly disagree’.
The interview ended with the participant providing demographic information: age, highest level of formal education, and partner status. He was then provided with a printout of the information matrix to take home with him.
Follow‐up 1
After the decision‐aid interview, patients were tracked in the clinic database to identify when they made their actual treatment choice. At that time, they completed the first follow‐up interview, over the phone for all but the 10 patients as described below in the repeatability study. All participants had made their treatment decision at the time of the follow‐up 1 interview but none had started the treatment. As shown in Fig. 1, this interview included three assessments of which we only focus on the Treatment Preference Assessment in this report.
Follow‐up 2
We continued to track patients through the clinic database in order to identify when they actually started their treatments. The second follow‐up interview, also over the telephone, took place approximately 3 months after they started their treatments. At that time, the patients completed a final TPA and an assessment of regret. Regret was measured by O'Connor's Regret Scale. 30 The Regret Scale has five items, each requiring agree/disagree responses on a five‐point ordinal scale; an individual's regret score was the total of the Regret Scale, a range of 5 (low regret) to 25 (high regret). This report focusses on both the two final assessments.
Repeatability study
It is recognized that when decision makers are faced with an unusual type of decision, such as the decision about treatment for prostate cancer, 21 , 22 the decision maker often needs to discover what he/she values related to the decision, 31 suggesting that this type of decision results in particularly active differentiation. Thus, it would be expected that the attributes considered important to patients in this study would shift frequently, especially near the beginning of the decision process. In spite of the expected changes, we assessed our method of obtaining important attributes in order to get a sense of the stability of the responses that we obtained. We asked 10 patients to return to the location of the original interview for their first follow‐up interview (anywhere from one to several weeks later) and to repeat the listing procedure. The items listed on their final list (Post‐info list for those who had two options and Remaining‐options list for those who had three) of the original interview were then compared with those listed in the follow‐up interview.
Results
Participants
Of the 70 men who were invited, 60 (86%) chose to participate in the study; 33 were provided with three treatment options and 27 with two options. Participants had a mean age of 65.8 years, with a range of 41–76 years (SD 6.95). Regarding their education: 10% had less than high school education, 43% had completed high school, and 47% had some post‐secondary education. Regarding their partner status: 88% were living with a partner, 5% were single and 7% were widowed or ‘other’ marital status.
Repeatability study
The 10 patients who participated in the repeatability study identified a mean of 4.3 (range 2–8) important attributes at the end of the original interview and a mean of 4.5 (range 3–7) in the follow‐up interview. Of the total number of items identified at both times, a mean of 69.2% appeared on both lists; a mean of 85.6% of original interview attributes were repeated in the follow‐up interview. Thus, the overwhelming majority of attributes identified as important at the original interview continued over the time to be identified as important; changes over time (either due to our measurement method or to a real shift in what was important) were more often in the direction of adding new attributes to the list.
Insight into cognitive processes
Objective 1 – attributes important to the decision
There was a wide range in the number of attributes important to individual patients, in the actual attributes chosen, and in their rank order. Overall, the 60 participants identified 33 different items as important to their decisions when they were selecting their most preferred option; one additional item was identified only when they were considering which option to drop. Thus, overall, 34 different attributes were identified as important to the decisions of these 60 patients.
Figure 2 shows the 33 different important attributes identified by the group when patients were considering their final options (Remaining‐options list for those offered three options and Post‐Info list for those offered two options) and the proportion of patients that identified each of the attributes as important, ranked 1 (most important) to 4 and greater. As seen in the figure, for the 60 patients, 18 different attributes were considered the most important attributes to the decision. The wide variation in important attributes is further demonstrated by the fact that only two attributes were important to more than 50% of patients (effect on bladder functioning, and effect on bowel functioning). Interestingly, we analysed the attributes with respect to each treatment option: for each attribute we calculated the proportion of patients offered that treatment option who identified that attribute as important and found similar proportions across the three treatment options. In addition, there was no difference in the number of attributes important to patients who were offered 3 treatment options (mean 4) to those offered 2 options (mean 4.3), [t(58) < 1]. Table 3 shows both the median and range in number of important attributes on each of the three lists focussed on choosing a preferred option.
