Abstract
Objective Does trust in physicians aid or hinder patient autonomy? We examine the relationship between trust in the recipient's doctor, and desire for a participative role in decisions about medical treatment.
Design We conducted a cross‐sectional survey in an urban Canadian teaching hospital.
Setting and participants A total of 606 respondents in three clinics (breast cancer, prostate cancer, fracture) completed questionnaires.
Variables studied The instrument included the Problem Solving Decision Making (PSDM) Scale, which used two vignettes (current health condition, chest pain) to categorize respondents by preferred role, and the Trust‐in‐Physician Scale.
Results Few respondents preferred an autonomous role (2.9% for the current health condition vignette and 1.2% for the chest pain vignette); most preferred shared decision‐making (DM) (67.3% current health condition; 48.7% chest pain) or a passive role (29.6% current health condition; 50.1% chest pain). Trust‐in‐physician yielded 6.3% with blind trust, 36.1% with high trust, 48.6% moderate trust and 9.0% low trust. As hypothesized, autonomous patients had relatively low levels of trust, passive respondents were more likely to have blind trust, while shared respondents had high but not excessive trust. Trust had a significant influence on preferred role even after controlling for the demographic factors such as sex, age and education.
Conclusions Very few respondents wish an autonomous role; those who do tend to have lower trust in their providers. Familiarity with a clinical condition increases desire for a shared (as opposed to passive) role. Shared DM often accompanies, and may require, a trusting patient–physician relationship.
Keywords: decision‐making, participation, patient–physician relationships, trust
Background
How does patients’ trust in their physician relate to their preferred role in medical decision‐making?
Over the past decades, there has been a major shift in the doctor–patient relationship from its former emphasis on paternalism, to a new recognition of the importance of an informed, autonomous patient. 1 , 2 , 3 , 4 , 5 , 6 , 7 As the literature has suggested, this shift in the locus of decision‐making from the doctor to the patient was heavily reinforced by the legal requirement for informed consent. 8 A growing literature has examined preferred roles in making treatment decisions, 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 how policy might promote more active participation 21 , 22 , 23 and how best to place this within the context of the therapeutic relationship. 24
Nonetheless, the relationship between trust and the doctor–patient relationship remains somewhat contentious. 25 , 26 , 27 , 28 Trust is a complex concept, which includes both technical (expertise) and interpersonal (e.g. communication, respect) elements. As reviewers of the concept have noted, there is no commonly shared understanding of what trust means or whether it is desirable. 29 , 30 Some claim that trust is a barrier to the optimal relationship between the provider and care recipient; they believe that recipients should be aggressive and engaged information seekers, who shop carefully among competing providers, define their own needs, play an active role in their own treatment and take responsibility for their treatment decisions. 26 Others are wary of this model, on such grounds that people with critical illnesses are known to depend on their physicians strongly, and can benefit from strong relationships which help them to deal with fear and uncertainty. 31 Others suggest that the personal relationship between the recipient and the provider is the context in which the treatment is chosen and carried out; distrust in their physician would be yet another stress and drain of energy for ill people, and would deprive them of a major potential source of information. 32 An emerging consensus suggests that trust is an important element of clinical encounters, regardless of the role care recipients wish to assume. 14 , 20 , 33
This study attempts to add empirical evidence to better understand the relationship between people's trust in their physician and their desire for a participative role in decisions about their medical treatment. It builds upon our previous research, which differed somewhat from other studies of patient roles in decision‐making (DM) by suggesting that the conceptualization of ‘participation’ should distinguish between two elements of choice. Recognizing that there is variation in nomenclature across subfields, we employ the term ‘problem solving’ (PS) to refer to situations in which there is one correct answer, and for which preferences are irrelevant. For example, results of an X‐ray cannot vary to respond to an individual's preference that her arm is not broken. In contrast, the term DM is used to refer to tasks which may indeed require prior PS, but also involve weighing the relative importance of potential outcomes. 34 , 35 , 36 , 37
In this study, we hypothesize that interpersonal trust‐in‐physician will be correlated with the desire for participation, such that people with high levels of trust will want to hand over control of PS and DM to the provider; people with moderate levels of trust will want to hand over PS only; whereas people with low levels of trust will prefer to keep control of both PS and DM.
