Abstract
Objective We explored the influence of different but factual scenarios about prostate‐specific antigen (PSA) screening on men's interest in having PSA screening to detect early prostate cancer.
Design Cross‐sectional, representative community survey.
Setting and participants A total of 514 men (89% response fraction) aged 50–70 years randomly selected from a telephone directory database in Sydney, Australia.
Main variables studied Demographic, health and psychological variables.
Main outcome variables Interest in undergoing screening in response to five unspecified scenarios and, elsewhere in our interview, a specified scenario in which PSA screening was mentioned explicitly.
Results When presented with a scenario describing a lack of evidence underpinning the efficacy of screening for an unspecified cancer, 61.2% of men reported that they ‘probably’ or ‘definitely’ wanted to undergo screening for an unspecified cancer. Similar proportions reported that they ‘probably’ or ‘definitely’ wanted to undergo screening even at the risk of unmasking indolent cancer (60.9%) or without expert consensus about the value of screening (62.8%). Greatest interest in screening was elicited in that scenario describing life‐time risk of dying from prostate cancer (72.6%) (P < 0.001). Significantly fewer indicated they would ‘probably’ or ‘definitely’ want to undergo screening for a cancer for which there was uncertainty about treatment efficacy and known side‐effects (46.1%) (P < 0.001). Increasing age was a consistent predictor of positive interest in screening. When asked later in our survey specifically about PSA screening, 68.1%‘probably’ or definitely’ wanted PSA screening.
Conclusion Public health policy makers need to ensure that men are provided with the scope of medical evidence germane to prostate cancer screening and treatment, thereby potentially improving prostate cancer screening decisions.
Keywords: community survey, decision‐making, intention to screen, prostate‐specific antigen screening
Introduction
The early detection of prostate cancer is controversial, given the debate surrounding prostate‐specific antigen (PSA) testing. Further, some have argued that the natural history of prostate cancer, which tends to be slow growing, raises questions about the value of screening for this disease. There is no evidence yet from randomized‐controlled trials that early detection of prostate cancer by PSA screening reduces disease‐specific mortality. 1 While two randomized studies of early detection and standardized treatment are currently underway in Europe and the US, results are not expected until 2006–2008. 2 , 3 At present, expected gains in survival are speculative. Further, the risk of detecting clinically insignificant (indolent) cancer through screening and the risk of permanent urinary, sexual and bowel dysfunction in men who subsequently undergo treatment for prostate cancer are substantial. 1 Hence, deciding whether or not to have PSA screening has been described as a ‘toss‐up’ 4 in which the anticipated but not yet proven reduction in disease‐specific mortality may be outweighed by exposure to real and irreversible harm. Medical authorities do not recommend PSA screening but, instead, advise that men are fully apprised of the evidence pertaining to the pros and cons of PSA screening before deciding themselves whether or not to be screened. 1 , 5 , 6 , 7 , 8 , 9 , 10
Debate continues about how ‘informed participation’ in PSA screening can be best achieved. 11 , 12 Striking the balance appears fraught. From the consumer perspective, too little is understood of men's perceptions of this controversy. Few studies have addressed the influence of specific messages on their interest in PSA screening. Sarfati et al. 13 have examined the effect of the framing of numerical data about the benefits of an unspecified screening test on decisions to participate among a population‐based sample of adult men and women. However, less research has been conducted to examine reactions to ‘framing’ about harms of screening. The extent to which key messages about the controversy have an impact on men's interest in PSA screening is especially unclear. While studies have recently explored women's aspects of decision‐making relating to mammographic screening, 14 men's reactions to PSA screening have not been examined.
Hence, we designed a community survey to determine the effect of different scenarios about PSA screening on men's interest in undergoing a test for cancer. Specifically, we wished to determine men's interest in screening in response to facts about the pros and cons of screening. Our study was particularly novel in first presenting scenarios about PSA screening although not disclosed as such. We did not disclose the scenarios as related to PSA screening as we did not want the scenarios to be affected by emotional reactions to PSA screening and prostate cancer, given the emotive climate in which PSA screening currently occurs. 4 We also included a scenario specifying PSA screening for prostate cancer.
Methods
Setting, participants and recruitment
We recruited a representative community sample of men aged between 50 and 70 years without a history of prostate cancer and fluent in English as part of a larger study reported elsewhere. 15 A total of 6998 telephone numbers from 29 contiguous postcodes in Sydney, Australia were randomly selected from a publicly available telephone directory database. To maximize response rates, we also extracted address listings from this database in order to mail a letter about our study to each selected household in advance of telephone contact. Men were informed that we were conducting a ‘community health study’, asking them about ‘their attitudes to medical tests, such as those for cancer screening’.
At least six call attempts were made by one of 12 trained interviewers from a non‐profit market research company 16 to establish contact with each household. Interviewers sought to determine if a man aged 50–70 years resided in each household. Once a potentially eligible household was identified, at least five attempts were made to speak with participants. In households in which more than one potentially eligible participant resided, the respondent was selected at random.
Once consent was obtained, the interviewer proceeded to administer our 25‐min computer‐assisted telephone (CATI) structured interview. Each consenting participant first was asked his age to verify his eligibility. If participants declined to provide their age, they were asked to indicate the range within which their ages fell. A diagnosis of prostate cancer was ascertained by interviewers during the early stages of the interview. The interview was terminated at the point when men disclosed a diagnosis of prostate cancer. Interviews were conducted during June and August 2000.
Outcome measures
Unspecified scenario‐based assessment of men's interest in PSA screening
We presented five scenarios to participants. Each scenario described ‘screening tests for the early detection of cancer’. While each scenario presented true facts about PSA testing, scenarios as scripted did not communicate specifically either that the test was PSA or that the cancer was that of the prostate.
As an introduction to these unspecified scenarios, interviewers first asked men to imagine tests were available for ‘five different cancers’, further advising that the tests ‘aim to find the cancer early to increase the chances of cure’. These tests then were described as ‘painless, simple and quick to have’ and, to avoid socio‐economic bias, ‘free’ at the point of participation. For each scenario, men were informed that you have the choice to have the test or not. Men then were asked to indicate the extent to which they would want to have the screening test based on the information given (‘definitely want the test’, ‘probably want the test’, ‘not mind whether you have the test or not’, ‘be unlikely to want this test’, ‘definitely want this test’). This approach was identical to that used by Sarfati et al. 13 in their research on men's and women's reactions to framing of the benefits of screening using data derived from mammography trials.
