Abstract
Background Colorectal cancer screening guidelines in the United States recommend that decisions about screening should incorporate patient preferences, but little is known about how patients make the trade‐offs inherent in choosing one of the five currently recommended screening programmes.
Study population Forty‐eight primary care patients at average risk for colorectal cancer who completed an experimental shared decision‐making intervention based on a multicriteria decision analysis.
Methods Descriptive analysis of priorities assigned to decision criteria describing the advantages and disadvantages of the five currently recommended colorectal screening programmes in the United States. Criteria were divided into four major criteria – avoid cancer, avoid screening side‐effects, avoid false positive test results and the combined importance of other considerations – and three subcriteria: the number of screening tests, test preparation and the test itself. Cluster analysis was used to identify common combinations of priorities within each set of criteria.
Results Patients assigned widely variable priorities to both the criteria and subcriteria: the average range of priorities was 46 on a 100 point priority scale. Cluster analysis identified six different combinations of priorities for the major criteria and four for the subcriteria. The differences in priorities assigned to both the criteria and subcriteria in the clusters were statistically significant with P < 0.0001.
Conclusions Even within a small group of patients, preferences vary widely regarding trade‐offs involved in choosing among the currently recommended colorectal cancer screening programmes in the United States. These results provide empiric support for recommendations to utilize a shared decision‐making process when making colorectal cancer screening decisions and highlight the need for additional research into how average risk patients view the trade‐offs inherent in choosing a colorectal cancer screening programme.
Keywords: colorectal neoplasms prevention and control, decision support techniques, practice guidelines, shared decision making
Introduction
Colorectal cancer is the fourth most common cancer worldwide and the second most common in developed countries. 1 The large burden of illness associated with colorectal cancer, along with recent studies showing that routine screening in average risk populations can reduce colorectal cancer morbidity and mortality, have resulted in calls for routine colorectal cancer screening in many developed countries. 2 , 3 , 4 , 5 , 6 In the United States, colorectal cancer prevention is a national health care priority. 7
Current colorectal cancer prevention efforts in the United States are based on screening, but there is no consensus regarding the best screening method to use. As there is no clearly superior screening strategy when all factors that could affect screening decisions are considered, current guidelines endorse several options and recommend that clinical decisions about screening be made through a shared decision‐making process that actively involves patients and incorporates their individual values and preferences. 8 , 9 , 10 , 11 Adherence to these guidelines therefore involves making a decision about the relative advantages and disadvantages of the recommended alternatives. 12
This recommendation is consistent with ethical principles for screening healthy populations that define a good decision as one that appropriately reflects the preferences and values of the patient involved. 13 , 14 , 15 , 16 Implementing shared decision making in busy practice settings, however, is hard to do. It is especially difficult in complex situations, like colorectal cancer screening, that involve making trade‐offs between the advantages and disadvantages of several alternatives over multiple considerations.
A better understanding of how patients view the trade‐offs inherent in choosing a colorectal cancer screening programme would help to clarify the practical implications of adopting a shared decision‐making approach to screening decisions and be useful in the creation of new guidelines in the United States and elsewhere. This information could also facilitate shared decision making in busy practice settings by contributing to the development more effective ways of assessing patient preferences and incorporating them into the clinical decision‐making process.
Currently, however, little is known about how patients view the trade‐offs involved in colorectal cancer screening decisions. In an earlier study, Ling et al. 17 asked patients to identify their preferred screening test and the test characteristic that most influenced their preference but did not examine how the patients made the trade‐offs among the different test characteristics. Other previous studies of patient preferences have either asked patients to choose a preferred screening programme without exploring the reasons behind their choice 18 , 19 , 20 or examined only a limited number of the trade‐offs about a single screening test (faecal occult blood testing). 21 The goal of this study was to address this issue by examining how a group of patients established priorities when making trade‐offs between the advantages and disadvantages of the five currently recommended colorectal cancer screening programmes in the United States: annual faecal occult blood tests, flexible sigmoidoscopy every 5 years, combined annual faecal occult blood tests and flexible sigmoidoscopy every 5 years, double contrast barium enema every 5 years, and colonoscopy every 10 years.
Methods
Study population
The study population consisted of 48 patients who, as members of the experimental group in a randomized‐controlled trial of a colorectal cancer screening decision aid, assessed the relative priorities of factors influencing the selection of a colorectal cancer screening programme. The full details of this study have been reported. 22 Pertinent details are summarized below.