As other research suggests that there is likely to be considerable variation in the desire for participation as a function of factors such as age, education, and whether the disease is chronic (because patients more experienced with their illness would have more time to become well informed), 13 , 38 , 39 , 40 , 41 , 42 , 43 , 44 we also investigated the influence of trust on a person's preferred role controlling for a respondent's socio‐demographic characteristics such as age and education.
Methods
Study population
The study received ethics approval from the Human Subjects Review Committee at the University of Toronto. The study population comprised patients at three outpatient clinics (breast cancer, prostate cancer and fracture) of a Toronto (Canada) teaching hospital. The hospital was primarily selected for its size, proximity and ease of access; it treats a large number of patients from the Toronto and surrounding area. The clinics were selected to ensure that the sample included both men and women, with a range of ages, and a mixture of severities of illness. The design allowed comparisons between predominantly male and predominantly female cancer patients. The fracture clinic population not only included patients with fractures, but also patients with spina bifida and low back pain. Participants were identified through a daily patient caseload sheet provided by the clinic staff at each site because the waiting rooms were also used by patients attending other clinics not involved in the study.
Study participants were approached by the research assistant, using a standard script approved by the Human Subjects Review Committee, and asked to complete a questionnaire, which included the Problem Solving Decision Making (PSDM) Scale and the Trust‐in‐Physician Scale. The script clarified that participation was purely voluntary, that none of their caregivers would see their responses, that their decision about whether or not to participate would not have any effect on their care, and that all responses would be anonymous. Agreement to participate was agreed to constitute informed consent. To ensure that anonymity could not be breached, no names were collected.
As there was no rationale for accepting any particular set of prior probabilities for the distribution of responses, formal power calculations were deemed premature. To ensure an expected value of at least five observations in each cell for cross tabulations, and at least 20 observations per independent variable for linear regressions/analysis of variance, the goal was to recruit at least 200 responses per site in order to ensure adequacy of sample size for statistical comparisons and allow us to perform subanalyses. Data collection began in January 1997 and was completed in April 1997. Although the data is now 7 years old, there is no reason to believe that the patterns of relationships have changed in the interim.
The inclusion criteria that were used were: patients had to be over 18 years of age, speak English, agree to participate, and be attending a clinic that had agreed to participate. This last criterion implies that the participants were patients of clinicians who were comfortable with having their patients approached to participate in this study. Our respondents thus represented the views of the subset of people who used the particular clinics where the study was conducted, and omits individuals whose trust in physicians was so low that they would not have sought out care from a hospital‐based clinic. The study was conducted in Canada; respondents would be fully insured for all hospital and physician care. Using a single cross‐sectional survey also limited our ability to examine changes over time. We recognize that it is likely that individuals captured by our survey were at different stages of their illness trajectory and that individual viewpoints may change over time. The study asked about trust in the physician they would be seeing at that clinic visit; we did not collect data about the length of time they had known that physician. In addition, some variables were potentially confounding; for instance, sex and clinic are confounded for two of the three sites because all respondents in the breast cancer clinic were females, and all respondents in the prostate centre were males.
Scales
Problem Solving Decision Making Scale
The PSDM Scale, which has been validated in other studies 35 , 36 was used to measure preferred role. The PSDM divides participation into two tasks, PS and DM. A short vignette is presented, and respondents are asked who should decide for each of a series of tasks, written to encompass both PS and DM activities. The four PS tasks are:
-
1
Who should determine (diagnose) what the likely cause of your symptoms are?
-
2
Who should determine what the treatment options are?
-
3
Who should determine what the risks and benefits for each treatment option are?
-
4
Who should determine how likely each of these risks and benefits are to happen?
The two DM tasks are:
-
1
Given the risks and benefits of these possible treatments, who should decide how acceptable those risks and benefits are for you?
-
2
Given all the information about risks and benefits of the possible treatments, who should decide what treatment option should be selected?
The response categories for all tasks use a five‐point Likert scale with (1) the doctor alone; (2) mostly the doctor; (3) both equally; (4) mostly me; (5) me alone.
