Abstract
Background Patients vary widely in their preferences and capacity for participating in treatment decision‐making. There are few interventions targeting patient understanding of how doctors make decisions and shared decision‐making. This randomized trial investigates the effects of providing cancer patients with a package designed to facilitate shared decision‐making prior to seeing their oncologist.
Patients and methods Sixty‐five female cancer patients were randomized to receive either the package (booklet and 15‐min video) or a booklet on living with cancer, before their initial consultation. Participants completed questionnaires prior to the intervention, immediately after the oncology consultation, and 2 weeks and 6 months later. The first consultation with the oncologist was audio‐taped and transcribed.
Results Patients receiving the package were more likely than controls to declare their information and treatment preferences in the consultation, and their perspectives on the costs, side‐effects and benefits of treatment. Doctors introduced considerably more new themes in the consultations with intervention subjects than they did with controls; no other differences in doctor behaviour were noted.
Conclusions This short intervention successfully shifted patient and doctor behaviour closer to the shared decision‐making model, although it did not alter patients’ preferences for information or involvement.
Keywords: information and involvement preferences, intervention, medical decision‐making, modeling, patient education, randomized controlled trial
Introduction
Attitudes to doctor–patient roles in clinical decision‐making have changed radically over the past 20 years, with most western doctors now expected to adhere to models promoting patient autonomy, rather than the paternalistic stance adopted in the past. 1 The putative benefits of facilitating patient involvement in decision‐making are patient empowerment, increased understanding and more mutually satisfactory clinical decisions. A number of studies have shown that among cancer patients, those offered choices in their treatment show better psychological adjustment. 2 , 3 , 4 In one study, patients who believed they were more responsible for treatment decisions and perceived they had more choice in treatment selection went on to have better health‐related quality of life. 5 There is also some evidence to suggest that patients who perceive their physicians are making an effort to facilitate their involvement in decision‐making tend to be more involved in that process 6 and are more satisfied with their physicians. Patients who come to appreciate the evidence‐base, which underlies good clinical decision‐making may also be more informed about the logic and potential benefits of participation in randomized clinical trials, should they be eligible. In spite of wide clinician acknowledgement of randomized clinical trials as the ‘gold standard’, many reports refer to the trivial proportion of patients who participate in randomized trials. 7 , 8 , 9 , 10
However, patients do not always behave in accordance with shared decision‐making models, or appear to desire such interactions. Most western cancer patients do expect to be informed about their illness, their likely prognosis and about the available treatment options. However, people vary markedly in their preference for involvement in clinical decision‐making and many still prefer to leave decisions about treatment to their doctor. Patients are more inclined to leave treatment decisions to their doctor than non‐patients; for example, Degner and Sloan 11 found that 59% patients vs. 36% of non‐patients preferred to leave treatment decisions to the doctor. Most studies report that slightly under one‐half of patients desire shared decision‐making, one‐third prefer the doctor to make the decision and one‐fourth prefer to have control over decision‐making. 12 , 13
These figures have led some to question the value and ethics of shared decision‐making. There are a number of possible explanations for patients expressing a preference for deferment of decisional autonomy to their doctors. First, there are substantial challenges to the cancer patient being informed and involved in decision‐making. 14
Having formulated questions that are answerable, patients must synthesize the relevant evidence, estimate the expected benefits and costs of each option based on the evidence and then judge the relative value of the expected outcomes to determine if the benefits are worth the harms and costs. Many patients have no experience of being involved in making treatment decisions and may feel overwhelmed by such a task. Secondly, patients may not be aware that involvement in decision‐making is an option, or that their doctor would endorse such a role. Thirdly, patients may not know what the term ‘shared decision‐making’ means and therefore have difficulty expressing a valid preference for involvement. 15
One study showed that as cancer patients grew more experienced, particularly if their disease was well‐controlled, they tended to obtain more information and greater involvement over time. 16 Doctors are also reported to influence patients’ expressed information and involvement preferences. For example, in the same study, patient preferences for receiving more or less confronting information after the consultation varied as a function of the doctor they saw during the consultation. Thus, some doctors more actively encourage patients to be involved, and empower them to do so.
Previous interventions encouraging patient involvement have focused on increasing patient questioning, e.g. 17 , 18 or have targeted doctor behaviours. 19 To our knowledge, no interventions have targeted patient understanding of how their doctors make treatment decisions, or provided patients with a model of patient behaviour in shared decision‐making. Providing people with a framework of clinical decision‐making might allow them to feel more able to participate in that decision‐making. Modelling has been shown in other contexts to be a powerful tool to encourage new behaviour that is unfamiliar to the viewer. 20 It provides both a framework for the behaviour (what it looks like) and demonstrates that it is acceptable and appropriate, especially if the model(s) are perceived as similar to the viewer and people of high status. 20
The primary aim of this project was to evaluate a strategy designed (a) to promote shared decision‐making in treatment decisions for cancer patients who prefer this role and (b) to allow patients who had not previously considered this issue to come to a clear preference regarding their role. Specifically, this randomized trial investigated the effect of providing cancer patients with a package designed to facilitate shared decision‐making prior to seeing their oncologist for the first time. We expected that the intervention would result in patients seeking more information and more active involvement in decision‐making. Further, we expected that the intervention would change patient behaviour in the medical consultation, with intervention subjects more often declaring their consultation preferences than controls. Patient outcomes (satisfaction with decision‐making, decisional conflict, anxiety, depressed mood) were also expected to be improved by the intervention. Doctor behaviour and doctor satisfaction with the consultation were also examined in this study.
Materials and methods
Participants
This project was conducted with full human research ethics committee approval. Female cancer patients were consecutively recruited from eight medical oncologists (five male, three female) employed in one of six teaching hospitals in the Sydney Metropolitan or NSW country areas. Women were eligible for the study if they had been recently diagnosed with cancer (any site or stage) and were seeing a medical oncologist for the first time to discuss treatment options, were over 16 years of age, able to speak and read English, and were not too ill to complete the questionnaires. Only women were recruited, as gender was thought likely to influence response to the intervention.