Figure 2.
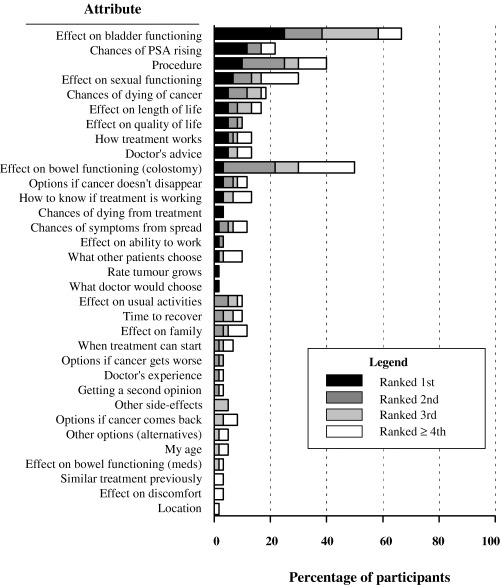
Ranking of attributes important to the decision. The figure illustrates the treatment attributes that were listed and identifies the proportion of patients that ranked each attribute as first, second, third, and fourth or lower in importance to their decision. The overall length of each bar indicates the proportion of all patients identifying that attribute as important.
Table 3.
A summary of the important attributes identified by each participant Pre‐info, Post‐info and when considering Remaining options
| Pre‐info list | Post‐info list | Changes between Pre‐info and Post‐info lists (n = 60) | Remaining‐option list1 | Changes between Post‐info and Remaining‐options lists1 (n=33) | |||
|---|---|---|---|---|---|---|---|
| Attributes dropped | Attributes added | Attributes dropped | Attributes added | ||||
| Participants providing responses [n (%)] | 60 (100) | 60 (100) | 27 (45) | 29 (48) | 33 (100) | 13 (39) | 8 (24) |
| Number of attributes [median (range)] | 4 (1–16) | 4 (1–10) | 2 (1–8) | 1 (1–4) | 2 (0–4) | 1 (1–3) | 2 (1–3) |
| Participants who identified non‐board attributes [n (%)] | 25 (42) | 32 (53) | 10 (30) | ||||
1Only patients with three treatment options completed the Remaining‐options list (n = 33).
The Drop‐list of attributes (attributes important to dropping the least‐preferred option) was only relevant to the 33 patients who were offered three options; of those patients, one man could not provide a reason to drop one of the options, thus there were responses from 32 patients. The median number of attributes underlying the drop for the 32 patients was 2 (range 1–4), including 10 of these patients who reported non‐board items (median 1, range 1–3). Nineteen of the patients (58%) identified at least one attribute on their drop lists that was not identified on any of their other lists and for 11 of the patients (33%), none of the attributes on their drop list was on any of the other lists. The most common attribute underlying the drop was ‘the procedure involved’ and that was important to 14 patients (42.4%). One attribute was important to the drop that was not identified on any of the lists focussed on choosing the most preferred option: ‘economic factors’.
We note that we were concerned that the large number of items in the board presentation might overwhelm patients’ ability to identify items beyond those presented that were important to them. Table 3 shows the proportion of patients who identified items not presented on the board (‘‘non‐board items’’) in their lists of attributes important to selecting the preferred option. As the table shows, a sizable proportion of patients identified such items on each of the lists. Overall, 37 of the 60 patients (61.6%) identified non‐board items in at least one of their important attribute lists.
Objective 2 – cognitive challenges
Insight into the cognitive challenges faced by patients was gleaned from their responses to the three items of the Decisional Conflict Scale identified in the methods. At the end of the decision‐aid interview 92% of the participants (strongly) agreed that they were clear about the importance of the benefits of the options and 88% (strongly) agreed that they were clear about the importance of the risks and side‐effects of the options. However, 47% (strongly) agreed that it was hard for them to decide if the benefits or the risks were important to them in the decision‐making.