To determine preferred role, mean scores are computed separately for the PS and DM dimensions for each respondent, and placed into one of three classifications: hand over (mean score on that dimension <3); share (mean score between 3 and 3.99); or keep (mean score ≥ 4). These classified PS and DM scores are then used to place respondents into one of three categories, as shown in Fig. 1. Passive patients wish to hand off both PS and DM, autonomous patients want to retain some control of both PS and DM (keep PS, and share or keep DM), while shared patients want to hand off or share PS but share or keep DM. It was considered theoretically implausible for an individual to wish to assume control for PS but not for DM.
Figure 1.
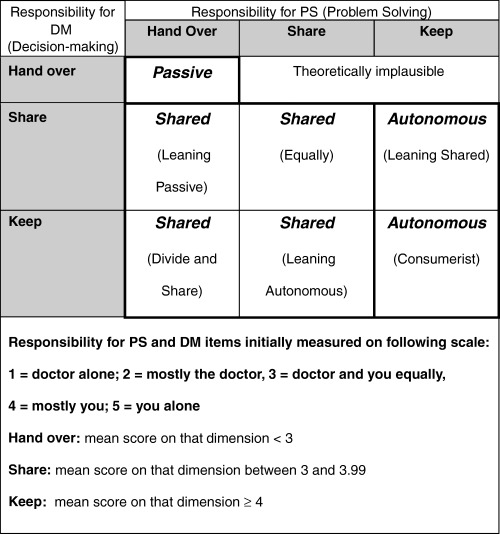
Categorization of preferred roles.
The version of the PSDM used in this study employed two brief vignettes. The current health vignette asked about DM for the patient's current health condition (defined as the condition for which they were attending the clinic). The chest pain vignette read: ‘suppose you had mild chest pain for three days and decided that you should visit your doctor about this.’ The chest pain vignette has been used in a number of studies, and allows comparison of the results in a particular patient population with results from other studies. It deals with a situation which could be life‐threatening, and about which most patients would not feel as expert. As such, it is hypothesized that there will be a greater willingness to handover control to the physician in the chest pain vignette than in the current health vignette. The scale shows favourable psychometric properties. In this sample, Cronbach's α for the four‐item PS component of the PSDM was 0.87 (current health vignette) and 0.90 (chest pain vignette). (It was not computed for the two‐item DM component.)
To control for amount of prior information about the chest pain vignette, respondents were also asked to indicate ‘How much experience have you had with the clinical situation described in the above scenario? (Please circle all letters that apply)’ with Yes/No answers possible for each of: (A) I have had personal experience with it; (B) I know of family members or close friends who have experienced it; (C) I have read/heard about it; and/or (D) I do not know much about it. To assess perceived knowledge about current health condition, respondents were asked to indicate, on a five point scale, how knowledgeable they felt about each of: your current health condition; the available treatment options; the risks and benefits of these options; which treatment you prefer.
Trust‐in‐Physician Scale
To measure patients’ trust in their physicians, the Trust‐in‐Physician Scale was used. This validated instrument measures a patient's interpersonal trust in his or her physician. 30 , 45 , 46 , 47 , 48 The scale assesses whether the patient had confidence in the dependability, knowledge and reliability of the information provided by physicians; these dimensions would appear particularly relevant in determining preferred roles in making treatment decisions. This scale has shown excellent psychometric characteristics, with Cronbach α above 0.85 in several studies. 30 , 45 , 46 This 11‐item scale was scored on a five‐point Likert scale with response categories ranging from (1) strongly disagree to (5) strongly agree; to avoid response set bias, some of the items are reversed before scoring. Anderson and Dedrick divided the resulting scores into three categories: low trust (scores <3); moderate trust (scores averaging from 3 to 3.99); and high trust (scores averaging from 4 to 5). For this research, high trust was further subdivided into two categories, where scores averaging between 4 and 4.99 were categorized as high and scores averaging 5.0 were categorized as blind trust. The Cronbach α for the 11‐item trust scale, with items reversed, was 0.91 in our sample.
Statistical analysis
Statistical analysis employed SAS‐PC. The chi‐square test (proc FREQ) was used to assess bivariate associations between socio‐demographic factors and the trust and preferred role categories; the relationship between mean knowledge score and preferred role was assessed using anova for unbalanced data (proc GLM).