Our five unspecified scenarios were as follows:
-
•
Scenario 1 (lack of definitive evidence for efficacy of screening): ‘There is no definite proof that having this test for finding cancer early saves lives. In 10 years’ time, research will tell us whether having the test makes a difference to how long a person will live.’
-
•
Scenario 2 (risk of detecting indolent cancer): ‘This type of cancer can be very severe or almost harmless. In some people cancer cells do not progress or spread to threaten life. This test is not yet accurate enough to tell the difference between a cancer that will progress to threaten life and one that won't.’
-
•
Scenario 3 (life‐time risk of dying from cancer): ‘Around 1.4% of people will die from this cancer before the age of 75.’
-
•
Scenario 4 (scientific and expert equipoise): ‘Some doctors say having this test will reduce the risk of dying from a certain kind of cancer. Other doctors disagree. There is no clear scientific evidence to support either view.’
-
•
Scenario 5 (risks of side‐effects and uncertain efficacy): ‘Having this test may find cancer early, before it causes symptoms. However, the available treatments are not proven to cure the disease. At least 30% of people having treatment will get side‐effects.’
Thus, these scenarios encapsulated five key dimensions of the controversy surrounding PSA screening. Quantitative information in scenarios 3 and 5 were obtained from the most recently available data. 17 , 18 , 19 Following the methods of Sarfati et al., 13 who did not specify their scenarios as relevant to a particular cancer or screening tests, we did not specify these scenarios as depicting facts about PSA screening for prostate cancer. Hence, we wished to keep the scenarios as distinct as possible to assess the effect of each fact in isolation of any presumptions men may have had about PSA testing or prostate cancer. Further, we wished to avoid carry‐over effects in which men's responses to the previously presented scenario would influence their response to the following presented scenario.
The CATI system randomly presented these five scenarios to participants. The order of the response scale was also randomized for each participant. Interviewers were instructed to record whether participants made any comment about prostate cancer during the presentation of the scenarios, as this would have indicated that they might have realized that these scenarios represented PSA screening.
Specified scenario‐based assessment of men's interest in PSA screening
Later during the interview, men explicitly were asked to indicate the degree to which they wanted to undergo PSA screening during the next 12 months as follows:
-
•
‘A blood test called a PSA test can check for prostate cancer. This test measures a substance in the blood called prostate‐specific antigen or PSA for short. Higher than expected levels of PSA might mean a man has prostate cancer. A screening PSA test may be ordered even though a man does not have symptoms and is considered ‘healthy’; the test aims to find prostate cancer early before it causes symptoms’.
Response options were identical as to those for the unspecified scenarios. They were then asked to provide reasons for their answers that were recorded verbatim by interviewers.
Potential predictors of interest in screening – unspecified scenarios
Control preferences scale
Participants were asked to indicate their preferences generally for involvement in medical decision‐making, using the measure developed by Degner and Sloan. 20 In this question, men were asked to select one of the five statements that best matched their preference. On the basis of their responses, they were classified as preferring either a ‘passive’, ‘collaborative’ or ‘active’ role in medical decision‐making. 20 The Control Preferences Scale has established psychometric properties in both patient and community samples. 20 The modified version as applied in this interview has been previously deployed in other Australian research. 14
Chance health locus of control scale
Using a six‐point Likert scale (‘strongly agree’ to ‘strongly disagree’), participants indicated their level of agreement to each of the following three statements:
-
•
People who never get sick are just lucky
-
•
There is no such thing as bad luck in health
-
•
Staying well is often a matter of chance.
These three statements comprise the chance subscale of the Abbreviated Lau‐Ware Health Locus of Control Scale. 21 The CATI system randomized presentation of these three statements. Scores for each item range from 1 to 6, corresponding to the responses ‘strongly agree’ to ‘strongly disagree’, respectively. Scores for the first and third items are reversed. Hence, scores on the higher end of the scale indicate a greater tendency to attribute health status to chance or ‘luck’. 21 , 22 This scale has sound psychometric properties. 21 , 22
Demographic characteristics
Our interview included demographic questions. Information on marital status (married or living as married, single, widowed or as divorced/separated), highest education qualification attained, current occupation, or occupation before retirement, country of birth and language usually spoken at home also was collected, using standard questions. Current or previous occupation was coded by MG using the second edition of the Australian Standard Classifications of Occupations. 23 Occupational skill level was then determined using a standardized procedure (managers and administrators and professional groups, skill level 1; associate professionals, skill level 2; tradespersons and advanced clerical and service workers, skill level 3; intermediate clerical, sales and service workers and intermediate production and transport workers, skill level 4; elementary clerical, sales and service workers and labourers and related workers, skill level 5).
Potential predictors of interest in screening‐specified scenario
In addition to variables outlined above, additional variables were identified as potential predictors for men's interest in screening when PSA was specified.
Lower urinary tract symptoms
A modified version of the American Urological Association Symptom Index (AUA‐SI) 24 was used to assess the presence of lower urinary tract symptoms (LUTS). The measure assessed whether or not men had experienced any of the following symptoms over the past month (‘Yes’ or ‘No’): incomplete emptying of bladder after urination; frequency; intermittency; difficulty postponing urination; weak urinary stream; having to push or strain to begin urination; nocturia.
Bother and worry associated with LUTS
Men reported any of the seven urinary symptoms were first asked how ‘bothered’ they were by these symptoms (‘not bothered’ to ‘bothered a lot’) and, second, how worried they were that ‘these symptoms could indicate a serious health problem’ (‘not worried’ to ‘worried a lot’).
Knowing or ever knowing a person with prostate cancer
Men were asked if ‘any family members had ever been diagnosed with prostate cancer’ (‘Yes’, ‘No’) and whether they knew ‘anyone else who has prostate cancer’ other than a family member (‘Yes’, ‘No’).
Perceived susceptibility to prostate cancer and worry about prostate cancer
Questions on perceived susceptibility were asked in order to obtain a measure of this construct as described by the Health Belief Model. 25 First, men were asked to estimate their ‘risk of developing prostate cancer’ compared with ‘an average man of your own age’ (‘less than average’ to ‘higher than average’). Secondly, they were asked to the extent to which they were worried that they ‘could develop prostate cancer’ (‘not worried’ to ‘worried a lot’).
Previous participation in PSA screening
Men were asked if they ever had a test to check if they had prostate cancer (‘Yes’, ‘No’) and to specify which type of test they had undergone.