Design
The study was a two‐arm, randomized controlled trial of a package comprising of a booklet and a videotape designed together to increase understanding of clinical decision‐making and to empower women to become more active in the consultation if they preferred this role. We were not able to explore the separate effect of these interventions because of feasibility issues, but rather sought to determine if as a package, they had an impact on patient outcomes.
Materials
Booklets
An eight page booklet titled ‘How treatment decisions are made’ was developed in consultation with an international panel of experts in the fields of evidence‐based medicine, psycho‐oncology and consumer involvement. The package was designed to operate as an ‘advanced organizer’ that lays the framework for the patient's understanding of the overall structure of the decision‐making process. Our package was designed to provide both a structuring of knowledge regarding clinical decision‐making and sufficient cues to activate the learning achieved in the context of the consultation. 21
The booklet describes decision‐making in the context of evidence‐based medicine, treatment options and patient preferences. It describes: (a) the importance of evaluating treatments before they are widely used, using historical examples where failure to do so resulted in medical disasters, (b) different stages of research which are conducted to evaluate the safety and efficacy of new treatments; (c) levels of evidence, (d) how the doctor decides which treatment to recommend, (e) the importance of patient involvement in treatment decision‐making, if that is desired and (f) a list of suggested questions to ask the doctor about treatment options. The booklet was assessed for readability using the Flesch‐Kincaid Grade Level in Word for Windows and achieved a reading age of less than grade 10. The booklet was piloted on 24 female cancer patients for acceptability, salience and clarity. All 24 patients felt the booklet was useful, salient and clearly expressed. We also showed the booklet to a panel of experts, including two patient advocates, two experts in evidence‐based medicine, two oncologists and two psycho‐oncologists. Some suggested changes to wording were introduced following this process. Finally, the booklet was included in a package provided to 164 cancer patients prior to their first meeting with an oncologist in a separate study. 22 Patients were asked to rate the perceived usefulness and ease of understanding of the material, and whether they found it anxiety provoking. About 60% of the patients reported that it made no difference to their anxiety levels and one‐third reported a reduction in anxiety. A few patients reported it made them anxious. About one‐third reported finding these materials extremely useful, one‐third very useful and one‐third a little useful. About 50% of both groups found the material very easy to understand, with about one‐third reporting them to be easy and 13% reasonably easy.
An NSW Cancer Council booklet titled ‘Living with Cancer’ was used for the control arm, to control for additional attention received. This is a booklet describing general issues in cancer (such as the impact of a cancer diagnosis and support services available) and is readily available to cancer patients. It contains no reference to questions to ask the doctor, or to how people make treatment decisions, and therefore was a true control in the sense of including no ‘active’ elements. Standard practice in participating institutions was to provide no additional reading at this time, although this varied.
Videotapes
Individual 15‐min videotapes were made of the eight experienced medical oncologists participating in the study, discussing treatment options with an actor. The videotapes were designed to model different patient styles, and to demonstrate that oncologists are comfortable with both active and passive styles. The oncologists responded naturally (i.e. unscripted) to the trained actor, who played the role of a patient with early stage breast cancer who had recently undergone surgical treatment for her cancer, and was attending her first consultation with a medical oncologist. She adopted, in random sequence, three different consultation participation styles: an active patient who wanted to be given all information and wanted to make the treatment decisions; a passive patient who wanted minimal information and for their doctor to make treatment decisions; and an intermediate patient who wanted all relevant information and to participate in collaborative decision‐making. These patient typologies were developed on the basis of a literature review and consensus process with an expert panel of medical oncologists and psychologists involved in cancer care. A more detailed description of the patient typologies is provided in Brown et al. 23 A qualitative analyses conducted as part of a separate study, showed that doctors were clearly able to distinguish the different patient styles, and responded positively, but differently to each scenario. 23
Each of the eight videos contained the following introductory message: ‘This video shows a woman with cancer having her first consultation with the doctor you are about to see. The woman, called Chris Jones, uses three different styles of talking with her doctor. Each of these styles shows the way some women like to talk to their doctor, and one of these may suit you. You'll notice that the doctor is comfortable with whatever way Chris wants. We would like you to look closely at each of these three ways of talking to the doctor. What is important for you to notice is how Chris speaks with the doctor, not the detail of what she actually says. It doesn't matter what type of cancer you have, we believe this video will help you get the most out of your consultation with the doctor’. Consultation videotapes were edited in a standard manner to provide: an introductory segment (10 min) and a closing segment that included a discussion of treatment options (5 min). The amount of time doctors and each patient type spent talking were also standardized across videotapes.
Procedure
Eligible patients were identified by clinic nurses, who checked with their oncologist that they were suitable. Women were telephoned to ask for permission to release their details to the research co‐ordinator. Interested women were phoned by the co‐ordinator, who informed them of the study, obtained verbal consent, and arranged for the women to attend their first oncology appointment 30 min early, to allow time for study procedures. At this time, after obtaining written consent, participants completed the baseline questionnaire and were randomly allocated to either the experimental or control group. Patients in the experimental group were given the booklet ‘How treatment decisions are made’, and were then shown the 15‐min video of their oncologist discussing treatment options with an actor. Control subjects received a NSW Cancer Council booklet on living with cancer. The subsequent medical consultation was audio taped, transcribed and coded for analysis. After the consultation, oncologists were asked to indicate how satisfied they were with the consultation. Questionnaires were completed by patients immediately after the consultation, and 2‐weeks later. The follow‐up questionnaire was designed to detect any ongoing effects of the intervention.
Measures
Audio‐tape analysis
A coding system was developed based on the informed decision‐making and shared decision‐making models, to document patient and doctor behaviours indicative of active patient participation in the consultation and shared decision‐making. We applied the coding system to a pilot sample of 10 randomly selected consultations in order to refine the codes and generate new items where appropriate. The presence or absence of the following doctor and patient behaviours were coded:
Did the doctor: (a) set his/her agenda, (b) ask the patient's agenda, (c) discuss non‐medical psychosocial issues, (d) engage in social chit‐chat, (e) check patient preferences (for information and involvement in decision‐making), (f) give patient a choice of treatment, (g) refer to medical evidence and the quality of the evidence, (h) acknowledge differences in expert opinion, (i) check patient understanding, (j) invite patient questions, (k) elicit patient perspectives (on costs and benefits of treatment options), (l) offer delay in treatment decision‐making, (m) discuss the option of a second opinion, (n) offer supplementary (written) information.