The study sample was obtained from a consecutive series of patients being seen for routine appointments at two primary care Internal Medicine practices in Rochester, New York. Patients were included if they were at average risk of colorectal cancer (no personal or family history of colorectal cancer, adenomatous polyps or inflammatory bowel disease), were 49 years old, had normal mental status, understood English, were not too physically ill to participate, were willing, and were due for a colorectal cancer screening test (i.e. according to available patient records and direct patient report had not had faecal occult blood testing during the previous 11 months, a barium enema or flexible sigmoidoscopy during the past 5 years, or a colonoscopy during the past 10 years).
The analytic hierarchy process
The decision aid was based on the analytic hierarchy process (AHP), one of the most frequently used multicriteria decision analysis methods. Multicriteria decision analysis is the term used to describe a group of techniques that have been developed to make it easier for people to make consistently good decisions in situations that involve trade‐offs among the advantages and disadvantages of several options. 23 The AHP has a number of advantages over other multicriteria techniques including a firm theoretic basis, flexibility, relative ease of use, and a built‐in check on the consistency of the judgments made during the course of an analysis. These advantages have led to widespread use of the AHP in many practical applications. 24 , 25 , 26 , 27 , 28 It is well suited for decisions regarding colorectal cancer screening and other similar situations because it was specifically designed for decisions involving multiple decision makers that require the integration of hard and soft data, preferences and values. In these circumstances the AHP can help to facilitate shared decision making by providing a way to explicitly characterize and discuss the otherwise implicit preferences and opinions. The AHP has been successfully applied to a variety of medical decisions. 29 , 30 , 31 , 32 , 33 , 34 , 35
An AHP analysis starts with the creation of a conceptual representation of the decision, called the decision model, consisting of the goal, the alternatives and the considerations being used as criteria to judge how well the alternatives meet the goal. Comparisons are then made among the criteria to determine their relative priorities in meeting the goal and among the alternatives to determine their relative abilities to fulfil the criteria. The results are then combined to create a quantitative measure on a ratio scale that indicates how well each of the alternatives can be expected to meet the goal. To illustrate, the details of the AHP analysis used for this study are briefly described below. More complete descriptions of the AHP are available in the literature. 22 , 26 , 32 , 35 , 36 , 37
The study intervention
During the trial, patients met with a single study nurse at the practice site just before a scheduled doctor's appointment. After a brief introduction to colorectal cancer screening and a thorough description of the screening alternatives, they were asked to complete an AHP analysis of the screening decision based on guidelines and data published by a multidisciplinary expert panel in 1997. 38
The decision model used for the analysis is shown in Fig. 1. The goal was defined as ‘Choose the best approach to colorectal cancer screening’. The alternatives were the five recommended screening programmes discussed earlier and ‘wait and see’, an alternative representing no screening at the current time. All screening programmes, except colonoscopy every 10 years, included a follow‐up colonoscopy if the initial test was abnormal. The decision criteria, shown on the middle levels of the model, were based on differences among the screening options that were identified and discussed in the guidelines.
Figure 1.

The decision model. Flex Sig, flexible sigmoidoscopy; yrs, years.
Patients began their analyses by comparing the priorities of the criteria in meeting the goal. The criteria (called performance measures in the patient information) were defined as follows:
-
1
Avoid colorectal cancer. A good screening programme is one that increases your chances of not getting cancer; the better the programme, the higher are your chances of avoiding colorectal cancer in the future.
-
2
Avoid major side‐effects from screening tests. Side‐effects are health problems that are caused by medical treatment or diagnostic tests. This performance measure means that a cancer screening programme should be safe and not, in itself, be harmful to your health. Better programmes have higher chances of having no side‐effects. Side‐effects from screening tests vary in severity. At this point we have included only the two most serious side‐effects which are both big problems that require further evaluation and treatment in the hospital. The first is intestinal perforation, which is when one of the screening tests tears a hole in the intestine. This requires immediate surgery to patch the hole and can cause a serious infection in the abdomen. The second major side‐effect is bleeding that is serious enough to require a blood transfusion and observation in the hospital. Although most people recover, both of these side‐effects can be fatal.
-
3
Avoid false positive tests. A false positive is a screening test that is positive or abnormal (in this case indicating that a polyp or cancer is present) when there really is no polyp or cancer present. In other words a false alarm. A false positive test can cause a lot of anxiety. It also means that one has to go for additional testing that you really did not need.
-
4
Other considerations: the number of times you are screened if you follow the screening programme from now until you are 80, what you need to do to prepare for the tests and what the tests themselves are like.