Preferences for involvement in decisions about PSA screening
Men selected one of five responses from an adapted version of the Degner and Sloan's Control Preferences Scale 20 in which men were asked to suppose that they had the choice to be screened for prostate cancer using a PSA test. As for analyses of the generic item asked earlier in the CATI, answers were classified as wanting either a ‘passive’, ‘collaborative’ or ‘active’ role as is standard. 20
Men's perceptions of the benefit of PSA screening
Men were asked to indicate the degree to which they were convinced of the benefit of PSA screening (‘very unconvinced of the benefit’, ‘somewhat convinced of the benefit’, ‘neither convinced nor unconvinced’, ‘somewhat convinced’, ‘very convinced’). 26
Knowledge
As described elsewhere, 15 14 questions were posed to assess respondents’ knowledge about prostate cancer. These questions assessed seven domains of knowledge: efficacy of PSA screening (n = 2 items); test accuracy (n = 3 items); controversy about PSA screening (n = 1 item); natural history of prostate cancer (n = 2 items); early‐stage prostate cancer as an asymptomatic illness (n = 2 items); risk factors for prostate cancer (n = 2 items); treatments side‐effects (n = 2 items). Items were presented in random order and were posed as either ‘true/false’ (n =0 items) or multiple choice format (n = 4 items). Correct responses to the knowledge items were summed to produce a total score and divided by the number of items (n = 14). A knowledge score was then computed by multiplying this number by 100.
Men's views towards PSA screening
As described elsewhere, 15 seven attitudinal‐type statements, the order of which was randomized, were presented to determine men views towards PSA screening. The statements comprised two subscales based on Rakowski et al.’s 27 construct of decisional balance: reasons for (n = 4 items) and against (n = 3 items) undergoing PSA screening. Men were asked to indicate the extent to which they agreed or disagreed with each statement, using a five‐point Likert scale (‘strongly agree’ to ‘strongly disagree’). Mean subscale scores were calculated and then a difference score (reasons for – reasons against) was computed. Hence, positive differences on this measure (range 1–4) indicated views were weighted for PSA screening, negative scores indicated a view against (range −4 to −1), while a score of 0 indicated an equal weighting for and against screening.
Quality control of interviewing
A random sample of approximately 10% of each interviewer's telephone calls were monitored throughout the interview process to ensure that interviewers adhered to the wording on the CATI screen.
Data analysis
Men's responses to the scenarios were the outcome measures for analyses. Wilcoxon's signed rank tests were applied to conduct ‘within group’ comparisons on men's responses to each of the unspecified scenarios, in turn. In addition, Wilcoxon's signed rank tests were applied to compare responses to each of the unspecified scenarios and the scenario specifying the test as PSA.
Multivariate logistic regression analyses were conducted to determine significant and independent predictors of ‘definitely wanting’ a screening test. Potential predictors considered for these analyses were educational qualifications (without a school certificate/formal qualifications vs. school certificate, higher school or leaving certificate, Technical and Further Education (TAFE) qualifications vs. university degree); occupational skill level (1 and 2 vs. 3, 4 and 5); employment status (currently employed vs. unemployed/retired/unable to work); marital status (married or living as married vs. other); country of birth (Australia vs. other); language usually spoken at home (English vs. other); self‐reported health status (excellent/good vs. fair/poor); current smoker (Yes vs. No); ever diagnosed with cancer (Yes vs. No); previous history of vascular disease (heart attack or stroke) (Yes vs. No); preferences for involvement in medical decision‐making (passive vs. other). Age and chance health locus of control scores were also modelled as continuous variables. These variables were selected as previous research has indicated that these factors may affect screening interest and/or participation. 26 , 28 , 29
When determining predictors of men's interest in screening specified as PSA, the following variables were assessed as potential predictors as follows: number of LUTS; bother associated with LUTS (no symptoms or not bothered vs. a little to a lot bothered); worry associated with LUTS (no symptoms or not worried vs. a little to a lot); worry about prostate cancer (not worried vs. a little to a lot worried); perceived risk of developing prostate cancer (higher than average vs. other); ever knowing a man with prostate cancer, either a relative or other individual (Yes vs. No); convinced about the benefit of PSA screening (very or somewhat unconvinced or neither convinced nor unconvinced vs. somewhat convinced vs. very convinced); preference for involvement in decision‐making (passive vs. other) specific to PSA screening decisions; knowledge about prostate cancer and PSA screening (continuous measure) and views towards PSA screening (continuous measure). Worry about prostate cancer and LUTS and bother associated with LUTS have been previously shown to be related to PSA screening participation. 28 , 29
Variables univariately associated with outcomes at the P < 0.25 level were selected for entry into multivariate models, to appropriately control for confounding. 30 Using a non‐automated backward selection procedure, a variable was removed from the model if it did not meet statistical significance and if its removal did not appreciably affect the parameter estimates of other variables in the model. 28 A variable with a P < 0.05 was considered statistically significant. Hence, the odds ratio (OR) for any variable in the final model was adjusted for other significant covariates.
Sample size
A previous telephone interview of male residents of Sydney in which 2000 households had been contacted yielded a completed interview for 143 males (7.2%) aged 50–69 years. 28 Hence, it was estimated that 7000 households would need to be contacted in order to yield 500 interviews for our study. A sample size of 500 was sufficient to estimate proportions so that the 95% confidence intervals for these estimates did not exceed ±5% of the true value. This sample size also was sufficient to conduct subgroup comparisons of responses between men on categorical predictor variables given a power of 0.80 and an α‐level of 0.05. 31 For example, the sample size allowed categorical comparisons of responses of men aged 50–59 and 60–69 years, assuming a difference of 10–15% between categories indicating that they would ‘definitely want’ to be tested, and assuming that 45–48% for participants are older than 60 years. 28
Ethical approval
The study was approved by the institutional ethics committee CSAHS Ethics Review Committee (RPAH zone) and the Human Ethics Committee of The University of Sydney.
Results
Recruitment
Figure 1 shows recruitment and participant flow throughout the study. Of the 585 men meeting our eligibility criteria, 521 completed interviews (raw response fraction of 89.1%). There was no significant difference in the ages of men who refused to participate in the study and declared their age and those who consented (P = 0.26). Of those 521 consenting to the interview, seven had prostate cancer that excluded them from our study. This reduced the final sample size to 514.
Figure 1.