Did the patient: (a) present her agenda, (b) declare her preferences (for information and involvement), (c) declare her perspectives (on costs and benefits of treatment, (d) portray self in an active role (e.g. ‘I will receive the chemotherapy’ vs. ‘You will give me the chemotherapy’).
Binary (yes/no) assessments were made for each of the above items. More global judgements regarding the degree to which patients were active in the consultation (low, moderate, high) and the extent to which doctors encouraged this in the patient (low, moderate, high) were also subjectively determined. The number of occasions the doctor or patient introduced new themes, number of patient questions asked, and total word counts for doctors, patients and family/friend were also computed.
Coders were blinded to randomization except in two consultations where patients referred to the intervention in the consultation, so blinding was broken. Coder 1 coded all audiotapes. Ten consultations were coded by the same rater on two occasions, 6 months apart to establish intra‐rater reliability, and eight different consultations were re‐coded by another trained rater to establish inter‐rater reliability). Intra‐rater reliabilities for ratings made by the same rater on the 21‐item consultation coding system were mostly high (mean = 0.92; range 0.36–1.0). Inter‐rater reliabilities were also high (mean = 0.85; range 0.5–1.0). For the full range of reliability statistics, see Table 1.
Table 1.
Reliability data for global consultation behaviours*
| Inter‐rater (n = 8) | Intra‐rater (n = 10) | |
|---|---|---|
| Given choice of treatment | 1.000 (0.005) | 1.000 (0.002) |
| Treatment delayed | 0.500 (0.102) | 1.000 (0.002) |
| Information preferences declared | 0.500 (0.102) | 0.800 (0.010) |
| Involvement preferences declared | 1.000 (0.005) | 0.615 (0.035) |
| Treatment preferences declared | 1.000 (0.005) | 1.000 (0.002) |
| Decisional preferences declared | 0.714 (0.035) | 1.000 (0.002) |
| Cost perspectives declared | 0.750 (0.028) | 1.000 (0.002) |
| Benefit perspectives declared | 0.500 (0.102) | 1.000 (0.002) |
| Agenda setting by patient/doctor | 1.000 (0.000) | 1.000 (0.000) |
| Second opinion discussed | 1.000 (0.005) | 1.000 (0.002) |
| Reference to medical evidence | 0.600 (0.064) | 1.000 (0.002) |
| Reference to quality of evidence | 0.357 (0.284) | |
| Expert differences acknowledged | 1.000 (0.005) | 1.000 (0.002) |
| Written information sought/offered | 1.000 (0.005) | |
| Understanding sought/offered | 1.000 (0.005) | 1.000 (0.002) |
| Patient questions invited | 1.000 (0.002) | |
| Psychosocial issues discussed | 0.696 (0.053) | 0.727 (0.023) |
| Social chit‐chat | 1.000 (0.005) | 1.000 (0.002) |
| Decision reached during consult | 1.000 (0.008) | 1.000 (0.000) |
| Agency displayed by patient† | 1.000 (0.001) | 0.950 (0.000) |
| Agency encouraged by doctor† | 0.746 (0.014) | 0.946 (0.000) |
*Kappas could not be reported when binary categories were constants [i.e. raters used only one of the two (binary) categories for these variables].
†Kappa binary variables and gamma for non‐binary variables.
Questionnaires
Information preferences were assessed by the method described by Cassileth et al. 24 This self‐report measure assesses preferences for both the amount of detail and type of information (only necessary information, only good news or all news, good or bad), and has good internal reliability, with a Cronbach's α level of 0.89, and item‐total correlations ranging from 0.47 to 0.71.
Preferences for decisional control 11 were assessed using a validated and reliable question from previous studies in cancer patients. Patients indicated whether they wanted to play an active, passive or collaborative role with their doctor when making treatment decisions. Five options offered were: (1) the doctor should make the decisions using all that is known about the treatments; (2) the doctor should make the decisions but strongly consider my needs and priorities; (3) the doctor and I should make the decisions together on an equal basis; (4) I should make the decisions, but strongly consider the doctor's opinion and (5) I should make the decisions using all I know or learn about the treatments. (6) Not applicable; no decision was made.
Achievement of information preference and desired role in decision‐making were assessed using adaptations of the above items, in which patients indicated their perception of what actually happened in the consultation, as opposed to their preferences.
Patient satisfaction with consultation was assessed using the Patient Satisfaction with Consultation Scale (25 items), adapted from Roter 17 and Korsch et al., 25 which has proven sensitivity in previous studies with cancer patients. 26 Responses to each statement are scored from 1 to 5, from disagree completely to agree completely, with high scores indicating greater satisfaction with the consultation.
Patient satisfaction with decision‐making was assessed using a validated and reliable five‐item scale 27 from previous studies in cancer patients; responses to each statement are scored from 1 to 5, from strongly disagree to strongly agree, with high scores indicating greater satisfaction with decision‐making. Statements included: I feel I have made an informed choice; my decision shows what is important for me; I expect to stick with my decision; I am satisfied with my decision. Patients were also asked to indicate how satisfied they were with the decision‐making process (very dissatisfied to very satisfied).
Decisional conflict was assessed using O'Connor's Decisional Conflict Scale (16 items); 28 responses to each statement are scored from 1 to 5, from strongly agree to strongly disagree, with high scores indicating higher decisional conflict.
Depressive symptoms were assessed using the Beck Depression Inventory (short form; 13 items), a standardized self‐report questionnaire that assesses occurrence of depressive symptoms over the past week. 29 Items are rated on a scale from 0 to 3, from neutral feelings about a statement to depressed feelings.