After the criteria were defined, the patients were shown age‐adjusted estimates (in 5‐year increments) for the cumulative likelihood of cancer, side‐effects and false positives, and the cumulative number of screening tests associated with each screening programme through age 85, assuming they would be screened from their current age until age 80. The data estimates were derived from the screening simulation study that was included in the original guideline. 38 For example, a 55‐year‐old patient was shown estimated outcomes for 25 annual faecal occult blood tests, six flexible sigmoidoscopies and double contrast barium enemas (one every 5 years), and three colonoscopies (one every 10 years). The number of screening tests and the test preparation(s) and procedure(s) included in each screening programme were also described. Numeric data were shown as odds displayed in both data tables and bar graphs. A sample of the data table used for 55‐year‐old patients is shown in Table 1.
Table 1.
Outcome data used by 55‐year‐old patients
| Wait and see | Annual FOBT | Flexible sigmoidoscopy every 5 years | Annual FOBT and flexible sigmoidoscopy every 5 years | Barium enema every 5 years | Colonoscopy every 10 years | |
|---|---|---|---|---|---|---|
| Avoid colorectal cancer* | 19 : 1 | 34 : 1 | 31 : 1 | 49 : 1 | 62 : 1 | 60 : 1 |
| Avoid major screening test side‐effects* | Sure | 104 : 1 | 1146 : 1 | 99 : 1 | 255 : 1 | 76 : 1 |
| Avoid false positive screening test results* | Sure | No chance | 235 : 1 | No chance | 3 : 1 | 99 : 1 |
| Number of screening tests | 0 | 25 | 6 | 36 | 6 | 3 |
| Preparation† | None | Special diet for 2–3 days | Enema | Special diet for 2–3 days and enema | Complete bowel prep | Complete bowel prep |
| The procedure† | None | Stool collection | 30 min with short endoscope | Stool collection and short endoscope | Enema with X‐rays | Long endoscope |
‘Sure’, certainty that the criterion will be achieved, i.e. if no screening tests are done it is certain that there will be no screening test side effects and no false positive test results; ‘No chance’, certainty that criterion will not be met, i.e. at least one false positive result will occur, based on the simulation data; FOBT, faecal occult blood tests.
*Data shown are the cumulative odds through age 85 of avoiding cancer, side‐effects and false positive screening tests based on screening from age 55 to age 80 used in the original study.
†In the original study, the details of the test preparations and procedure were fully explained in addition to this summary.
The patients then compared the relative importance of the four ‘major’ criteria (avoid cancer, avoid side‐effects, avoid false positive screening tests and the other considerations) relative to the goal of choosing the best screening test. They were first asked if the two criteria being compared were equally important in choosing a colorectal cancer screening programme. If not, they were asked to identify the more important criterion and to indicate how much more important it was using the following scale: slightly, moderately, between moderately and strongly, strongly, between strongly and very strongly, very strongly, between very strongly and extremely, or extremely. Separate pairwise comparisons were made for every possible pair of criteria (avoid cancer vs. avoid side‐effects, avoid cancer vs. avoid false positives etc.), a total of six comparisons. After all the comparisons were completed, they were converted to a 1–9 scale and arranged to create a comparison matrix. A normalized ratio scale indicating the relative priority of each criterion was then derived by calculating the normalized right principle eigenvector of the matrix. This procedure is analogous to taking the average of all of the direct and indirect comparisons among the criteria that are contained in the matrix and adjusting the results so that the total sums to 1.
The patients then repeated this comparison process to determine the relative importance of the three subcriteria and to compare the abilities of the alternatives to fulfil the criteria and subcriteria using the age‐adjusted outcome estimates and other information described above.
The results of the comparisons were then combined to determine which screening option was most consistent with the patients’ preferences. This was performed by multiplying the scores indicating how well the alternatives fulfilled the criteria by the priorities assigned to the criteria and summing the results, a procedure that is analogous to calculating a weighted average. In the original study, these results were discussed with the patients immediately before a scheduled visit with their physician.
Analysis of criteria priorities
As noted above, the decision criteria were based on key differences among the recommended screening programmes. Analysis of the priorities assigned to the criteria, therefore, provides a way of obtaining insight into how the patients viewed the trade‐offs involved in choosing among them.
As multiple considerations are involved, it is important to understand how patients made both the individual trade‐offs and each group of related trade‐offs. For this reason, a two‐step analysis was performed consisting of a descriptive analysis of the priorities assigned to each criterion using descriptive statistics followed by a cluster analysis of the priorities assigned to the four major criteria and the three subcriteria.