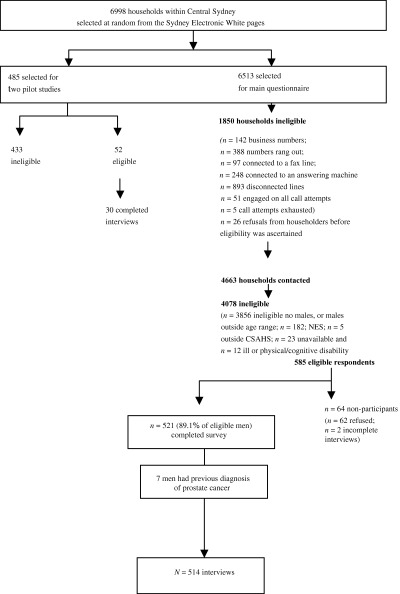
Participant recruitment and flow.
Table 1 displays the age distribution of the sample. This was no different to that for men from the population defining the sampling frame.
Table 1.
Comparison age of study participants with age of male residents of Central Sydney Area Health Service (CSAHS) Census data
| Age group (years)* | Number of respondents (%) | Male population of Central Sydney (2000 census) (%) | Test statistic (P‐value) |
|---|---|---|---|
| 50–54 | 175 (35.0) | 15 063 (33.3) |
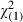 = 0.64 (0.42) = 0.64 (0.42) |
| 55–59 | 133 (26.6) | 11 780 (26.0) |
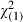 = 0.09 (0.76) = 0.09 (0.76) |
| 60–64 | 108 (21.6) | 9885 (21.9) |
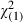 = 0.03 (0.87) = 0.03 (0.87) |
| 65–69 | 84 (16.8) | 8500 (18.8) |
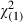 = 1.30 (0.25) = 1.30 (0.25) |
| Total | 500* | 45 228 |
*Fourteen men aged 70 years were excluded for this analysis. The analyses compare differences in proportions (number of respondents over total respondents) for each age group.
Table 2 displays additional demographic and health characteristics of our sample. The majority was married or living as married (n = 385; 74.9%), currently employed (n = 361; 70.2%) and usually spoke English at home (n = 448; 87.2%). Over half (n = 276; 53.7%) were born in Australia and more than one‐third held a university qualification (n = 192; 37.4%). With respect to men's preferences for decisional control, only one‐quarter (24.9%; n = 128) were considered ‘passive’, while 127 (24.7%) preferred an ‘active’ role. The majority (n = 258; 50.2%) preferred to ‘collaborate’ with their doctors during medical decision‐making. One respondent was ‘unsure’ (Table 2).
Table 2.
Demographic and health characteristics male participants without prostate cancer (n = 514)
| Characteristic | n (%) |
|---|---|
| Marital status | |
| Living as married | 385 (74.9) |
| Single | 73 (14.2) |
| Widowed | 12 (2.3) |
| Divorced/separated | 43 (8.4) |
| Refused | 1 (0.2) |
| Highest educational qualification attained | |
| Did not finish primary school | 12 (2.3) |
| Finished primary school, but did not attend high school | 28 (5.4) |
| Left high school before completing school certificate | 44 (8.6) |
| School certificate or equivalent | 70 (13.6) |
| Higher school certificate or equivalent | 75 (14.6) |
| TAFE* qualification | 91 (17.7) |
| University degree | 192 (37.4) |
| Refused | 2 (0.4) |
| Country of birth | |
| Australia | 276 (53.7) |
| Other | 237 (46.2) |
| Refused | 1 (0.2) |
| Language spoken at home | |
| English | 448 (87.2) |
| Other | 65 (12.6) |
| Refused | 1 (0.2) |
| Employment status | |
| Retired/receiving benefit | 145 (28.2) |
| Employed | 361 (70.2) |
| Unemployed | 7 (1.4) |
| Refused | 1 (0.2) |
| Occupation | |
| Managers and administrators | 84 (16.3) |
| Professionals | 162 (31.5) |
| Associate professionals | 48 (9.3) |
| Tradespersons and related workers | 71 (13.4) |
| Advanced clerical and service workers | 2 (0.4) |
| Intermediate clerical, sales and service workers | 33 (6.4) |
| Intermediate production and transport workers | 32 (6.2) |
| Elementary clerical, sales and service workers | 22 (4.3) |
| Labourers and related workers | 22 (4.3) |
| Insufficient detail provided for accurate coding | 37 (7.2) |
| Refused | 1 (0.2) |
| Occupational skill level | |
| 1 | 246 (47.9) |
| 2 | 48 (9.3) |
| 3 | 73 (14.2) |
| 4 | 65 (12.6) |
| 5 | 44 (8.6) |
| Unable to be reliably classified/refused | 38 (7.4) |
| Self‐reported health status | |
| Excellent | 98 (19.1) |
| Good | 283 (55.1) |
| Fair | 107 (20.8) |
| Poor | 26 (5.1) |
| Current smoker | |
| Yes | 82 (16.0) |
| No | 432 (84.0) |
| Previous heart attack or stroke | |
| Yes | 44 (8.6) |
| No | 470 (91.4) |
| Control Preferences Scale (medical decisions in general) | |
| Active | 127 (24.7) |
| Shared or collaborative | 258 (50.2) |
| Passive | 128 (24.9) |
| Unsure | 1 (0.2) |
| Health locus of control scale | |
| Mean (SD) | 3.3 (0.93) |
*Technical and Further Education.
Of a possible score of six, chance health locus of control scores were an average of 3.3 (SD =0.93) indicating that, overall, respondents tended not to attribute health status to chance (median 3.3; IQ = 2.7–4.0) (Table 2).
Men's responses to unspecified scenarios
Table 3 displays men's responses to our five unspecified scenarios. As described in Methods, scenario 1 presented men with information about a screening test for cancer for which there was a lack of evidence for its efficacy. Over 60% (n = 315; 61.3%) of men indicated that they would ‘probably’ or ‘definitely’ want this screening test. The most popular screening test was that described in scenario 3, however. Specifically, over 70% of respondents stated that they either would ‘probably’ (35.0%, n = 180) or ‘definitely’ want the test (37.5%, n = 193). Less than half (46%) stated that they would ‘probably’ or ‘definitely’ want the test described in scenario 5 (Table 3).
Table 3.