State anxiety was measured using the Spielberger State‐Trait Anxiety Inventory (STAI, 20 items); 30 participants are asked to rate each item as to whether it describes how they feel at the moment from ‘not at all’ to ‘very much so’, with higher scores representing greater anxiety levels.
Demographic data, information and involvement preferences, depressed mood, state anxiety and health locus of control were assessed before the consultation. Information and involvement preferences, satisfaction with the consultation, decisional conflict, state anxiety and whether a treatment decision was reached, were assessed immediately after the consultation. Doctors were asked to indicate their satisfaction with the consultation at this time. Two weeks later, patient satisfaction with decision‐making, depression, state anxiety and whether a treatment decision was reached, were assessed.
Statistical analysis
A small sample size was achieved because of difficulties in recruiting patients to this complex study. Thus analyses were univariate. Chi‐square analyses were used to determine effectiveness of the intervention in influencing patients’ consultation behaviour. As a result of the small sample size and limited variability of data, the following variables were recoded into dichotomous categories: information preferences (few or all details; little or all information), involvement preferences (passive or active involvement), doctor satisfaction (low or high), married (yes or no) and education (tertiary qualifications or none). Other variables were analysed as continuous variables. Two‐tailed, independent groups Mann–Whitney U‐tests were used to detect differences between groups on: number of patient questions, number of new themes raised, ratio of doctor to patient talk, and time since surgery. Effects of the intervention on patient outcomes (decisional conflict, depression, state anxiety) were also assessed using Mann–Whitney U‐tests. Two‐tailed, independent groups Student's t‐tests were used to detect age differences between groups. Intra‐ and inter‐rater reliability analyses are also provided for ratings made by a single rater on two occasions, and by two different raters on a single occasion. Analyses were conducted using the Statistical Package for the Social Sciences (SPSS).
Results
Participants
Seventy‐one people were approached to be included in the study. Sixty‐five (92%) people agreed to complete the initial questionnaire. Of the six who declined participation, four were recently informed they had metastatic disease. Demographic and disease features of participants are shown in Table 2. The mean age of participants was 53 years (range: 25–81 years; SD = 12.7 years). Mean time since surgery for the primary cancer was 10.6 weeks (range: 1–162 weeks; SD = 25.7 weeks). The majority of participants had early stage disease, and the most common diagnosis was breast cancer. Forty‐five (70%) patients attended the consultation with a family member or friend. Forty‐six (71%) patients were currently married, and 26 (40%) held some tertiary qualification.
Table 2.
Demographic and disease features of participants
| Variable | Intervention group | Control group |
|---|---|---|
| Age [mean (SD)] | 51 (12) | 54 (13) |
| Time since surgery (weeks) | 8 (14) | 12 (32) |
| Stage of disease (%) | ||
| Early | 89 | 87 |
| Advanced | 11 | 13 |
| Diagnosis (%) | ||
| Breast cancer | 71 | 68 |
| Other | 29 | 32 |
| Marital status (%) | ||
| Single | 20 | 9 |
| Married/de facto | 74 | 77 |
| Widowed/divorced | 3 | 14 |
| Education level (%) | ||
| 10 years or less | 50 | 54 |
| 12 years | 3 | 11 |
| >12 years (tech) | 13 | 23 |
| >12 years (uni) | 33 | 11 |
| Religion (%) | ||
| Yes | 89 | 74 |
| No | 11 | 26 |
| Country born (%) | ||
| Australia | 83 | 74 |
| Other | 17 | 26 |
| Family member present at consult (%) | ||
| Yes | 73 | 70 |
| No | 27 | 30 |
Thirty subjects were randomized to receive the package, and 35 were given the control information booklet. There were some dropouts during the study: all participants completed the pre‐consultation questionnaire. Sixty‐two (95%) patients completed the questionnaire immediately after the consultation; completion did not vary as a function of group designation (z = −1.6, P = 0.10). Fifty‐three subjects (82%) completed the questionnaire at 2 weeks; completion did not vary with group designation (z = −1.6, P = 0.10). Intervention and control subjects did not differ with respect to age, marital status, education level, diagnosis or stage of disease, although there was a trend for time since surgery to be longer in control subjects (8.0 vs. 12.7 weeks; z = −1.8, P = 0.06).
Response to the intervention
1 Information and involvement preferences. Information and involvement preferences were assessed prior to the intervention and after the consultation with the oncologist. Most subjects wanted to be provided with as many details as possible, both before (80%) and after (84%) the consultation; and most people wanted as much information as possible, both before (86%) and after (89%) the consultation. Preferences for information were not altered by the intervention; the lack of statistical differences here may have been the result of poor score variability and ceiling effects.
Before the consultation, most subjects wanted decision‐making to be shared (52%), although a proportion did want the doctor to make the decision but strongly consider their needs and priorities (27%), or they wanted to make the decision themselves but strongly consider the doctors opinion (16%). Involvement preferences changed over time (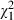 = 18.0, P = 0.00), but were not altered by the intervention. Thus, involvement preferences were similar in intervention and control group subjects both before (
= 18.0, P = 0.00), but were not altered by the intervention. Thus, involvement preferences were similar in intervention and control group subjects both before (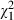 = 0.0, P = 0.97) and after the intervention (
= 0.0, P = 0.97) and after the intervention (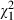 = 0.2, P = 0.67), while intervention subjects (
= 0.2, P = 0.67), while intervention subjects (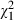 = 8.6, P = 0.00) and controls (
= 8.6, P = 0.00) and controls (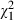 = 18.0, P = 0.00) changed their involvement preferences to a similar degree. No net change in preference was noted, with approximately equal numbers of subjects in each group changing preferences over time from an active to a passive role, and changing from a passive to a more active role in decision‐making.
= 18.0, P = 0.00) changed their involvement preferences to a similar degree. No net change in preference was noted, with approximately equal numbers of subjects in each group changing preferences over time from an active to a passive role, and changing from a passive to a more active role in decision‐making.
Results on outcome measures for the intervention and control groups at each assessment point are shown in Table 3.
Table 3.