The term cluster analysis refers to a group of techniques that use mathematical algorithms to uncover groups in data. The purpose of a cluster analysis is to create a classification scheme that promotes a better understanding of the similarities and differences among a group of subjects based on a series of experimental observations. The clustering method chosen for this study was the medoid partitioning procedure developed by Kaufman and Rousseeuw. This method is based on calculating the Euclidian distance between data points in n‐dimensional space and grouping them so as to maximize the differences between groups. The success of the classification in achieving this goal is measured by calculating the silhouette value, which ranges from −1 to 1. Guidelines for interpreting silhouette values suggest that values between −1 and 0.25 indicate that no substantial structure has been found, between 0.26 and 0.50 that a weak structure has been found, between 0.51 and 0.70 that a reasonable structure has been found, and between 0.71 and 1 that a strong structure has been found. The optimum number of clusters for a set of data is determined by creating classifications with different numbers of clusters and selecting the one with the highest average silhouette score. 39
Separate cluster analyses were performed for the four major criteria and the three subcriteria. The statistical significance of the resulting classifications was assessed by using anova to compare the mean values of the criteria in the different clusters. To adjust for multiple comparisons, statistical significance was defined as P < 0.01.
All statistical calculations were performed using NCSS. 39
Results
The mean age of the study population was 64.9 years and ranged from 50 to 81 years. There were 26 women and 22 men. Forty‐seven (98%) were white. Eight patients (17%) had not completed high school, 18 (37.5%) were high school graduates, 16 (33%) had completed some college courses, and six (12.5%) had graduated from a 4‐year college study.
The study intervention was well received by patients. The mean response to the question ‘Did you understand the interview’ on a five‐point Likert scale ranging from 5 (Yes, fully understood) to 1 (No, did not understand at all) was 4.72. The mean response on a similar scale ranging from 5 (strongly agree) to 1 (strongly disagree) was 4.85 to the question ‘Did you like the interview’ and 4.81 to the question ‘Doctors should use [this type of interview] routinely’.
The priorities assigned to the decision criteria are summarized in 2, 3. The means and ranges of the priorities were: avoid cancer 56% (26–72%), avoid side‐effects 24% (4–51%), avoid false positives 10% (4–31%), other considerations 10% (3–31%), frequency 28% (6–72%), preparation 19% (5–62%), and test procedure 54% (9–80%).
Figure 2.
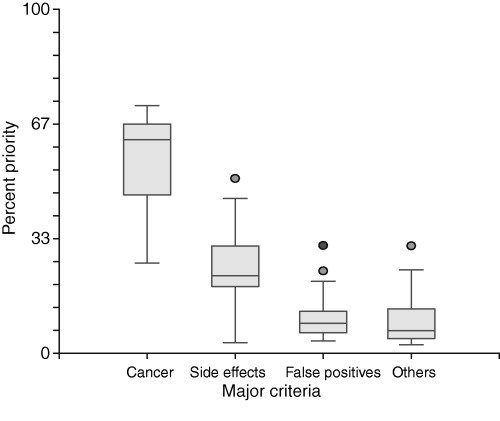
Box plot showing the priorities assigned by patients to the four major criteria. The horizontal line in the shaded box indicates the median. The box itself indicates the 25th to 75th percentiles. The vertical lines extending from the box indicate the 95% range. Priorities outside this range are indicated with the round dots.
Figure 3.
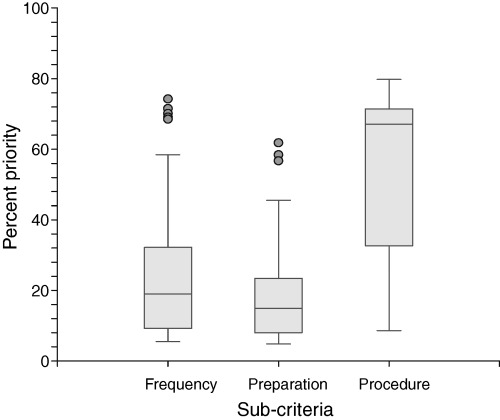
Box plot showing the priorities assigned by patients to the three subcriteria. The horizontal line in the shaded box indicates the median. The box itself indicates the 25th to 75th percentiles. The vertical lines extending from the box indicate the 95% range. Priorities outside this range are indicated with the round dots.
The best cluster analysis classification of the priorities assigned by patients to the major criteria had six clusters containing 7, 12, 14, 4, 2 and 9 patients and a silhouette value of 0.36. The best classification for priorities assigned to the subcriteria had four clusters containing 5, 11, 21 and 11 patients and a silhouette value of 0.59. These results are summarized in 4, 5.
Figure 4.