Scenario‐based assessment of men's preferences for undergoing cancer screening (within‐group comparison)
| Item | Definitely not want the test | Be unlikely to want the test | Not mind whether you have the test or not | Probably want this test | Definitely want this test |
|---|---|---|---|---|---|
| Unspecified scenarios | |||||
| Scenario 1 (lack of evidence underpinning efficacy of screening) | 28 (5.4) | 80 (15.6) | 91 (17.7) | 157 (30.5) | 158 (30.7) |
| Scenario 2 (risk of detecting indolent cancer) | 32 (6.2) | 93 (18.1) | 76 (14.8) | 160 (31.1) | 153 (29.8) |
| Scenario 3 (risk of dying from cancer) | 28 (5.4) | 46 (8.9) | 67 (13.0) | 180 (35.0) | 193 (37.5) |
| Scenario 4 (lack of expert consensus) | 35 (6.8) | 71 (13.8) | 85 (16.5) | 171 (33.3) | 152 (29.6) |
| Scenario 5 (treatment uncertainty and side‐effects from treatment) | 74 (14.4) | 147 (28.6) | 56 (10.9) | 129 (25.1) | 108 (21.0) |
| Specified scenario | |||||
| Interest in PSA screening | 20 (3.9) | 39 (7.6) | 105 (20.4) | 160 (31.1) | 190 (37.0) |
Values are given as n (%)
PSA, prostate‐specific antigen.
Multivariate analyses: predictors of ‘definitely’ wanting a screening test unspecified scenarios
Table 4 displays independent predictors of ‘definitely’ wanting the screening test as described in each unspecified scenario.
Table 4.
Multivariate predictors of ‘definitely wanting’ a screening test for cancer for scenarios 1 to 5
| Variable | Unadjusted odds ratio (95% CI) | P‐value | Adjusted odds ratio (95% CI) | P‐value |
|---|---|---|---|---|
| Scenario 1 | ||||
| Age (years)* | 1.06 (1.02–1.09) | <0.001 | 1.05 (1.02–1.09) | 0.002 |
| 50–59† | 1.00 | 1.00 | ||
| 60–70 | 1.93 (1.32–2.82) | 1.85 (1.25–2.73) | ||
| Educational qualifications | ||||
| Without SC | 1.77 (1.01–3.11) | 0.009 | 1.43 (0.80–2.55) | 0.03 |
| SC/TAFE/HSC | 1.89 (1.23–2.91) | 1.78 (1.15–2.74) | ||
| University qualification† | 1.00 | 1.00 | ||
| Scenario 2 | ||||
| Age (years) | 1.04 (1.01–1.08) | 0.01 | NA | NA |
| 50–59† | 1.00 | |||
| 60–70 | 1.56 (1.07–2.29) | |||
| Scenario 3 | ||||
| Educational qualifications | ||||
| Left before SC | 1.69 (0.99–2.88) | 0.02 | 1.57 (0.90–2.75) | 0.03 |
| SC/HSC/TAFE | 1.74 (1.16–2.61) | 1.71 (1.14–2.57) | ||
| University degree† | 1.00 | 1.00 | ||
| Employment status | ||||
| Not employed | 1.67 (1.13–2.45) | 0.01 | 1.60 (1.07–2.39) | 0.02 |
| Employed† | 1.00 | 1.00 | ||
| Current smoker | ||||
| Yes† | 1.00 | 0.09 | 1.00 | 0.051 |
| No | 1.55 (0.93–2.59) | 1.67 (0.99–2.82) | ||
| Scenario 4 | ||||
| Age (years) | 1.05 (1.02–1.09) | 0.002 | 1.05 (1.01–1.08) | 0.02 |
| 50–59† | 1.00 | 1.00 | ||
| 60–70 | 1.79 (1.22–2.62) | 1.68 (1.14–2.48) | ||
| Self‐reported health status | ||||
| Excellent/good† | 1.00 | 0.006 | 1.00 | 0.007 |
| Fair/poor | 1.81 (1.19–2.74) | 1.66 (1.08–2.53) | ||
| Scenario 5 | ||||
| Age (years) | 1.07 (1.03–1.11) | <0.001 | 1.07 (1.03–1.10) | 0.001 |
| 50–59† | 1.00 | 1.00 | ||
| 60–70 | 2.23 (1.45–3.42) | 2.13 (1.38–3.29) | ||
| Has ever had heart attack or stroke | ||||
| Yes | 2.35 (1.22–4.52) | 0.01 | 1.98 (1.01–3.86) | 0.052 |
| No† | 1.00 | 1.00 | ||
*Age was modeled as a continuous variable. Age is also presented as a categorical variable to facilitate interpretation of the effect age has on the outcomes’.
†Referent group.
Scenario 1, lack of evidence underpinning efficacy of screening; scenario 2, risk of detecting indolent cancer; scenario 3, lifetime risk of dying from cancer; scenario 4: lack of expert consensus on the efficacy of early detection; scenario 5, uncertainty of benefit from treatment and risk of developing side‐effects from treatment.
Compared with respondents with less interest in screening, those who ‘definitely wanted’ the screening test for which there was no definite proof yet that early detection saves lives (scenario 1) were significantly and independently more likely to be older [adjusted odds ratio (AOR) = 1.05; 95%CI 1.02–1.09; P = 0.002] and without university qualifications (P =0.002) (Hosmer Lemeshow goodness‐of‐fit 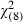 = 12.21; P = 0.14; Table 4).
= 12.21; P = 0.14; Table 4).
Increasing age was the only significant independent predictor for respondents ‘definitely’ wanting the test in which there was a risk of detecting indolent cancer (scenario 2) (OR =1.04; 95%CI 1.01–1.08) (P = 0.01) (Table 4).
Respondents who did not have a university qualification (P = 0.03) and who were not currently employed (AOR = 1.60; 95%CI 1.07–2.39) (P = 0.02) were significantly and independently more likely to ‘definitely’ want the screening test for a cancer for which the life‐time risk of dying was 1.4% (scenario 3) (Hosmer Lemeshow goodness‐of‐fit 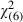 = 2.42; P =0.88; Table 4).
= 2.42; P =0.88; Table 4).
Older men (AOR = 1.05; 95%CI 1.01–1.08) (P = 0.02) and those with ‘fair’ or ‘poor’ health (AOR = 1.66 95%CI 1.08–2.53) (P = 0.007) were significantly and independently more likely to ‘definitely want’ to undergo a screening test despite non‐consensus among experts (scenario 4) (Hosmer Lemeshow goodness‐of‐fit 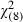 =3.10; P = 0.93; Table 4).
=3.10; P = 0.93; Table 4).
Similarly, older men (AOR 1.07; 95%CI 1.03–1.10) (P = 0.001) were significantly more likely to want a screening test despite the lack of evidence for the efficacy for treatment and the risk of treatment complications (scenario 5) (Hosmer Lemeshow goodness‐of‐fit 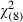 = 7.96; P =0.44; Table 4).
= 7.96; P =0.44; Table 4).