Mean scores on psychological measures for intervention and control group subjects (standard deviations)
| Intervention | Control | |
|---|---|---|
| Depression | ||
| Baseline | 15.8 (3.0) | 16.4 (3.7) |
| 2 weeks | 16.2 (4.5) | 15.9 (3.7) |
| 6 months | 16.5 (4.5) | 14.6 (1.4) |
| State anxiety | ||
| Baseline | 40.2* (11.2) | 48.6 (12.2) |
| After consult | 38.0 (12.1) | 40.8 (12.7) |
| 2 weeks | 32.3* (10.9) | 40.3 (12.2) |
| 6 months | 34.4 (13.2) | 35.7 (9.4) |
| Locus of control (baseline) | ||
| Internal LOC | 19.8 (9.9) | 19.0 (6.5) |
| External LOC | 28.8 (4.6) | 28.7 (5.3) |
| Chance LOC | 19.8 (6.6) | 19.9 (7.9) |
| Decisional conflict | ||
| After consult | 37.8 (4.8) | 36.6 (3.0) |
| Satisfaction | ||
| After consult | 22.6 (1.8) | 22.7 (2.2) |
*P < 0.01.
2 Decisional conflict. Immediately after the consultation, 41 (67%) patients indicated that a decision had been made about the type of treatment they were to receive (usually chemotherapy or hormone therapy); all but one had made the decision 2 weeks later. Decisional conflict was assessed immediately after the consultation and was similar in intervention subjects (mean = 37.8) and controls (mean = 36.6; z = −0.4, P = 0.66).
3
Satisfaction. Immediately after the consultation, patient satisfaction with the consultation was high, and was similar in intervention subjects (median = 22.6) and controls (median = 22.7; z = −0.6, P = 0.54). Patient satisfaction with decision‐making 2 weeks later was high, with 51 (94%) indicating they were satisfied to very satisfied, and none indicating dissatisfaction on the single‐item scale; satisfaction ratings (using the five‐point scale) were similar in intervention subjects (median = 4.5) and controls (median = 4.4; z = −0.4, P = 0.67). Doctor satisfaction was also high, with 52 (90%) doctors indicating they were satisfied to very satisfied with the medical consultation, and none indicating dissatisfaction. Doctor satisfaction with the consultation did not vary as a function of participants’ group designation (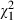 = 0.2, P = 0.64).
= 0.2, P = 0.64).
4 Psychological measures. State anxiety was assessed before and after the consultation, and 2 weeks later. Anxiety measures were high before the consultation (mean = 44.7), declined immediately after the consultation (mean =39.4), and declined further 2 weeks later (mean = 37.0). Anxiety levels were significantly higher in controls (mean = 48.6) than in intervention subjects at baseline (mean = 40.2; z =−2.5, P = 0.01) and 2 weeks later (40.3 vs. 32.3; z = −2.7, P = 0.01), although no differences were detected immediately after the consultation (40.8 vs. 38.0; z = −0.9, P = 0.39).
Depressed mood was assessed before the consultation, and 2 weeks later. Depression scores were high before the consultation (mean = 16.7), and declined a little 2 weeks later (mean = 15.9). Depression scores were similar in intervention subjects (mean = 16.5) and controls at baseline (mean = 16.9; z = −0.5, P = 0.60), and at 2 weeks (16.5 vs. 15.4; z = −0.4, P = 0.69).
Consultation behaviour
Fifty‐two of the 65 medical consultations had a clear audiotape; three consultations were not taped because of recorder malfunction and 10 tapes were inaudible because of outside noise (unfortunately building construction occurred during the data collection period). Thus consultations between medical oncologists and 23 intervention subjects and 29 controls were transcribed and coded. Quantitative analyses indicated that the package did impact positively on patient behaviour in the consultation, and also tended to impact on doctor behaviour. Mean scores and percentages of behaviours coded are shown in Table 4.
Table 4.
Mean scores (SD) and percentages of global consultation behaviours for intervention and control group subjects
| Intervention | Control | |
|---|---|---|
| Patient question count | 8.8 (8.6) | 8.1 (7.9) |
| Kin question count | 4.4 (3.0) | 3.9 (3.7) |
| Doctor new themes | 23.5 (10.5)* | 18.0 (9.5) |
| Patient new themes | 7.9 (5.9) | 6.8 (5.1) |
| Kin new themes | 2.9 (2.6) | 2.8 (2.5) |
| Total words doctor | 3350.5 (1515.6) | 3048.4 (1596.6) |
| Total words patient | 1238.5 (585.3) | 1373.1 (924.1) |
| Total words kin | 254.4 (200.1) | 320.5 (335.5) |
| Agenda setting by patient/doctor (%) | 47.8 | 48.3 |
| Information preferences declared (%) | 65.2* | 41.4 |
| Involvement preferences declared (%) | 30.4 | 17.2 |
| Treatment preferences declared (%) | 60.9* | 37.9 |
| Understanding sought/offered (%) | 73.9 | 82.8 |
| Patient questions invited (%) | 87.0 | 86.2 |
| Given choice of treatment (%) | 56.5 | 41.4 |
| Treatment delayed (%) | 47.8 | 44.8 |
| Reference to medical evidence (%) | 87.0 | 72.4 |
| Reference to quality of evidence (%) | 26.1 | 31.0 |
| Expert differences acknowledged (%) | 21.7 | 24.1 |
| Cost perspectives declared (%) | 73.9** | 44.8 |
| Benefit perspectives declared (%) | 60.9** | 31.0 |
| Second opinion discussed (%) | 17.4 | 20.7 |
| Written information sought/offered (%) | 43.5 | 55.2 |
| Psychosocial issues discussed (%) | 39.1 | 34.5 |
| Social chit‐chat (%) | 39.1 | 41.4 |
| Decision reached in consult (%) | 56.5 | 67.9 |
| Decisional preferences declared (%) | 65.2 | 72.4 |
| Agency displayed by patient | ||
| Low (%) | 34.8 | 48.3 |
| Moderate (%) | 26.1 | 13.8 |
| High (%) | 39.1 | 37.9 |
| Agency encouraged by doctor | ||
| Low (%) | 43.5 | 41.4 |
| Moderate (%) | 26.1 | 24.1 |
| High (%) | 30.4 | 34.5 |
*P < 0.1; **P < 0.05.