Results
Of the 611 patients identified by clinic staff as eligible to participate in the study, 606 completed and returned a questionnaire while in the clinic, for a response rate of 99%. Three of the five non‐participating patients were excluded because of language difficulty; there were two refusals. The very high response rate appears to have resulted from a combination of relatively long waiting times in the clinic, with few other distractions. Table 1 reports key demographic characteristics of our sample: mean age was 56 (SD 15.5), with a range from 16 to 90; 48.8% were male; 42.1% had a high school education or less and 58.7% were classified as having a chronic health condition, defined as living with their current health condition at least 6 months.
Table 1.
General characteristics of respondents
| Characteristic | n | % |
|---|---|---|
| Gender (n = 602) | ||
| Male | 294 | 48.8 |
| Female | 308 | 51.2 |
| Age (n = 602) | ||
| 34 and under | 65 | 10.8 |
| 35–49 | 133 | 22.1 |
| 50–64 | 207 | 34.4 |
| 65+ | 197 | 32.7 |
| Education (n = 601) | ||
| Elementary school | 59 | 9.8 |
| Some high school | 91 | 15.1 |
| Graduated high school | 103 | 17.1 |
| Some post‐secondary | 95 | 15.8 |
| Graduated university | 176 | 29.3 |
| Some graduate training | 77 | 12.8 |
| Health (self‐reported) (n = 599) | ||
| Excellent | 29 | 4.8 |
| Very good | 143 | 23.9 |
| Good | 239 | 39.9 |
| Fair | 148 | 24.7 |
| Poor | 40 | 6.7 |
| Chronic condition? (n = 589) | ||
| No | 243 | 41.3 |
| Yes | 346 | 58.7 |
Preferred role
Preferred role was categorized as passive, shared or autonomous (Fig. 1). As shown in Table 2, few respondents preferred an autonomous role (2.9% for the current health condition vignette and 1.2% for the chest pain vignette). As predicted, respondents were more likely to wish a shared role for the current health condition than for the chest pain vignette. For their current health condition, 67.3% wished a shared approach, with 29.7% preferring a passive role. In contrast, responses for the chest pain vignette, while often shared, showed a shift towards a more passive role, with 48.7% still falling into the shared category, and 50.1% of respondents preferring a passive role. Few respondents fell into the boundary classifications (e.g. no respondents fell into the ‘autonomous‐leaning shared’ category for either vignette). Only one response fell into the theoretically implausible category (for the current health condition scenario only); this response was treated as missing for the remainder of the analysis.
Table 2.
Categorization of preferred roles, by vignette
| Current health condition vignette | Chest pain vignette | |||
|---|---|---|---|---|
| Preferred role | n | % | n | % |
| Passive | 177 | 29.7 | 296 | 50.1 |
| Shared | 401 | 67.3 | 288 | 48.7 |
| Leaning passive | 142 | 23.8 | 156 | 26.4 |
| Shared equally | 9 | 1.5 | 4 | 0.7 |
| Leaning autonomous | 35 | 5.9 | 17 | 2.9 |
| Divide and share | 215 | 36.1 | 111 | 18.8 |
| Autonomous | 17 | 2.9 | 7 | 1.2 |
| Leaning shared | 0 | 0 | 0 | 0 |
| Autonomous/consumerist | 17 | 2.9 | 7 | 1.2 |
| Theoretically implausible | 1 | 0.2 | 0 | 0 |
| Total | 596 | 100 | 591 | 100 |
For all other computations in this paper, the ‘theoretically implausible’ response was set to missing and excluded from further analysis.
There was a statistically significant difference in perceived knowledge about current health condition by perceived role (Table 3), with the autonomous seeing themselves as far more knowledgeable, and those wishing a passive role indicating less knowledge.
Table 3.
Mean knowledge score, by preferred role for current health condition vignette
| Mean knowledge score | Passive | Shared | Autonomous | Total | R 2 |
|---|---|---|---|---|---|
| Your current health condition | 2.9 | 4.2 | 5.0 | 3.8 | 0.24 |
| The available treatment options | 2.6 | 4.0 | 5.0 | 3.6 | 0.26 |
| The risks and benefits of these options | 2.8 | 3.8 | 4.9 | 3.4 | 0.27 |
| Which treatment you prefer | 2.3 | 3.8 | 4.9 | 3.8 | 0.24 |
All comparisons were statistically significant, using PROC GLM for analysis of variance, at P < 0.001.