Men's responses to specified scenarios about PSA screening
When asked explicitly about PSA screening, 190 respondents (37.0%) indicated that they would ‘definitely’ want to undergo testing, while 31.1% (n = 160) indicated that they would ‘probably’ want to be tested. Men were significantly more likely to ‘definitely’ want PSA screening than to so indicate in response to the unspecified tests depicted in scenario 1 (lack of evidence underpinning efficacy of screening), scenario 2 (risk of detecting indolent cancer), scenario 4 (lack of expert consensus) and scenario 5 (treatment uncertainty and side‐effects from treatment) (P’s = 0.02 −<0.001). A similar proportion of men indicated ‘definitely’ wanting a PSA test and the unspecified test depicted in scenario 3 (risk of dying from cancer) (P = 0.30).
Table 5 provides the reasons nominated by those 350 respondents who held positive views about PSA screening in the next 12 months. Almost one‐fifth wished to be tested because of their perceived risk of developing prostate cancer (17.1%, n = 60), either because of their age (9.1%, n = 32), the presence of urinary symptoms (2.9%, n = 10), a family history of prostate cancer (4.3%, n = 15), or other reasons not specified (0.9%, n = 3). A further 50 respondents (14.3%) reported that early detection was a ‘good thing’ or wished to have a prostate check without offering a more specific reason. Wanting ‘peace of mind’ or ‘reassurance’, reporting PSA testing as a useful ‘precautionary or preventive measure’, indicating PSA testing is ‘convenient’, ‘wanting to know’, and already undergoing regular testing were reasons each offered by approximately 10% of respondents.
Table 5.
Reasons for positive views about prostate‐specific antigen (PSA) screening (n = 350)
| Reason | n (%) |
|---|---|
| Perceives to be at risk of developing prostate cancer | 60 (17.1) |
| Due to age | 32 (9.1) |
| Due to urinary symptoms | 10 (2.9) |
| Due to a family history | 15 (4.3) |
| Other, not specified | 3 (0.9) |
| Early detection is a ‘good thing’/ to have a check‐up | 50 (14.3) |
| For peace of mind or reassurance | 33 (9.4) |
| As a precautionary or preventive measure | 32 (9.1) |
| Is already regularly tested | 37 (10.6) |
| Wants to know whether or not has cancer | 28 (8.0) |
| Test is convenient/easy/painless | 26 (10.3) |
| Worried or concerned about prostate cancer | 9 (2.6) |
| Is due for a check‐up | 15 (4.3) |
| Participation in study prompted interested in PSA screening | 12 (3.4) |
| Doctor recommends PSA screening | 11 (3.1) |
| Feels need for preventive measures due to past health problems | 10 (2.9) |
| Other | 27 (7.7) |
Table 6 shows the reasons given by those 156 participants who held negative views about PSA screening. ‘Feeling well’ and not having ‘any symptoms’ of prostate cancer were the most common reasons for not wishing to have a PSA test (n = 28; 17.9%). Only 13 respondents (8.3%) provided reasons suggesting they were aware of the controversy surrounding testing, such as the side‐effects of treatment and the inaccuracy of PSA assaying. For example, one respondent stated that ‘until there is a decent cure without side‐effects, I will take my chances’.
Table 6.
Reasons for negative or indifferent views about prostate‐specific antigen (PSA) screening (n = 156)
| Reason | n (%) |
|---|---|
| Feeling well/have no symptoms/ there is no need for testing | 28 (17.9) |
| Would be tested if doctor advised it | 21 (13.5) |
| Not worried about prostate cancer | 17 (10.9) |
| Negative implications of testing | 13 (8.3) |
| Neutral feelings about testing | 12 (7.7) |
| Recently had test | 10 (6.4) |
| Reassured by previous negative test result | 9 (5.8) |
| Rather not know if had cancer | 7 (4.5) |
| Doctor advised against PSA testing | 5 (3.2) |
| Mistrust of doctors | 4 (2.6) |
| Other health priorities | 3 (1.9) |
| Other | 12 (7.7) |
| Respondent gave a reason in support of being tested | 14 (9.0) |
| Reason not given | 1 (0.6) |
Multivariate analyses: predictors of ‘definitely’ wanting a screening test specified scenario
A greater number of LUTS was independently associated with an increasing likelihood of ‘definitely’ wanting a PSA screening test (AOR = 1.15 95%CI 1.02–1.29; P = 0.02) (see Table 7). Men who were at least a ‘little worried’ about prostate cancer were also significantly more likely to ‘definitely want’ a PSA screening test than those who were not worried (AOR = 1.71; 95%CI 1.14–2.56; P = 0.01) as were those who personally knew someone who had been diagnosed with prostate cancer (AOR = 1.60; 95%CI 1.07–2.39; P = 0.02) and those who were ‘somewhat’ or ‘very’ convinced about the benefits of screening PSA tests (AOR = 1.15; 95%CI 0.71–1.88 and AOR = 3.74; 95%CI 2.16–6.46, respectively; P < 0.001). Positive views towards PSA screening predicted ‘definite’ interest in PSA screening (AOR = 1.47; 95%CI 1.16–1.86; P = 0.001). Men who were more likely to attribute health status to chance or luck, as evidenced by relatively high scores on the chance health locus of control scale were also less likely to ‘definitely want’ a PSA screening test during the next 12 months, an association which was marginally significant (AOR = 0.81, 95%CI 0.66–1.01; P = 0.06) (Hosmer‐Lemeshow 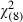 = 4.37; P = 0.82).
= 4.37; P = 0.82).
Table 7.
Multivariate predictors of ‘definitely’ wanting a prostate‐specific antigen (PSA) screening test in next 12 months
| Variable | Unadjusted odds ratio (95%CI) | P‐value | Adjusted odds ratio (95%CI) | P‐value |
|---|---|---|---|---|
| LUTS* | 1.20 (1.08–1.34) | 0.001 | 1.15 (1.02–1.29) | 0.02 |
| 0 or 1 symptoms† | 1.00 | 1.00 | ||
| Two or more symptoms | 1.82 (1.26–2.64) | 1.53 (1.01–2.30) | ||
| Worried about prostate cancer | ||||
| A little to a lot | 1.85 (1.28–2.68) | 0.001 | 1.71 (1.14–2.56) | 0.01 |
| Not worried† | 1.00 | 1.00 | ||
| Know person with prostate cancer | ||||
| Yes | 1.79 (1.23–2.59) | 0.002 | 1.60 (1.07–2.39) | 0.02 |
| No† | 1.00 | 1.00 | ||
| Convinced of benefit of PSA screening | ||||
| Very/somewhat unconvinced to neither† | 1.50 (0.95–2.36) | <0.001 | 1.00 | <0.001 |
| Somewhat convinced | 5.32 (3.23–8.75) | 1.15 (0.71–1.88) | ||
| Very convinced | 1.00 | 3.74 (2.16–6.46) | ||
| Views towards screening* | 1.83 (1.48–2.26) | <0.001 | 1.47 (1.16–1.86) | 0.001 |
| −4 to 2‡ | 1.00 | 1.00 | ||
| 2.1–4 | 2.39 (1.65–3.46) | 1.68 (1.12–2.52) | ||
| Chance health locus of control score* | 0.84 (0.69–1.02) | 0.07 | 0.81 (0.66–1.01) | 0.06 |
| 1–3† | 1.00 | 1.00 | ||
| 4–6 | 0.71 (0.50–1.03) | 0.70 (0.47–1.05) | ||
*Modelled as continuous.