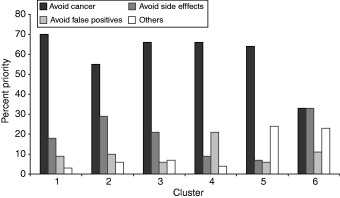
The mean priority assigned to the four major criteria in each of the six decision clusters. The total of criteria priorities in any single cluster equals 100%.
Figure 5.
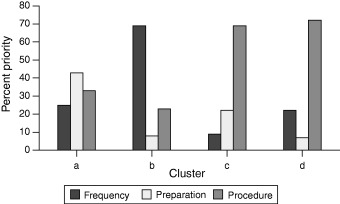
The mean priority assigned to the three other criteria in each of the four decision clusters. The total of criteria priorities in any single cluster equals 100%.
As shown in Fig. 4, when assigning priorities among the major criteria, all patients considered avoiding cancer a high priority. It has the highest priority in five clusters and in the sixth it was tied with avoiding side‐effects for most important criterion. There was, however, variation among the clusters in both the relative priorities of the criteria and their rank order. Clusters 1 and 2 have the same rank order – avoid cancer, avoid side‐effects, avoid false positives and others – but differ by a factor of 2 in the relative importance of avoid cancer vs. avoid side‐effects: in cluster 1 avoiding cancer is six times more important than avoiding side‐effects, whereas in cluster 2 it is only three times more important. Avoiding cancer is the dominant criterion in clusters 3, 4 and 5 but the importance of the other three criteria vary. Cluster 3 is similar to cluster 1 except that the combined importance of the other considerations is more important than avoiding false positives. In clusters 4 and 5, instead of avoid side‐effects, avoiding false positives and the combined importance of the other considerations are the second most important criteria respectively.
The four clusters describing different combinations of priorities assigned to the subcriteria are shown in Fig. 5. In each, the rank ordering of the criteria and their relative priorities are different. In cluster a, preparation was considered most important followed by the procedure and screening frequency. The priorities of the three subcriteria, however, are fairly similar. In contrast, the other clusters have a dominant and two relatively minor subcriteria. In cluster b, the dominant subcriterion is screening frequency, followed by the procedure and test preparation. In cluster c, procedure is the dominant subcriterion, followed by test preparation and screening frequency. In cluster d, procedure is again the dominant subcriterion, but this time screening frequency is second and test preparation least important.
For both the major criteria and the subcriteria, anova shows that the differences in mean priorities assigned to the criteria and subcriteria in the different clusters were highly statistically significant with F‐ratios ranging from 24.46 to 210.48 and all P values <0.0001. These results are summarized in Table 2.
Table 2.
Analysis of variance results, cluster mean priorities
| Criterion | Mean square between clusters | Mean square within clusters | F‐ratio* | P‐value |
|---|---|---|---|---|
| Major criteria | ||||
| Avoid cancer | 0.1600 | 0.0024 | 65.89 | <0.0001 |
| Avoid side‐effects | 0.0760 | 0.0028 | 27.62 | <0.0001 |
| Avoid false positives | 0.0230 | 0.0009 | 25.80 | <0.0001 |
| Other considerations | 0.0340 | 0.0014 | 24.46 | <0.0001 |
| Subcriteria | ||||
| Frequency | 0.7230 | 0.0034 | 210.48 | <0.0001 |
| Preparation | 0.2660 | 0.0048 | 55.85 | <0.0001 |
| Procedure | 0.6710 | 0.0058 | 114.73 | <0.0001 |
*Differences from mean squares shown are due to rounding.
Discussion
Increasing emphasis on explicit, evidence‐based guidelines has led to a greater awareness that, in many cases, no single approach to patient management is clearly superior when all pertinent objectives are considered. In these situations, patient management decisions depend on judgments comparing the advantages and disadvantages of the alternative management strategies. Traditionally, trade‐offs between the risks and benefits of management options have been made by the people developing clinical practice guidelines. Recently, however, it has become increasingly recognized that these judgments should reflect the preferences of patients whose lives will be affected by the decision. 13 , 40 For this reason, guideline developers have started to identify sets of acceptable management alternatives and recommend that patients be actively involved in the process of selecting which alternative is most appropriate for them. 15
The results of this study provide evidence to support the more theoretical considerations that led to this recommendation. Despite a relatively small number of patients, both individual preferences and preference patterns regarding issues that influence the choice of a colorectal cancer screening programme varied widely. These findings emphasize the importance of adopting a shared decision‐making approach when selecting a colorectal screening programme for individual patients.