Only 96 respondents (16.2%) had personally experienced the situation described in the chest pain vignette, although 52.2% had friends or families who had experienced it and 59.1% had read about it. On balance, 61.8% indicated that they did not know much about the situation in this scenario. The relationship between personal experience with the chest pain scenario and preferred role for that vignette was not statistically significant. However, there was a highly significant difference between respondents believing that they did not know much about the chest pain scenario, and preferred role (42.8% of the autonomous, 51.2% of the shared and 72.5% of the passive claimed to know little about this scenario).
Trust
Of the 601 respondents who completed the Trust‐in‐Physician Scale, 6.3% had blind trust, 36.1% had high trust, 48.6% moderate trust and 9.0% low trust. Sex, education and age were all significantly related to trust (Table 4); whether the current health condition had existed for at least 6 months or not. Although blind trust was not common, it was seen more frequently among female than male respondents, among those with less education, and those over 65 years of age. In contrast, it was never found among those with post‐secondary education, or under 35 years of age.
Table 4.
Relationship between trust category and sex, age and education
| Low (%) | Moderate (%) | High (%) | Blind (%) | Total [n(%)] | |
|---|---|---|---|---|---|
| Full sample | 9.0 | 48.6 | 36.1 | 6.3 | 601 (100) |
| By sex1 (n = 600) | |||||
| Male | 9.2 | 53.4 | 34.4 | 3.1 | 294 (100.1) |
| Female | 8.8 | 43.8 | 37.9 | 9.5 | 306 (100) |
| By age group2 (n = 600) | |||||
| <35 | 12.5 | 64.1 | 23.4 | 0 | 64 (100) |
| 35–49 | 9.1 | 54.5 | 31.8 | 4.5 | 132 (99.9) |
| 50–64 | 10.6 | 50.2 | 32.4 | 6.8 | 207 (100) |
| 65+ | 6.1 | 37.6 | 47.2 | 9.1 | 197 (100) |
| By education group3 (n = 599) | |||||
| Elementary school | 3.4 | 16.9 | 57.6 | 22 | 59 (99.9) |
| Some high school | 4.4 | 33 | 48.4 | 14.3 | 91 (100.1) |
| Graduated high school | 3.9 | 44.1 | 44.1 | 7.8 | 102 (99.9) |
| Some post‐secondary | 4.3 | 51.1 | 40.4 | 4.3 | 94 (100.1) |
| Graduated university | 13.6 | 61.4 | 25 | 0 | 176 (100) |
| Some graduate training | 20.8 | 63.6 | 15.6 | 0 | 77 (100) |
Chi‐square values were statistically significant for 1trust and sex (P < 0.005); 2trust and age group (P < 0.0003); 3trust and education group (P < 0.0001).
Trust and preferred role were significantly associated (P < 0.0001) for both the current health condition and chest pain vignettes. This relationship exists in both directions. As shown in 2, 3, those with blind trust overwhelmingly tend to prefer a passive role (97.3% for chest pain, 81.1% for current health condition vignettes). Preference for a passive role declines as trust diminishes, with moderately high rates for those with high trust (67.0% passive for chest pain, 44.7% for current health condition), and lower rates for those with moderate trust (35.4% chest pain, 14.5% current health), and those with low trust (26.4% chest pain, 14.8% current health). Preference for a shared role follows the reverse pattern. The few wishing an autonomous/consumerist role also show low levels of trust.
Figure 2.
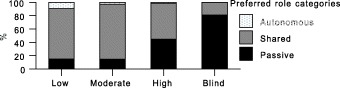
Preferred role by Trust: current health vignette.
Figure 3.
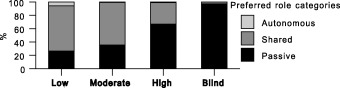
Preferred role by Trust: chest pain vignette.
Similarly, as shown in 4, 5, the majority of respondents wishing a passive role had high or blind trust, whereas for those classified as autonomous, the majority had moderate to low trust. The results do not show causality, but imply that desire for an autonomous role may be related to a sense among these individuals that providers cannot be trusted to perform the problem‐solving tasks properly.