Objective 3(a) – changes in important attributes over decision process
Table 3 also describes the changes that occurred between the lists, providing us with some insight into the differentiation processes. A great number of important attributes were changed during the decision process: almost half of the patients (45%) added to, and a similar proportion (48%) dropped attributes from, the Pre‐Info list when they listed their important attributes on the Post‐Info list. Of the patients offered only two options, 78% changed at least one attribute between the two lists. Similarly, almost one‐third of those completing the Remaining‐options list dropped attributes from their Post‐Info list and 25% added more. Overall, 49 (81.7%) patients changed, at some point in the interview, the attributes that they reported as important to the selection of their treatment choice.
Objective 3(b) – differentiation and consolidation: changes in treatment ratings
Over the three assessments during the decision‐aid interview, 43 (71.7%) patients changed at least one of their TPA ratings of the treatment options offered to them. Between the interview and follow‐up 1, 45 patients (75%) changed at least one of their TPA scores, and between follow‐up 1 and follow‐up 2, 34 (57%) changed at least one of their scores. Of the 13 who did not change their scores in the first post‐interview period, 11 (85%) had scored their preferred option at 4 (maximum) and all other options at 0 (minimum), suggesting they had completed their differentiation and consolidation by the earlier time point; the same was true for 16 of the 22 (73%) who did not change their TPAs in the second time interval. Only six patients (10%) started the interview off with the maximum separation between TPA scores of their options and never changed the scores.
Figure 3 shows the average TPA scores for the most preferred option and for that of its closest competitor across the 56 patients who had completed the TPAs at all five time points it was assessed in the study, the three in the interview – when changes would usually reflect differentiation – and in each of the two follow‐up interviews, when changes reflect consolidation. As seen in the figure, the difference between mean TPA score for the most preferred option and that of its nearest competitior grew significantly over the five time points: the score of the most preferred option increased gradually over the entire time, while that of its closest competitor dropped [F(4,220) = 16.6, P < 0.001]. Thus, as Diff Con suggests, the difference in the attractiveness of the most preferred option when compared with its nearest competitor continued to increase over the whole of the study.
Figure 3.
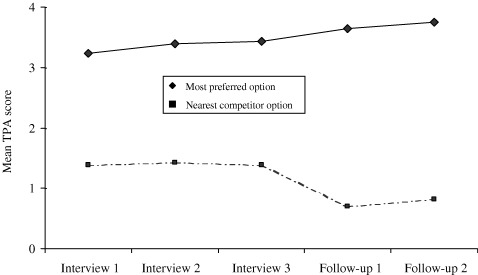
Mean TPA scores for most preferred option and for its nearest competitor during the study. The figure illustrates that the mean TPA score for the most preferred option increased in a linear manner throughout the decision‐aid interview, and continued to do so after the actual decision was made. The figure also illustrates how the mean TPA score for the nearest competitor dropped in a step fashion between the end of the decision‐aid interview and follow‐up 1, when the treatment decision was made.
Objective 4(a) – cognitive processes associated with stability of preferred treatment option
At the beginning of the interview 17 (28%) of the patients did not have a clear treatment preference, as indicated by ties in top TPA scores; by the end of the interview, only five of them still did not have a treatment preference – three of them changed their TPA scores, but this did not resolve the tie. Five additional patients had TPA scores that were tied at the end of the interview but not earlier. Thus, at the end of the interview, 50 (83%) patients had a clear treatment preference; of the 10 patients who did not have a clear preference, nine had been offered all three treatment options. It is interesting to note that while the changes in TPA scores [described in the results addressing Objective 3(b)] suggest that three‐fourths of our patients were actively changing their attitudes towards the options offered to them, those changes were not suggested when looking at which option patients preferred over the course of the interview; of the 43 who had a treatment preference at the beginning of the interview, 32 (74% of that group) did not change that preference during the interview.