Patient behaviour
Intervention subjects were more likely to declare their preferences and perspectives during the consultation than were control subjects. They were more likely to declare their perspectives on the costs and side‐effects (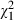 =4.4, P = 0.04) and benefits of treatment (
=4.4, P = 0.04) and benefits of treatment (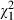 =4.6, P = 0.03), and they were more likely to declare their preferences for information (
=4.6, P = 0.03), and they were more likely to declare their preferences for information (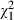 =2.9, P = 0.09) and treatment during the consultation (
=2.9, P = 0.09) and treatment during the consultation (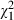 = 2.7, P = 0.10). However, intervention subjects were not more likely to declare their involvement preferences than controls (
= 2.7, P = 0.10). However, intervention subjects were not more likely to declare their involvement preferences than controls (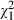 = 1.3, P = 0.26), or to declare which of the patient participatory roles they wanted to adopt depicted in the video (
= 1.3, P = 0.26), or to declare which of the patient participatory roles they wanted to adopt depicted in the video (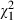 =0.3, P = 0.58). The degree to which patients spoke of themselves in an active (vs. passive) manner did not vary as a function of group designation (
=0.3, P = 0.58). The degree to which patients spoke of themselves in an active (vs. passive) manner did not vary as a function of group designation (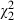 = 1.6, P = 0.46).
= 1.6, P = 0.46).
Total number of words used by intervention subjects (mean = 1238.5) was similar to that used by controls (mean = 1373.1, z = 0.0, P = 0.96), and there was no difference in the ratio of doctor to patient talk between intervention subjects (mean = 3.1) and controls (mean = 3.4; z = −1.0, P = 0.33). CDM subject question counts (mean = 8.8) and those of controls (mean = 8.1; z = −1.0, P = 0.33) were also similar. Intervention subjects (mean = 7.9) did not introduce more new themes than controls during the consultation (mean = 6.8; z = −0.5, P = 0.65).
Doctor behaviour
Doctors tended to introduce more new themes in consultations with intervention subjects (mean = 23.5) than with controls (mean = 18.0; z = −1.9, P = 0.06).
Otherwise, doctors did not behave differently towards the two groups of subjects, suggesting that doctors were largely unaware of individual participant's group designations. This is corroborated by the findings that doctors correctly guessed group designation in only six (20%) consultations, and control group designation in only nine (26%) consultations.
Total number of words used by doctors in consultations with intervention subjects (mean = 3350.5) was similar to that used in consultations with controls (mean = 3048.4; z = −0.7, P = 0.47).
Relationship between involvement preference and behaviour in the consultation
Univariate statistics explored the relationship between the patient's involvement preference as stated before the consultation, and their behaviour in the consultation. Only one variable was associated with involvement preference. Patients who preferred to make the decision themselves or to share decisions, were significantly more likely to initiate a declaration of their information preferences (χ 2 = 5.9, P < 0.05).
Discussion
This randomized trial investigated the effect of providing cancer patients with a package (both written materials and video), designed to facilitate shared decision‐making, prior to seeing their oncologist for the first time. To our knowledge, while a large body of work has been undertaken on the benefits of shared decision‐making 31 , 32 and strategies to facilitate patient involvement in decision‐making 33 – notably decision aids 34 – this is the first intervention evaluated that has specifically targeted patient knowledge of the clinical decision‐making process, and attempted to model for patients, different participatory styles.
Contrary to expectation, the intervention did not impact on some patient outcomes. Patients in the intervention arm were not more satisfied with the consultation, less depressed, and did not experience less decisional conflict than those in the control arm. We had hypothesized that if the intervention successfully empowered patients to adopt their preferred role, and to take on a more active role than they might otherwise have felt capable of, then treatment decisions might be easier and psychological outcomes less severe. An earlier study by Fallowfield et al. 2 reported that patients seeing a surgeon who offered choice had significantly reduced psychological morbidity for many months after the treatment decision was made.
Intervention subjects’ information and involvement preferences were also not changed as a result of the intervention. We had expected that patients who lacked confidence in their ability to contribute to treatment decisions might gain knowledge, skills and confidence from the intervention, and therefore shift to preferring a more active role. In fact, few patients changed their involvement preferences at all during the study, with a substantial proportion (one‐third) initially preferring the doctor to take the major role. This percentage is not dissimilar to those reported by earlier observational studies. 12 , 13
One interpretation of these results is that patient reports of involvement preferences alone may be insufficient to detect the impact of interventions that target patient activity in the consultation. The stated preferences and behaviours of patients in this study were not concordant. Thus, patients who declared a preference for shared decision‐making or a dominant role in decision‐making were not more likely to declare these preferences in the consultation, declare their views on costs or benefits or use language that portrayed themselves in an active role (agency). These behaviours have been described as important components of the shared decision‐making consultation and would therefore be expected to occur more commonly when patients are pursuing this goal. 35 Recently, concerns have been raised about the use of quantitative role label measures, such as those used here, to elicit preferences for treatment decision‐making. 15 , 36 These concerns include the lack of detailed information describing how many authors derived their role labels; a lack of standardization across measures, which has resulted in variation in the number of roles and the role descriptions used in the different instruments; and questions about their reliability and validity. Further research effort is needed to develop better and more sensitive ways to explore patient views on decision‐making.
Despite the stated involvement preferences remaining unchanged, the package did impact positively on some patient behaviour in the consultation: subjects receiving the package were more likely to declare their information and treatment preferences, and were more likely to declare their perspectives on the costs, side‐effects and benefits of treatment. Some doctor behaviour was also affected by the intervention, with doctors introducing considerably more new themes during the consultations with intervention subjects than with controls. Considering the relatively small sample size, these results are encouraging, and suggest that interventions like this may be able to overcome some of the substantial barriers to shared decision‐making in cancer consultations. Nevertheless, many of the behaviours we thought might be affected by the intervention were not. As good inter‐rater and intra‐rater reliability for the coding was obtained, this does not appear to be a function of unreliable coding. Future work needs to explore in greater detail the immediate impact of the interventions on knowledge, attitudes and confidence.