Figure 4.
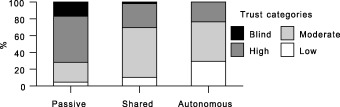
Trust by preferred role: current health vignette.
Figure 5.
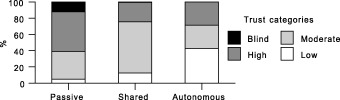
Trust by preferred role: chest pain vignette.
Analysis of variance using the SAS GLM procedure reveals that the significant relationship between trust and preferred role persists after controlling for the demographic factors of sex, age and education (data not shown). There was also some minor variation across clinics (data not shown). However, clinic may be in part a proxy for sex (100% males were from the prostate clinic, 0% of the breast clinic, and 46.5% of respondents from the fracture clinic).
Discussion
Participation in DM encompasses several dimensions. The concept of preferred role employed in this study differentiated between a desire to be involved in problem‐solving tasks, and a desire to be involved in making treatment decisions. Although most of our respondents rejected a purely passive role, they also showed little desire to take full charge. Recognizing that we asked about hypothetical preferences rather than the results of their actual clinical encounters, it is noteworthy how very few respondents preferred an autonomous/consumerist role in DM. Instead, we found a high preference for taking a shared approach. This preference for a shared role is higher for the current health condition vignette than for the chest pain scenario. One interpretation of this difference is that increased knowledge about a health condition is likely to shift preferences from passive to shared. Further indirect support for this contention arises from the increased willingness to participate in the hypothetical chest pain vignette among those who felt more knowledgeable, although the direction of causality for this relationship clearly cannot be established from a survey‐based study.
Our results do not support the more extreme models of consumer autonomy. We found that most respondents in this study trusted their provider; less than 10% of the sample indicated low levels of trust. However, this did not lead to passivity; most also wished to participate in making decisions about their current health condition. Although desire for a passive role was significantly associated with higher levels of trust, and desire for an autonomous role with low levels, those wishing a shared role also exhibited high (but not blind) trust levels. Shared DM often accompanied a trusting patient–physician relationship. Analysis of the distinction between PS and DM elements of treatment choice thus suggests that trust may be an essential component of preferred role. Our respondents overwhelmingly wished to hand over PS to physicians. Underlying this preference, however, are assumptions about the ability of providers to carry out these activities well, both in the technical sense of properly assimilating and interpreting data, and in the interpersonal one of acting in the best interests of their patients and clearly communicating findings and options. Such a relationship is opposed to the caveat emptor model of purchaser and provider. It is noteworthy that expertise, caring and communication are key components of how interpersonal trust in physicians has been defined in the literature. Our results thus suggest that, far from being a barrier to patient autonomy, warranted trust may indeed be an integral element of the physician–patient relationship.
Acknowledgements
The study was funded by the Social Sciences and Humanities Research Council of Canada, grant 410970299. An earlier version of this research formed the PhD thesis of Natasha Sharpe. We appreciate the assistance of Ann Pendleton with data entry and analysis, and of Cathy Bezic for secretarial assistance.