They also suggest that routine assessment of patient preferences regarding the trade‐offs involved in picking a screening programme is an important component of shared decision making in this situation. While several tools are currently available to help patients understand the key differences among the currently recommended screening tests, 41 , 42 we do not yet have valid and reliable methods for assessing patient priorities and integrating them into clinical decisions about screening that are feasible for routine use. The results of the cluster analysis suggest that, although patient preferences vary, it may be possible to group them into a relatively small number of common preference patterns that could contribute to the development of rapid methods of assessing decision priorities in busy practice settings.
The identification of patient priority patterns could also help future guideline developers to determine which screening options should be recommended. Comparison of the advantages and disadvantages of potential options against a panel of common patient preference patterns could help determine if an option should be recommended for general consideration, only for certain sets of priorities, or not at all. A major advantage of this type of analysis is that it would help avoid omitting a screening programme from practice guidelines that is the ideal choice for groups of patients whose preferences differ from the majority point of view, such as those represented by cluster 6.
The approach taken in this study differs from previous studies that have asked patients to select a preferred colorectal cancer screening test after reviewing information about several options. Three recent studies using this format also found that patient preferences vary. 18 , 19 , 20 However, none collected data about the reasons why patients preferred one test over the others.
At least two studies have assessed factors that influence patient preferences for colorectal cancer screening options. Ling et al. 17 used a decision aid to provide information to the patients about the five recommended screening tests and was then asked to rank order them in terms of preference and also rate the importance of various test features in establishing their preferences. They found variation among patients in their preferred screening test although the majority preferred either annual faecal occult blood testing (43%) or colonoscopy every 10 years (40%). There was also variability in the test features patients identified as most important in establishing their preferences. The most important test feature was test accuracy, identified as the most important factor by 54% of the patients. However, six other features – frequency of testing, discomfort, complications, inconvenience, time and need for further testing – were rated most important by at least 3% of the respondents. The results of this study are consistent with those of Ling et al. and provide new information about how patients view the relative importance of factors that influence choices among these five colorectal cancer screening tests.
In the other study, Salkeld et al. 21 used discrete choice modelling to assess patient preferences for trade‐offs between the benefits (measured as the number of colorectal cancer deaths prevented), harms (measured as the number of unnecessary colonoscopies), and different test result notification policies associated with two different faecal occult blood tests. The study intervention was limited to these three considerations to reduce the number of comparisons needed to complete the analysis. About two‐thirds of their sample was willing to make trade‐offs between the benefits and harms and, as a group, indicated willingness to accept up to 853 colonoscopies per 10 000 patients screened biennially for every colorectal cancer death prevented. Because of the differences in methods used, it is difficult to directly compare the results of this study with those of Salkeld et al. It is worth noting, however, that multicriteria decision‐making methods like the AHP are not as severely limited as discrete choice methods in the number of considerations that can be included in an analysis. 43 This allowed us to incorporate both more considerations and more alternatives in our study and provide information that is more directly applicable to screening decisions now being faced by patients in the United States.
This study has several limitations that may affect its validity and generalizability. The first is that patient preferences were obtained from a relatively small number of patients in a single practice setting. Therefore, the patient preference clusters identified in this sample may not capture the full spectrum of patient views or be the same as if they were derived from larger numbers of patients seen in a variety of settings. For these reasons the patient preference clusters identified in this study must be considered preliminary. The extent of variation observed in this small sample, however, suggests that substantial variation exists among average risk patients and that further investigation is warranted.
The second limitation is that the analysis was restricted to the decision criteria that were contained in the original model, which was based on differences among the screening alternatives that were identified in the screening guidelines current at the time of the study. Including additional criteria, however, is likely to increase the amount of variation found. The amount of variation found within this small sample indicates a need for additional research to define the considerations that should affect colorectal cancer screening decisions.
A third limitation is that, because of the constraints of the original study design, the reproducibility of decision priorities over time was not assessed. The stability of patient preferences for criteria affecting screening and other preventive interventions over time is another important area for future research.
In conclusion, the results of this study indicate that patient priorities for key trade‐offs involved in colorectal cancer screening decisions posed by current US guidelines vary widely. These findings support guideline recommendations that a shared decision‐making approach be used to select the most appropriate screening programme for each patient based on their preferences and values. They also emphasize the need for additional research to define criteria that should be included in clinical decisions regarding colorectal cancer screening for average risk patients, to examine how patients make trade‐offs among them, and to develop clinically feasible methods for eliciting patient preferences and facilitating shared decision making in busy clinical settings.
Acknowledgements
This project was supported in part by grant number R03 HS10728 from the Agency for Healthcare Research and Quality.