†Referent group.
‡Categories based on median split of data.
Discussion
This study sought to determine men's views about controversies in respect of PSA screening. Previous research has demonstrated that preferences for screening are influenced by the manner in which quantitative information describing the benefits of screening is framed. 13 Our study has examined whether or not men's interest in PSA screening is affected by qualitative messages about screening, in particular, how the potential negative impact of screening is communicated.
In general, a majority of men in our study were positive about screening tests for cancer, irrespective of the attribute of the test as described in our unspecified scenarios. While other investigators have expressed fears that communicating negative facts about PSA screening will deter men from undergoing PSA screening, 11 the majority of men in our study persisted in their interest in screening tests, irrespective of a lack of medical and scientific consensus, a lack of evidence to support its efficacy and despite the test detecting indolent cancer. As shown here, scenarios which communicate the life‐time incidence of developing cancer consistently appear to be the most persuasive influence on the public's interest in screening while those emphasizing side‐effects and uncertainties of treatment appear to dampen interest. Hence, messages concerning possible risks of cancer and the effects of treatment on quality of life impress more greatly than those conveying information about equivocal evidence underpinning screening efficacy. However, it is unclear whether men would assess these facts differently if asked to consider them as facts relevant to the one test for the one cancer. It remains unclear as to how best to examine how individuals integrate and weigh all relevant information about screening tests simultaneously. 13 , 32 Further, it is unclear how men's reactions may have differed had they been made aware that the scenarios were representing facts about PSA screening for prostate cancer.
Age was a consistent predictor of positive interest in undergoing screening for all but one unspecified scenario presented. Older men were more likely to report ‘definitely’ wanting a screening test, irrespective of the lack of evidence underpinning the efficacy of screening, the risk of detecting indolent cancer, lack of expert consensus concerning the benefits of screening or the risk of developing side‐effects from treatment. This finding suggests the concerns older individuals have about their health and the increasing salience of cancer as a potential health problem for them. Educational qualifications also predicted men's interest in undergoing a screening test where there was lack of evidence underpinning the efficacy of screening and when the lifetime risk of developing cancer was presented. Specifically, men reporting fewer qualifications were more likely to report interest than those holding a university degree. This finding contradicts those from other studies reporting that individuals of higher socio‐economic status are more likely to participate in PSA screening. 33 However, traditionally, screening programmes have aimed to increase uptake rather than promote informed participation 34 leaving open the question of how participation may be affected if individuals were informed of both the negative and positive impact of screening on health and the effect such messages would have on the profile of screening attendees. These findings suggest that education level may modify an individual's interpretation of information.
A significantly greater number of men endorsed PSA screening when compared with scenarios in which PSA screening was not specified. This suggests that men particularly attach emotional significance to prostate cancer, perceiving the disease and testing for it as salient to their own health‐care needs. However, men's reasons for wanting to undergo PSA screening were not always based on accurate views about risk. For example, men's perceived risks of developing prostate cancer because of urinary symptoms are not evidence‐based. 35 Men's uncritical acceptance of the view that early detection is important also appears persuasive when deciding to undergo screening. Similarly, men who held negative views towards PSA screening or appeared indifferent about it offered justifications for their views that were inconsistent with aims of screening. Men choose not to undergo screening most commonly because they felt reassured by a previous negative test result, or because they reported being asymptomatic. Such reasons manifest confusion among men about the aims of PSA screening.
In order to meet the demands of informed decision‐making, information about screening needs to be presented in a way that does not bias the decisions that individuals make. Qualitative information that is not quantified by statistics may be interpreted differently. 36 In the absence of available information quantifying the benefits and risks of PSA screening, GPs and policy makers need to pay attention to how they describe the known ‘knowns’ and the known ‘unknowns’ about PSA screening. Men's decisions about screening seem not to be affected by the potentially negative messages that screening may unmask indolent disease, is not supported by the evidence or lack of medical and scientific consensus of its benefit. Yet information about side‐effects of treatment is more likely to polarize men's views. It is not ethical to downplay the risks while emphasizing the potential benefits, nor is it defensible to over‐emphasize the risks of screening while minimizing the case for health gain. Public health policy makers need to ensure that men have access to the spectrum of relevant evidence‐based prostate cancer information in order to optimize a given man's screening decision.
Acknowledgements
The authors thank those men who freely gave their time to participate in this study and Andrew Searles, Helen Beddow and David Shellard from the Hunter Valley Research Foundation, for CATI services. At the time of the study, Melina Gattellari was supported by a Commonwealth Department of Education, Science and Training Australian Postgraduate Award (APA) and was a doctoral candidate at the School of Public Health, University of Sydney.