References
- 1. President's Commission . President's Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. Making Health Care Decisions: The Ethical and Legal Implications of Informed Consent in the Patient‐Practitioner Relationship, Vol. 1. Washington, DC: Government Printing Office, 1982. [Google Scholar]
- 2. Katz J. The Silent World of Doctor and Patient. New York: Free Press, 1984. [Google Scholar]
- 3. Beauchamp TL, Childress JF. Principles of Biomedical Ethics. New York, Oxford: Oxford University Press, 1989. [Google Scholar]
- 4. Kassirer JP. Incorporating patients’ preferences into medical decisions. New England Journal of Medicine, 1994; 330: 1895–1896. [DOI] [PubMed] [Google Scholar]
- 5. Pellegrino ED. Patient and physician autonomy: conflicting rights and obligations in the physician–patient relationship. Journal of Contemporary Health Law and Policy, 1994; 10: 47–68. [PubMed] [Google Scholar]
- 6. Coulter A. Paternalism or partnership? British Medical Journal, 1999; 319: 719–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Department of Health . Choice, Responsiveness and Equity. http://www.doh.gov.uk/choiceconsultation/research.htm (accessed October 2003). [Google Scholar]
- 8. Silverman DR. Narrowing the gap between rhetoric and the reality of medical ethics. Academic Medicine, 1996; 71: 227–237. [DOI] [PubMed] [Google Scholar]
- 9. Ende J, Kazis L, Ash AB, Moskowitz MA. Measuring patients’ desire for autonomy: decision making and information‐seeking preferences among medical patients. Journal of General Internal Medicine, 1989; 4: 23–30. [DOI] [PubMed] [Google Scholar]
- 10. Beisecker AE, Beisecker TD. Patient information‐seeking behaviors when communicating with doctors. Medical Care, 1990; 28: 19–28. [DOI] [PubMed] [Google Scholar]
- 11. Guadagnoli E, Ward P. Patient participation in decision making. Social Science and Medicine, 1998; 47: 329–339. [DOI] [PubMed] [Google Scholar]
- 12. Deber RB. The patient‐physician partnership: changing roles, and the desire for information. Canadian Medical Association Journal, 1994; 151: 171–176. [PMC free article] [PubMed] [Google Scholar]
- 13. Degner LF, Kristjanson LJ, Bowman D et al. Information needs and decisional preferences in women with breast cancer. Journal of the American Medical Association, 1997; 277: 1485–1492. [PubMed] [Google Scholar]
- 14. Kenny P, Quine S, Shiell A, Cameron S. Participation in treatment decision‐making by women with early stage breast cancer. Health Expectations, 1999; 2: 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Degner LF, Sloan JA. Decision making during serious illness: what role do patients really want to play? Journal of Clinical Epidemiology, 1992; 45: 941–949. [DOI] [PubMed] [Google Scholar]
- 16. Stiggelbout AM, Kiebert GM. A role for the sick role: patient preferences regarding information and participation in clinical decision making. Canadian Medical Association Journal, 1997; 157: 383–389. [PMC free article] [PubMed] [Google Scholar]
- 17. Brady TJ. The patient's role in rheumatology care. Current Opinion in Rheumatology, 1998; 10: 146–151. [DOI] [PubMed] [Google Scholar]
- 18. Entwistle VA, Skea ZC, O'Donnell MT. Decision about treatment: interpretations of two measures of control by women having a hysterectomy. Social Science and Medicine, 2001; 53: 721–732. [DOI] [PubMed] [Google Scholar]
- 19. Beaver K, Bogg J, Luker KA. Decision‐making role preferences and information needs: a comparison of colorectal and breast cancer. Health Expectations, 1999; 2: 266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caress A‐L, Luker K, Woodcock A, Beaver K. A qualitative exploration of treatment decision‐making role preference in adult asthma patients. Health Expectations, 2002; 5: 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Entwistle VA, Buchan H, Coulter A, Jadad A. Towards constructive innovation and rigorous evaluation: a new series on methods for promoting and evaluating participation. Health Expectations, 1999; 2: 75–77 (editorial). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Entwistle VA. Supporting and resourcing treatment decision‐making: some policy considerations. Health Expectations, 2000; 3: 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coutler A. Whatever happened to shared decision‐making? Health Expectations, 2002; 5: 185–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roter D. The medical visit context of treatment decision‐making and the therapeutic relationship. Health Expectations, 2000; 3: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keating NL, Green DC, Kao A, Gazmararian JA, Wu VY, Cleary PD. How are patients’ specific ambulatory care experiences related to trust, satisfaction, and considering changing physicians? Journal of General Internal Medicine, 2002; 17: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Englehardt H. The Foundations of Bioethics, 2nd edn. New York: Oxford University Press, 1996. [Google Scholar]