Presented in part at the 23rd annual meeting of the Society for Medical Decision Making, September 2000
References
- 1. Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. European Journal of Cancer, 2001. (Suppl. 8); 37: S4–S66. [DOI] [PubMed] [Google Scholar]
- 2. Pignone M, Rich M, Teutsch S, Berg A, Lohr K. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine, 2002; 137: 132–141. [DOI] [PubMed] [Google Scholar]
- 3. Walsh JM, Terdiman JP. Colorectal cancer screening: scientific review. Journal of the American Medical Association, 2003; 289: 1288–1296. [DOI] [PubMed] [Google Scholar]
- 4. Walsh JM, Terdiman JP. Colorectal cancer screening: clinical applications. Journal of the American Medical Association, 2003; 289: 1297–1302. [DOI] [PubMed] [Google Scholar]
- 5. Young GP, St John DJ, Winawer SJ, Rozen P. Choice of fecal occult blood tests for colorectal cancer screening: recommendations based on performance characteristics in population studies: a WHO (World Health Organization) and OMED (World Organization for Digestive Endoscopy) report. American Journal of Gastroenterology, 2002; 97: 2499–2507. [DOI] [PubMed] [Google Scholar]
- 6. Coebergh JW. Colorectal cancer screening in Europe: first things first. European Journal of Cancer, 2004; 40: 638–642. [DOI] [PubMed] [Google Scholar]
- 7. Adams K, Corrigan J (eds) Priority Areas for National Action: Transforming Health Care Quality. Washington DC: The National Academies Press, 2003. [PubMed] [Google Scholar]
- 8. Woolf S. The best screening test for colorectal cancer – a personal choice. New England Journal of Medicine, 2000; 343: 1641–1643. [DOI] [PubMed] [Google Scholar]
- 9. Walsh J, Terdiman J. Colorectal cancer screening: clinical applications. Journal of the American Medical Association, 2003; 289: 1297–1302. [DOI] [PubMed] [Google Scholar]
- 10. Smith RA, Cokkinides V, Eyre HJ. American Cancer Society Guidelines for the Early Detection of Cancer, 2005. CA A Cancer Journal for Clinicians, 2005; 55: 31–44. [DOI] [PubMed] [Google Scholar]
- 11. Winawer S, Fletcher R, Rex D et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale – update based on new evidence. Gastroenterology, 2003; 124: 544–560. [DOI] [PubMed] [Google Scholar]
- 12. Deber R, Kraetschmer N, Irvine J. What role do patients wish to play in treatment decision making? Archives of Internal Medicine, 1996; 156: 1414–1420. [PubMed] [Google Scholar]
- 13. Forrow L, Wartman S, Brock D. Science, ethics, and the making of clinical decisions. Implications for risk factor intervention. Journal of the American Medical Association, 1988; 259: 3161–3167. [PubMed] [Google Scholar]
- 14. Lee J. Screening and informed consent. New England Journal of Medicine, 1993; 328: 438–440. [DOI] [PubMed] [Google Scholar]
- 15. Sheridan S, Harris R, Woolf S. Shared decisionmaking about screening and chemoprevention. American Journal of Preventive Medicine, 2004; 26: 67–80. [DOI] [PubMed] [Google Scholar]
- 16. Walter L, Covinsky K. Cancer screening in elderly patients. A framework for individualized decision making. Journal of the American Medical Association, 2001; 285: 2750–2756. [DOI] [PubMed] [Google Scholar]
- 17. Ling BS, Moskowitz MA, Wachs D, Pearson B, Schroy PC. Attitudes toward colorectal cancer screening tests. Journal of General Internal Medicine, 2001; 16: 822–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leard L, Savides T, Ganiats T. Patient preferences for colorectal cancer screening. Journal of Family Practice, 1997; 45: 211–218. [PubMed] [Google Scholar]
- 19. Pignone M, Bucholtz D, Harris R. Patient preferences for colon cancer screening. Journal of General Internal Medicine, 1999; 14: 432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sheikh RA, Kapre S, Calof OM, Ward C, Raina A. Screening preferences for colorectal cancer: a patient demographic study. Southern Medical Journal, 2004; 97: 224–230. [DOI] [PubMed] [Google Scholar]
- 21. Salkeld G, Solomon M, Short L, Ryan M, Ward JE. Evidence‐based consumer choice: a case study in colorectal cancer screening. Australian and New Zealand Journal of Public Health, 2003; 27: 449–455. [DOI] [PubMed] [Google Scholar]