Certainly, other interventions targeting shared decision‐making have successfully altered patient outcomes and behaviour. A recent systematic review of decision aids 34 concluded that there is now clear evidence that patients receiving decision aids have higher knowledge, less difficulty in reaching a decision and more active participation in decision‐making. Similarly, in a number of studies, question prompt sheets have been shown to increase patient questioning and reduce anxiety. 18 It is not possible to assess whether these various interventions, targeting different problems and barriers to shared decision‐making, are differentially effective, or influence different outcomes. Future research might usefully directly compare different interventions to promote shared decision‐making and achieve involvement preferences.
Despite randomization, patients in the control arm were significantly more anxious at baseline than those who received the package, making it difficult to assess the impact of the intervention on reported state anxiety. Nonetheless, the fact that the intervention apparently did not increase anxiety (control subjects were still more anxious 2 weeks later) is reassuring. A review of studies of decision aids recently concluded that there was no evidence that decision aids increase anxiety, 34 which is in line with the current findings. However, in another study we conducted recently in which patients were provided with the booklet used in this study, a question prompt sheet and a booklet on patient rights (but not the video used here), patients were significantly more anxious before the consultation than those in the control arm. 22 Perhaps the emphasis in this study on becoming as involved as one wished, and the oncologists’ endorsement of all styles (rather than only the active style), prevented patients from feeling overwhelmed or burdened by excessive personal expectations. This may be an important consideration for future interventions in this area.
Limitations of the study
The sample size was small, limiting the study's power to detect effects. Recruitment was affected by the size of the intervention, which tended to impact on clinic time and organization. This is a concern for feasibility of routine administration of the intervention, although if integrated into clinic practice, this would be unlikely to remain problematical.
The data should be interpreted with caution, because there was a lack of score variability and common ceiling effects. For example, most patients already wanted to be provided with as many details and as much information as possible before the intervention, and most patients and doctors rated their satisfaction as high after the consultation. Some of the doctors participating in this study had previously participated in other studies focusing on doctor–patient communication, and therefore may have already been attuned to facilitating patient involvement. It is possible that the intervention may have had greater impact with a more naive group of doctors.
Patients in the intervention arm received written material and were exposed to a videotape modelling different levels of patient activity in the consultation. It is not possible from the study design to ascertain whether one component of the intervention was more influential than the other. To do so adequately would have required a four‐arm study (booklet alone, videotape alone, booklet plus videotape, or control booklet only) which was beyond the means of the current study. Unfortunately, we did not directly ask the women which component they preferred, or whether they felt that one had been more influential than another, which may have cast some light on this issue. It would be useful to know which of these materials impacted more on participants. In a separate study, 37 the booklet was evaluated without the videotape. Patients receiving the booklet were more active in the consultation than controls, suggesting that this component alone is effective, but this does not mean that the videotape was any less effective.
We did not determine if actual treatment decisions were affected by the intervention; however this is unlikely. In a separate data collection exercise with a subset of the sample, we interviewed women several months after their initial consultation, to ask what factors were most important in their decision‐making. 36 The majority emphasized ‘personal’ factors. These included the feeling that their doctor cared for, understood and respected them; that they could trust and have confidence in their doctor; that the doctor would give them enough time, that they would be listened to; and that the doctor would be open and honest. If these factors were felt to be present, many women were happy to accept the doctor's recommendation, confident that they would receive optimum treatment. Yet other women felt there was no decision to be made, they just wanted to get on with their treatment. Thus, their own activity in the consultation and involvement in the decision‐making process (and the intervention targeting these) were unlikely to have influenced what treatment they received.
Conclusion
This study showed that a short intervention delivered before an initial oncology consultation could influence patients’ and doctors’ behaviour in the consultation. Patients receiving the intervention behaved in ways more closely approximating the ideal shared decision‐making model.
Acknowledgements
The authors would like to thank the following oncologists for kindly participating in the study: Prof. Keiren Phadke, Dr David Goldstein, Dr Michael Boyer, Dr Amanda Goldrick, Dr Helen Wheeler, and Dr David Bell. The authors would also like to thank Judy Hood for her secretarial support of the study.
This work was supported by the National Health and Medical Research Council of Australia (Grant No. 970735).