Of the 50 who had a treatment preference at the end of the interview, 38 (76%) chose that preferred option as their actual treatment, and we describe their having the same preferences at the two time points as their preferences being consistent. Consistency of the preference was not associated with the size of the difference in TPA scores between the most preferred option and its nearest competitor, as measured either at the end of the interview (odds ratio 1.38, 95% CI: 0.80–2.37) or at the time of follow‐up 1 (odds ratio 0.87, 95% CI: 0.44–1.72). However, the consistency was associated with increasing differences in the TPA score between the most preferred option and the TPA score of its nearest competitor between the interview and follow‐up 1 (odds ratio 2.1, 95% CI: 1.40–3.14). Thus, it appears that the relationship between TPA scores and whether patients actually chose the option that had the highest TPA score at the end of the interview is complex. The association appears to be with the shift in the difference between the preferred option and its competitiors, rather than with the size of the difference at any particular point in time.
Objective 4(b) – cognitive processes associated with regret
The range of regret scores was 5–14 (recall that the scale is 5–25) with a mean of 8.4; six patients scored above 10. The degree of regret was not associated with the difference in the TPA scores of the most preferred option and it nearest competitor, as measured at either follow‐up 1 or at follow‐up 2 [both F(1,54) < 1]. However, regret scores were negatively associated with the shifts in the difference in the two TPA scores: regret scores decreased as the difference in TPA scores of the most preferred option and its nearest competitor increased from the interview to follow‐up 2 [F(1,54) = 4.27, P < 0.05] and from follow‐up 1 to follow‐up 2 [F(1,53) = 7.28, P < 0.01]. Thus, similar to the above association with shifts in TPA scores, it seems that patients experience less regret as the difference in the TPA scores between the two highest options increases. In other words, if differentiation is increasing, patients tend to feel less regret.
Discussion
Our cognitive approach to the design and implementation of a decision aid has provided us with some insights into the patients’ cognitive decision processes. The first insight is that there is wide variation from one patient to the next in attributes that affect their treatment decisions, consistent with the wide variation that we found in our retrospective survey of patient information needs for decision‐making. 24 The variation in patient values may not be particularly surprising (although we are not aware of it being documented specifically for the decision by others), but it does have important implications both for supporting patient involvement in this treatment decision and for studies of the population. Systems designed to support individual patient involvement must be able to accommodate the variation but in a manner that limits cognitive burden caused by addressing issues irrelevant to the individual; and, the two‐thirds of our patients who identified attributes not included in our original list suggests that our approach to this issue is reasonable. In studying the population, the wide variation implies that a large number of attributes are required to cover the concerns of the population – and, our repeatability study suggests that, if anything, our results may underestimate that number. The large number of concerns suggests that the relatively few health states considered in decision analyses models of this decision 32 , 33 , 34 , 35 miss salient aspects of the decision important to a large number of patients.
A second insight is how frequently patients changed their report of important attributes and changed their evaluations of the treatment options offered to them. We acknowledge that some of the changes we found may be due to random effects and perhaps to our methods, e.g. narrowing patients’ focus to two of the three options. But, even of those patients who were offered only two options, 78% modified the attributes important to their choice. Thus, it is likely that a large part of the observed changes was due to the patients still discovering which attributes were important to their decisions and how to integrate them. We point out that all our patients had had at least two consultations with specialists (a urologist and a radiation oncologist) before arriving at the decision‐aid interview. Thus, they had both more time and more professional input than many patients who face this type of treatment decision. We recognize that going to two specialists may have, in fact, confused them through receiving some contradictory information. However, most of the urologists and all the radiation oncologists in our area knew (and approved) the information that we provided. It is likely, therefore, that the doctors were less contradictory than patients typically face. It is also possible that some of the shift during the interview was due to the patients becoming less influenced by the physicians’ preferences; again, because of the physician awareness of our study, it is likely that such influence would be minimized relative to what patients might otherwise encounter. Regardless of its source, the extent of changes in attributes and in treatment ratings demonstrated in this study emphasizes the dynamic nature of the decision process.