- 27. Bayertz K, ed. Sanctity of Life and Human Dignity. Boston: Kluwer Academic Press, 1996. [Google Scholar]
- 28. Keyes W. Life, Death, and the Law: A Sourcebook on Autonomy and Responsibility in Medical Ethics. Springfield, IL: Thomas, 1995. [Google Scholar]
- 29. Hall MA, Dugan E, Zheng B, Mishra AK. Trust in physicians and medical institutions: what is it, can it be measured, and does it matter. The Milbank Quarterly, 2001; 79: 613–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pearson SD, Raeke LH. Patients’ trust in physicians: many theories, few measures, and little data. Journal of General Internal Medicine, 2000; 15: 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cassell E. Teaching the fundamentals of primary care. The Milbank Quarterly, 1995; 73: 373–405. [PubMed] [Google Scholar]
- 32. Brody H. The Healer's Power. New Haven: Yale University Press, 1992. [Google Scholar]
- 33. Brown RF, Butow PN, Henman M, Dunn SM, Boyle F, Tattersall MHN. Responding to the active and passive patient: flexibility is the key. Health Expectations, 2002; 5: 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Deber RB, Baumann AO. Clinical reasoning in medicine and nursing: decision making versus problem solving. Teaching and Learning in Medicine, 1992; 4: 140–146. [Google Scholar]
- 35. Kraetschmer N. Preferences of patients undergoing angiogram for participation in treatment decisions: coping style and the Problem Solving‐Decision Making Scale. Master of Science thesis, Graduate Department of Community Health, University of Toronto, Toronto, ON, 1994. [Google Scholar]
- 36. Deber RB, Kraetschmer N, Irvine J. What role do patients wish to play in treatment decision making? Archives of Internal Medicine, 1996; 156: 1414–1420. [PubMed] [Google Scholar]
- 37. Sharpe N. The impact of trust on roles patients wish to play in making medical decisions. Doctor of Philosophy thesis, Graduate Department of Community Health, University of Toronto, Toronto, ON, 1997. [Google Scholar]
- 38. Cassileth BR, Zupkis RV, Sutton‐Smith K, March V. Information and participation preferences among cancer patients. Annals of Internal Medicine, 1980; 92: 832–836. [DOI] [PubMed] [Google Scholar]
- 39. Strull WM, Lo B, Charles G. Do patients want to participate in medical decision making? Journal of the American Medical Association, 1984; 252: 2990–2994. [PubMed] [Google Scholar]
- 40. Weeks JC. Preferences of older cancer patients: can you judge a book by its cover? Journal of the National Cancer Institute, 1994; 86: 1743–1744. [DOI] [PubMed] [Google Scholar]
- 41. Siminoff LA, Fetting JH. Factors affecting treatment decisions for a life‐threatening illness: the case of medical treatment of breast cancer. Social Science and Medicine, 1991; 32: 813–818. [DOI] [PubMed] [Google Scholar]
- 42. Wartman SA, Morlock LL, Malitz FE, Palm EA. Patient understanding and satisfaction as predictors of compliance. Medical Care, 1983; 21: 886–891. [DOI] [PubMed] [Google Scholar]
- 43. Cassileth B, Zupkis R, Sutton‐Smith K, March V. Information and participation preferences among cancer patients. Annals of Internal Medicine, 1988; 92: 832–836. [DOI] [PubMed] [Google Scholar]
- 44. Greenfield S, Kaplan SH, Ware JE. Jr. Expanding patient involvement in care: effects on patient outcomes. Annals of Internal Medicine, 1985; 102: 520–528. [DOI] [PubMed] [Google Scholar]
- 45. Anderson LA, Dedrick RF. Development of the trust in physician scale: a measure to assess interpersonal trust in patient–physician relationships. Psychological Reports, 1990; 67: 1091–1100. [DOI] [PubMed] [Google Scholar]
- 46. Thom DH, Ribisl KM, Stewart AL, Luke DA, Stanford Trust Study Physicians . Further validation and reliability testing of the Trust in Physician Scale. Medical Care, 1999; 37: 510–517. [DOI] [PubMed] [Google Scholar]
- 47. Freeburger JK, Callahan LF, Currey SS, Anderson LA. Use of the Trust in Physician scale in patients with rheumatic disease: psychometric properties and correlates of trust in the rheumatologist. Arthritis and Rheumatism, 2003; 49: 51–58. [DOI] [PubMed] [Google Scholar]
- 48. Thom DH, Stanford Trust Study Physicians . Physician behaviors that predict patient trust. Journal of Family Practice, 2001; 50: 323–328. [PubMed] [Google Scholar]