References
- 1. Harris R, Lohr KN. Screening for prostate cancer: an update of the evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine 2002; 137: 917–929. [DOI] [PubMed] [Google Scholar]
- 2. de Koning HJ, Liem MK, Baan CA, Boer R, Schroder FH, Alexander FE. ERSPC. Prostate cancer mortality reduction by screening: power and time frame with complete enrollment in the European Randomised Screening for Prostate Cancer (ERSPC) trial. International Journal of Cancer 2002; 98: 268–273. [DOI] [PubMed] [Google Scholar]
- 3. Prorok PC, Andriole GL, Bresalier RS et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Controlled Clinical Trials 2000; 21: 273S–309S. [DOI] [PubMed] [Google Scholar]
- 4. Pauker SG, Kassierer JP. Contentious screening decisions: does choice matter? New England Journal of Medicine 1997: 336: 1243–1244. [DOI] [PubMed] [Google Scholar]
- 5. National Cancer Institute . Prostate Cancer Screening and Testing. http://www.nci.nih.gov/cancer_information/testing/. Accessed 28 July 2004. [Google Scholar]
- 6. Feightner JW. Screening for prostate cancer In: The Canadian Guide to Clinical Preventive Health Care, Canadian Task Force on Preventive Health Care. http://www.ctfphc.org/. [Google Scholar]
- 7. Canadian Cancer Society . Canadian Cancer Society Perspective on Early Detection and Treatment of Prostate Cancer. http://www.cancer.ca/ccs/internet/standard/0,3182,3172_428338_428444_langId‐en,00.html (accessed 31 December 2003). [Google Scholar]
- 8. National Health Service . Prostate Cancer Risk Management Prostate Cancer Risk Management. http://www.cancerscreening.nhs.uk/prostate/index.html. [Google Scholar]
- 9. Advisory Committee on Cancer Prevention . Position paper: recommendations on cancer screening in the European Union. European Journal of Cancer, 2000; 36: 1473–1478.13. [DOI] [PubMed] [Google Scholar]
- 10. The Cancer Council Australia . National Cancer Prevention Policy 2001–03. Sydney: The Cancer Council Australia, 2001. [Google Scholar]
- 11. Catalona WJ. Informed consent for prostate‐specific antigen screening. Urology 2003; 61: 17–19. [DOI] [PubMed] [Google Scholar]
- 12. Talcott JA. What patients should be told before agreeing to a blood test that could change their lives. Urology 2000; 61: 7–9. [DOI] [PubMed] [Google Scholar]
- 13. Sarfati D, Howden‐Chapman P, Woodward A, Salmond C. Does the frame affect the picture? A study into how attitudes to screening for cancer are affected by the way benefits are expressed. Journal of Medical Screening 1998; 5: 137–140. [DOI] [PubMed] [Google Scholar]
- 14. Davey HM, Barratt AL, Davey E et al. Medical tests: women's reported and preferred decision‐making roles and preferences for information on benefits, side‐effects and false results. Health Expectations 2002; 5: 330–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gattellari M, Ward JE (2004). A community‐based randomised controlled trial of three different educational resources for men about prostate cancer screening. Patient Education and Counseling (in press). [DOI] [PubMed] [Google Scholar]
- 16. Hunter Valley Research Foundation . http://www.hvrf.com.au/.
- 17. Coates MS, Tracey EA. Cancer in New South Wales Incidence and Mortality 1997. Sydney: NSW Cancer Council, 2000. [DOI] [PubMed] [Google Scholar]
- 18. National Health and Medical Research Council (NHMRC) . Evidence‐Based Recommendations for the Management of Localised Prostate Cancer. Draft Guidelines. http://www.health.gov.au/nhmrc/publications/cphome.htm. [Google Scholar]
- 19. Wasson JH, Cushman CC, Bruskewitz RC, Littenberg B, Mulley AG Jr, Wennberg JE, Prostate Disease Patient Outcome Research Team . A structured literature review of treatment for localized prostate cancer. Archives of Family Medicine 1993; 2: 487–493. [DOI] [PubMed] [Google Scholar]
- 20. Degner LF, Sloan HA. Decision making during serious illness: what role do patients really want to play? Journal of Clinical Epidemiology 1992; 45: 941–950. [DOI] [PubMed] [Google Scholar]
- 21. Lau RR. Origins of health locus of control beliefs. Journal of Personality and Social Psychology 1982; 42: 322–334. [DOI] [PubMed] [Google Scholar]
- 22. Marshall GN, Collins BE, Crooks VC. A comparison of two multidimensional health locus of control instruments. Journal of Personality Assessment 1990; 54: 181–190. [DOI] [PubMed] [Google Scholar]
- 23. Australian Bureau of Statistics (ABS) . Australian Standard Classifications of Occupations, 2nd edn. Canberra: AGPS, 1997. [Google Scholar]
- 24. Barry MJ, Fowler FJ Jr, O'Leary MP et al. The Measurement Committee of the American Urological Association . The American Urological Association symptom index for benign prostatic hyperplasia. Journal of Urology 1992; 148: 1549–1557. [DOI] [PubMed] [Google Scholar]
- 25. Janz NJ. Health Belief Model: a decade later. Health Education Quarterly 1984; 11: 1–47. [DOI] [PubMed] [Google Scholar]
- 26. Slevin TJ, Donnelly N, Clarkson JP, English DR, Ward JE. Prostate cancer testing: behaviour, motivation and attitudes among Western Australian men. Medical Journal of Australia 1999; 171: 185–188. [DOI] [PubMed] [Google Scholar]
- 27. Rakowski W, Andersen MR, Stoddard AM et al. Confirmatory analysis of opinions regarding the pros and cons of mammography. Health Psychology, 1997; 16: 433–441. [DOI] [PubMed] [Google Scholar]
- 28. Ward JE, Hughes AM, Hirst GHL, Winchester L. Men's estimates of prostate cancer risk and self‐reported rates of screening. Medical Journal of Australia 1997; 167: 250–253. [DOI] [PubMed] [Google Scholar]
- 29. Gattellari M, Young JM, Ward J. GP and patient predictors of PSA screening in Australian general practice. Family Practice 2003; 20; 289–293. [DOI] [PubMed] [Google Scholar]
- 30. Hosmer DW, Lemeshow S. Applied Logistic Regression. Brisbane: John Wiley and Sons, 2001. [Google Scholar]
- 31. Fleiss JL. Statistical Methods for Rates and Proportions. New York: Wiley, 1981. [Google Scholar]
- 32. Salkeld G, Solomon M, Short L, Ryan M, Ward JE. Evidence‐based consumer choice: a case study in colorectal cancer screening. Australian and New Zealand Journal of Public Health 2003; 27: 449–455. [DOI] [PubMed] [Google Scholar]
- 33. Nijs HGT, Essink‐Bot ML, DeKoning HJ, Kirkels WJ, Schörder FH. Why do men refuse or attend population‐based screening for prostate cancer? Journal of Public Health Medicine 2000; 22; 312–316. [DOI] [PubMed] [Google Scholar]
- 34. Austoker J. Gaining informed consent for screening is difficult – but many misconceptions need to be undone. British Medical Journal 1999; 319: 722–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Young J, Muscatello D, Ward JE. Are men with lower urinary tract symptoms at increased risk of prostate cancer? A systematic review and critique of the available evidence. BJU International 2000; 85: 1037–1048. [DOI] [PubMed] [Google Scholar]
- 36. Woloshin S. Schwartz LM. How can we help people make sense of medical data? Effective Clinical Practice 1999; 2: 176–183. [PubMed] [Google Scholar]