The fact that we found that patients were less likely to change TPA scores at later time points suggests that the decision does get consolidated. We recognize that the TPA measure could hide continuing shifts in important attributes reflective of continuing differentiation/consolidation. However, as Diff Con suggests, we observed an increasing proportion of patients over time whose TPA scores for their preferred option was the maximum score and the scores for all other alternatives was the minimum, and that both the stability of treatment choice and less regret were associated with that increase in difference in TPA scores. The pattern of changes that we observed raises questions about helping patients with their decision using a process that does not recognize differentiation which is extended in time; e.g. a simple application of decision analysis would produce utilities at a particular point in time. However, continuing differentiation could result in the patient's utilities shifting, possibly quite considerably as that which occurs when an attribute is in conflict with the general preference for a particular treatment. 36
A third insight relates to the fact that almost one‐third of our patients identified attributes when they considered dropping their least favoured option that were not otherwise mentioned. Discussions of treatment decision‐making do not typically include a distinction between selecting a most‐preferred and a least‐preferred option. As Diff Con and other process theories of decision‐making suggest, 9 however, early stages of decision‐making are frequently focussed on screening out options. These results suggest that when there are more than two options, if screening out options is not explicitly built into the process, important attributes may be overlooked and we may in turn increase the complexity of the decision for the patients.
A fourth insight was gained by focussing on differentiation and consolidation. Both the stability of the patients’ treatment choices and the extent of their regret after the decision appear to be related to whether the patients’ evaluations of the options were still increasingly in favour of their initial treatment preference. The reasons for the increasing divergence may be quite complicated; e.g. as already mentioned, Svenson 36 has noted that when an attribute is in conflict with the preferred option, we often reduce the importance of that attribute. In addition to differentiation that is inherent to all decisions, we recognize that some of the observed change in preference after the decision‐aid interview could be due to physician preferences swaying the patient. Although we do not know why the patients in this study were changing their assessments of the various treatment options, it does appear that increasing divergence in the assessments was associated with better stability and less regret, and we note that the observation provides support for Diff Con.
While we have gained some insight into how individual patients viewed particular attributes, the aid did not provide us with information about how patients actually weighed each attribute as they arrived at their preferred option. The fact that a high proportion of our participants found it hard to decide if risks or benefits were more important raises questions about whether the trade‐off exercises could be improved upon as a method for helping patients weigh and integrate the attributes that are important to them. These results suggest that further study of patients’ cognitive weighing and integrating processes is required. Part of our plan is to use qualitative research methods in ‘exit interviews’ to gain some insight into these processes.
O'Connor and colleagues’ review of decision‐aid studies 2 noted that decision aids were more likely to alter the choice of patients who were undecided before using the aid than those who had an initial inclination and our results are consistent with that observation. We would argue, however, that decision aids also offer potential benefits to patients who appear to have a preferred treatment option prior to using the aid. The most obvious benefit to an ‘already‐decided’ group is to ensure that their choice is based on fact and not on misconception. The potential benefits that result from the unique goal of decision aids to clarify patients’ values is less obvious. We argue that through values clarification, a decision aid can promote differentiation and consolidation, thereby reducing the likelihood that patients will experience regret. As already mentioned, Diff Con identifies that we often use post‐decisional restructuring to reduce the likelihood that we will experience regret. Our data demonstrate that although over half of our participants had a preferred treatment at the beginning of the interview – and did not change that preference – almost all showed evidence of differentiation/consolidation. Our evidence that increasing differentiation/consolidation was associated with less early regret suggests that patients with an apparent treatment preference could use the decision‐aid interview to consolidate their choices, and hence reduce the likelihood they will regret the decision later.
We conclude that our approach of basing decision‐aid development on a cognitive process theory of decision‐making helps us create a product that could both help patients and provide us with some insight into their cognitive processing that may, in turn, help us to be even more effective. Guided by Diff Con, our observations of differentiation/consolidation and its relationships to stability of choice and to regret suggest that values clarification exercises in decision aids may want to focus on encouraging differentiation processes. Our observations that decision processes are related to regret, even expressions of early regret, support the use of regret as a primary outcome for the evaluation of decision aids.