References
- 1. Emanuel EJ, Emanuel LL. Four models of the physician–patient relationship. Journal of the American Medical Association 1992; 267: 2221–2226. [PubMed] [Google Scholar]
- 2. Fallowfield LJ, Baum M, Maguire GP. Effects of breast conservation on psychological morbidity associated with diagnosis and treatment of early breast cancer. British Medical Journal 1992; 293: 1331–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fallowfield LJ, Hall A, Maguire GP, Baum, M. Psychological outcomes of different treatment policies in women with early breast cancer outside a clinical trial. British Medical Journal 1990; 301: 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morris J, Ingham R. Choice of surgery for breast cancer: psychosocial considerations. Social Science and Medicine 1988; 27: 1256–1262. [DOI] [PubMed] [Google Scholar]
- 5. Street RL, Voigt B. Patient participation in deciding breast cancer treatment and subsequent quality of life. Medical Decision Making 1997; 17: 298–306. [DOI] [PubMed] [Google Scholar]
- 6. Street RL Jr, Voigt B, Geyer C Jr, Manning T, Swanson GP. Increasing patient involvement in choosing treatment for early breast cancer. Cancer 1995; 76: 2275–2285. [DOI] [PubMed] [Google Scholar]
- 7. Friedman MA, Cain DF. National Cancer Institute sponsored cooperative group clinical trials. Cancer 1990; 65: 2376–2382. [DOI] [PubMed] [Google Scholar]
- 8. Silverman WA. Patients’ preferences and randomised trials. Lancet 1994; 343: 1586. [DOI] [PubMed] [Google Scholar]
- 9. Tate HC, Rawlinson JB. Randomised comparative studies in the ignorance and treatment of cancer in the United Kingdom: room for improvement. Lancet 1979; 2: 623–625. [DOI] [PubMed] [Google Scholar]
- 10. Yee JY, Breaux SR. Accrual of radiotherapy patients to clinical trials. Cancer 1983; 52: 1014–1016. [DOI] [PubMed] [Google Scholar]
- 11. Degner LF, Sloan JA. Decision‐making during serious illness: what role do patients really want to play? Journal of Clinical Epidemiology 1992; 45: 941–948. [DOI] [PubMed] [Google Scholar]
- 12. Brody H. Autonomy revisited: progress in medical ethics. Journal of the Royal Society of Medicine 1985; 78: 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sutherland HJ, Llewellyn‐Thomas HA, Lockwood GA, Tritchler DL, Till JE. Cancer patients: their desire for information and participation in treatment decisions. Journal of the Royal Society of Medicine 1989; 82: 260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anonymous Evidence‐based care: 2. Setting guidelines: how should we manage this problem? Canadian Medical Association Journal 1994; 150: 1417–1423. [PMC free article] [PubMed] [Google Scholar]
- 15. Davey HM, Barratt AL, Davey E, et al. Medical tests: women's reported and preferred decision‐making roles and preferences for information on benefits, side‐effects and false results. Health Expectations 2003; 5: 330–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Butow PN, MacLean M, Dunn SM, Tattersall MHN, Boyer M. The dynamics of change: cancer patients’ preferences for information, involvement and support. Annals of Oncology 1997; 8: 857–863. [DOI] [PubMed] [Google Scholar]
- 17. Roter D. Patient participation in the patient‐provider interaction: the effects of patient question‐asking on the quality of interaction, satisfaction and compliance. Health Education Monographs 1977; 5: 281–315. [DOI] [PubMed] [Google Scholar]
- 18. Brown RF, Butow PN, Dunn SM, Tattersall MHN. Promoting patient participation and shortening cancer consultations: a randomised trial. British Journal of Cancer 2001; 85: 1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joos SK, Hickam DH, Gordon GH, Baker LH. Effects of a physician communication intervention on patient care outcomes. Journal of General Internal Medicine 1996; 11: 147–155. [DOI] [PubMed] [Google Scholar]
- 20. Bandura A, Blanchard EB, Ritter B. The relative efficacy of desensitisation and modelling approaches for inducing behavioural, affective, and attitudinal changes. Journal of Personality and Social Psychology 1969, 13: 173–199. [DOI] [PubMed] [Google Scholar]
- 21. Patel VL, Groen GJ. Knowledge based solution strategies in medical reasoning. Cog Science 1986; 10: 91–116. [Google Scholar]
- 22. Butow P, Devine R, Boyer M, Pendlebury S, Jackson M, Tattersall MHN. A cancer consultation preparation package: changing patients but not doctors is not enough. Journal of Clinical Oncology, in press. [DOI] [PubMed] [Google Scholar]
- 23. Brown RF, Butow PN, Henman M, Dunn SM, Boyle F, Tattersall MH. Responding to the active and passive patient: flexibility is the key. Health Expectations 2002; 5: 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cassileth BR, Zupkis RV, Sutton‐Smith K, March V. Information and participation preferences among cancer patients. Annals of Internal Medicine 1980; 92: 832–836. [DOI] [PubMed] [Google Scholar]
- 25. Korsch BM, Gozzi EK, Francis V. Gaps in doctor–patient communication. Pediatrics 1968; 42: 855. [PubMed] [Google Scholar]
- 26. Butow P, Brown RF, Cogar S, Tattersall MHN, Dunn SM. Oncologists’ reactions to cancer patients’ verbal cues. Psycho-Oncology 2002; 11: 47–58. [DOI] [PubMed] [Google Scholar]
- 27. Holmes‐Rovner M, Kroll J, Schmitt N, et al. Patient satisfaction with health care decisions: the satisfaction with decision scale. Medical Decision Making 1996; 16: 58–64. [DOI] [PubMed] [Google Scholar]
- 28. O'Connor AM. Validation of a decisional conflict scale. Medical Decision Making 1995; 15: 25–30. [DOI] [PubMed] [Google Scholar]
- 29. Beck AT, Steer R, Garbin M. Psychometric properties of the Beck Depression Inventory: twenty‐five years of evaluation. Clinical Psychology Review 1988; 8: 77–100. [Google Scholar]
- 30. Spielberger CD. Manual for the State‐Trait Anxiety Inventory (Form Y). Palo Alto, CA: Consulting Psychologists Press, 1983. [Google Scholar]
- 31. Craig JC. Irwig LM. Stockler MR. Evidence‐based medicine: useful tools for decision making. Medical Journal of Australia 2001; 174: 248–253. [DOI] [PubMed] [Google Scholar]
- 32. Goyder E. Barratt A. Irwig LM. Telling people about screening programmes and screening test results: how can we do it better? Journal of Medical Screening 2000; 7: 123–126. [DOI] [PubMed] [Google Scholar]
- 33. Edwards A. Evans R. Elwyn G. Manufactured but not imported: new directions for research in shared decision making support and skills. Patient Education and Counseling 2003; 50: 33–38. [DOI] [PubMed] [Google Scholar]
- 34. O'Connor AM, Roston H, Fiset V, et al. Decision aids for patients facing health treatment or screening decisions: systematic review. British Medical Journal 1999; 319: 731–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ford S, Schofield T, Hope T. What are the ingredients for a successful evidence‐based patient choice consultation? A qualitative study. Social Science and Medicine 2003; 56: 589–602. [DOI] [PubMed] [Google Scholar]
- 36. Entwistle VA, Skea ZC, O'Donnell MT. Decisions about treatment: interpretations of two measures of control by women having a hysterectomy. Social Science and Medicine 2001; 53: 721–732. [DOI] [PubMed] [Google Scholar]
- 37. Henman MJ, Butow PN, Brown RF, Tattersall MHN. Lay constructions of decision‐making: perceptions of women with cancer. Psycho-Oncology 2002; 11: 295–306. [DOI] [PubMed] [Google Scholar]