- 22. Dolan J, Frisina S. Randomized controlled trial of a patient decision aid for colorectal cancer screening. Medical Decision Making, 2002; 22: 125–139. [DOI] [PubMed] [Google Scholar]
- 23. Belton V, Stewart T. Multiple Criteria Decision Analysis. Boston, Dordrecht, London: Kluwer Academic Publishers, 2002. [Google Scholar]
- 24. Golden B, Wasil E, Harker P. The Analytic Hierarchy Process. Applications and Studies. Berlin: Springer‐Verlag, 1989. [Google Scholar]
- 25. Zahedi F. The analytic hierarchy process – a survey of the method and its applications. Interfaces, 1986; 16: 96–108. [Google Scholar]
- 26. Saaty T. How to make a decision: the analytic hierarchy process. Interfaces, 1994; 24: 19–43. [Google Scholar]
- 27. Baker D, Bridges D, Hunter R et al. Guidebook to Decision‐making Methods. United States Department of Energy, 2001. . Available at http://emi‐web.inel.gov/Nissmg/guidebook_2002.pdf (Accessed on : 10 October 2005). [Google Scholar]
- 28. Vaidya OS, Kumar S. Analytic hierarchy process: an overview of applications. European Journal of Operational Research, 2005. (in press). [Google Scholar]
- 29. Peralta‐Carcelen M, Fargason CA Jr, Coston D, Dolan JG. Preferences of pregnant women and physicians for 2 strategies for prevention of early‐onset group B streptococcal sepsis in neonates. Archives of Pediatric and Adolescent Medicine, 1997; 151: 712–718. [DOI] [PubMed] [Google Scholar]
- 30. Carter K, Ritchey N, Castro F, Caccamo L, Kessler E, Erickson B. Analysis of three decision‐making methods: a breast cancer patient as a model. Medical Decision Making, 1999; 19: 49–57. [DOI] [PubMed] [Google Scholar]
- 31. Dolan JG, Bordley DR. Isoniazid prophylaxis: the importance of individual values. Medical Decision Making, 1994; 14: 1–8. [DOI] [PubMed] [Google Scholar]
- 32. Dolan JG, Bordley DR. Involving patients in complex decisions about their care: an approach using the analytic hierarchy process. Journal of General Internal Medicine, 1993; 8: 204–209. [DOI] [PubMed] [Google Scholar]
- 33. Dolan JG, Bordley DR, Miller H. Diagnostic strategies in the management of acute upper gastrointestinal bleeding: patient and physician preferences. Journal of General Internal Medicine, 1993; 8: 525–529. [DOI] [PubMed] [Google Scholar]
- 34. Dolan JG. Can decision analysis adequately represent clinical problems? Journal of Clinical Epidemiology, 1990; 43: 277–284. [DOI] [PubMed] [Google Scholar]
- 35. Dolan JG. Medical decision making using the analytic hierarchy process: choice of initial antimicrobial therapy for acute pyelonephritis. Medical Decision Making, 1989; 9: 51–56. [DOI] [PubMed] [Google Scholar]
- 36. Dolan JG, Isselhardt BJ Jr, Cappuccio JD. The analytic hierarchy process in medical decision making: a tutorial. Medical Decision Making, 1989; 9: 40–50. [DOI] [PubMed] [Google Scholar]
- 37. Forman E, Gass S. The Analytic Hierarchy Process – An Exposition. Operations Research, 2001; 49: 469–486. [Google Scholar]
- 38. Winawer SJ, Fletcher RH, Miller L et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology, 1997; 112: 594–642. [DOI] [PubMed] [Google Scholar]
- 39. NCSS . NCSS 2000 Program: Number Cruncher Statistical Systems. Kaysville, UT.: NCSS, 2000. [Google Scholar]
- 40. Eddy D. A Manual for Assessing Health Practices and Designing Practice Policies. Philadelphia, PA: American College of Physicians, 1992. [Google Scholar]
- 41. CDC . Facts on Screening. Screen for life: National Colorectal Cancer Action Campaign. CDC Cancer Prevention and Control. Available at: http://www.cdc.gov/cancer/screenforlife/fs_detailed.htm Accessed on: 1 May 2004. [Google Scholar]
- 42. National Cancer Institute . Colorectal cancer screening PDQ, National Cancer Institute; Available at http://www.nci.nih.gov/cancertopics/pdqScreening/colorectal/patient (Accessed on : 10 October 2005). [Google Scholar]
- 43. Scholl A, Manthey L, Helm R, Steiner M. Solving multiattribute design problems with analytic hierarchy process and conjoint analysis: an empirical comparison. European Journal of Operational Research, 2005; 164: 760–777. [Google Scholar]