Acknowledgements
We would like to thank Drs Charles Hayter, Bill Mackillop and Curtis Nickle for their role in consensus discussions regarding the information imparted to patients in the decision aid, to Drs Charles Hayter, Lawrence Paszat, Peter Froud, and Peter Dixon for their role in recruiting patients. The study was supported in part by the National Cancer Institute of Canada with funds contributed by the Canadian Cancer Society. D Feldman‐Stewart is supported by an Ontario Ministry of Health and Long‐Term Care Career Scientist Award. The results were presented in part to the Society of Medical Decision Making, Cincinnati, Ohio, September, 2000.
References
- 1. O'Connor AM, Fiset V, Rostom A, Tetroe JM, Entwistle V, Llewellyn‐Thomas HA, Holmes‐Rovner M, Barry M, Jones J. Decision aids for people facing health treatment or screening decisions. pp. 1–14, 1999. Chichester, UK: John Wiley & Sons Ltd. Cochrane Collaboration, Consumers and Communication Cochrane Review Group, Contact Paola Rio: E‐mail: rio@hna.ffh.vic.gov.au.
- 2. O'Connor AM, Fiset V, DeGrasse C, Graham ID, Evans W, Stacey D, Laupacis A, Tugwell P. Decision aids for patients considering options affecting cancer outcomes: Evidence of efficacy and policy implications. Journal of National Cancer Institute, 1999; monographs: 67–80. [DOI] [PubMed] [Google Scholar]
- 3. Simon HA. Rational choice and the structure of the environment. Psychological Review, 1956; 63: 129–138. [DOI] [PubMed] [Google Scholar]
- 4. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science, 1974; 185: 1124–1131. [DOI] [PubMed] [Google Scholar]
- 5. Norman DA, Bobrow DG. On data‐limited and resource‐limited processes. Cognitive Psychology, 1975; 7: 44–64. [Google Scholar]
- 6. Todd PA, Benbasat I. The influence of decision aids on choice strategies under conditions of high cognitive load. IEEE Transactions on Systems, Man and Cybernetics, 1994; 24: 537–547. [Google Scholar]
- 7. Todd P, Benbasat I. The influence of decision aids on choice strategies: an experimental analysis of the role of cognitive effort. Organizational Behavior and Human Decision Processes, 1994; 60: 36–74. [Google Scholar]
- 8. Todd P, Benbasat I. Inducing compensatory information processing through decision aids that facilitate effort reduction: an experimental assessment. Journal of Behavioral Decision Making, 2000; 13: 91–106. [Google Scholar]
- 9. Beach LR, Potter RE. The pre‐choice screening of options. Acta Psychologica, 1992; 81: 115–126. [Google Scholar]
- 10. Montgomery H. Toward a perspective theory of decision‐making and judgment. Acta Psychologica, 1994; 87: 155–178. [DOI] [PubMed] [Google Scholar]
- 11. Payne JW, Bettman JR, Coupey E, Johnson EJ. A constructive process view of decision‐making: multiple strategies in judgement and choice. Acta Psychologica, 1992; 80: 107–141. [Google Scholar]
- 12. Svenson O. Decision‐making and the search for fundamental psychophysical regularities: what can be learned from a process perspective? Organizational Behavior and Human Decision Processes, 1996; 65: 252–267. [Google Scholar]
- 13. Svenson O. Process descriptions of decision‐making. Organization Behavior and Human Performance, 1979; 23: 86–112. [Google Scholar]
- 14. O'Connor AM. A call to standardize measures for judging the efficacy of interventions to aid patients’ decision‐making. Medical Decision Making, 1999; 19: 504–505. [DOI] [PubMed] [Google Scholar]
- 15. Entwistle V, Sowden TA, Watt IS. Evaluating interventions to promote patient involvement in decision‐making: by what criteria should effectiveness by judged? Journal of Health Services Research Policy, 1998; 3: 100–107. [DOI] [PubMed] [Google Scholar]
- 16. Baron J. Nonconsequentialist decisions. Behavioral and Brain Sciences, 1994; 17: 1–42. [Google Scholar]
- 17. Bell D. Regret in decision‐making under uncertainty. Operations Research, 1982; 30: 961–981. [Google Scholar]
- 18. Loomes G, Sugden R. Regret theory: an alternative theory of rational choice under uncertainty. The Economic Journal, 1982; 92: 805–824. [Google Scholar]
- 19. Ritov I. Probability of regret: anticipation of uncertainty resolution in choice. Organization Behavior and Human Decision Processes, 1996; 66: 228–236. [Google Scholar]
- 20. Grove A. Taking on prostate cancer. Fortune, May 13, 1996; 55–71. [Google Scholar]
- 21. O'Rourke ME, Germino BB. Prostate cancer treatment decisions: a focus group exploration. Oncology Nursing Forum, 1998; 25: 97–104. [PubMed] [Google Scholar]
- 22. O'Rourke ME. Narrowing the options: the process of deciding on prostate cancer treatment. Cancer Investigation, 1999; 17: 349–359. [DOI] [PubMed] [Google Scholar]
- 23. Ausabel DP. Educational Psychology: A Cognitive View. New York, NY: Holt Rinehart & Winston, Inc., 1968. [Google Scholar]
- 24. Feldman‐Stewart D, Brundage MD, Nickel JC, Mackillop WJ. The information required by patients with early‐stage prostate cancer in choosing their treatment. British Journal of Urology International, 2001; 87: 218–223. [DOI] [PubMed] [Google Scholar]
- 25. Feldman‐Stewart D, Brundage M, Van Manen L. A decision aid for men with early‐stage prostate cancer: theoretical basis and a test by surrogate patients. Health Expectations, 2001; 4: 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feldman‐Stewart D, Kocovski N, McConnell B, Brundage M, Mackillop W. Perception of quantitative information for treatment decisions. Medical Decision Making, 2000; 20: 228–238. [DOI] [PubMed] [Google Scholar]
- 27. Degner LF, Sloan JA. The control preferences scale. Canadian Journal of Nursing Research, 1997; 29: 21–43. [PubMed] [Google Scholar]
- 28. O'Connor AM. Decisional Conflict Scale, 3rd edn. Document of the Loeb Health Research Institute. Available: http://www.lri.ca/programs/ceu/ohdec/measures.htm. 1998. [Google Scholar]
- 29. Graham I, O'Connor AM. Satisfaction with Preparation for Decision Making. Document of the Loeb Health Research Institute. Available: http://www.lri.ca/programs/ceu/ohdec/measures.htm. 1996. [Google Scholar]
- 30. Brehaut J, O'Connor AM, Wood T, Hack T, Siminoff LA, Gordon E, Feldman‐Stewart D. Measuring regret over different treatment decisions. Medical Decision Making, 2003; 22: 536. [DOI] [PubMed] [Google Scholar]
- 31. Fischhoff B, Slovic P, Lichtenstein S. Knowing what you want: measuring labile values In: Wallsten TS. (ed.) Cognitive Processes in Choice and Decision Behavior. Hillsdale, NJ: Lawrence Erlbaum Associates, 1980: 117–141. [Google Scholar]
- 32. Fleming C, Wasson JH, Albertsen PC, Barry MJ, Wennberg JE. A decision analysis of alternative treatment strategies for clinically localized prostate cancer. Journal of the American Medical Association, 1993; 269: 2650–2658. [PubMed] [Google Scholar]
- 33. Kattan MW, Cowen ME, Miles BJ. A decision analysis for treatment of clinically localized prostate cancer. Journal of General Internal Medicine, 1997; 12: 299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mold JW, Holtgrave DR, Bisonni RS, Marley DS, Wright RA, Spann SJ. The evaluation and treatment of men with asymptomatic prostate nodules in primary care: a decision analysis. Journal of Family Practice, 1992; 34: 561–568. [PubMed] [Google Scholar]
- 35. Walsh PC. A decision analysis of alternative treatment strategies for clinically localized prostate cancer. Journal of Urology, 1993; 150: 1330–1332. [PubMed] [Google Scholar]
- 36. Svenson O, Hill T. Turning prior disadvantages into advantages: Differentiation and consolidation in real‐life decision‐making In: Rayner R, Crozier WR, Svenson O. (eds). Decision Making: Cognitive Models and Explanations. London: Routledge, 1997. [Google Scholar]


