Abstract
Objective To examine who reported using unsolicited prostate cancer screening decision aids distributed as part of a randomized controlled trial, whether reported use varied by type of aid (video or pamphlet), and what affect reported use had on study outcomes.
Methods A total of 1152 men aged 50 and older from four medical facilities in the United States were randomly assigned to pamphlet, video or usual care (control). Materials were mailed 2 weeks prior to clinic appointments in general internal medicine. Outcomes were assessed by phone survey 1 week after appointments. Analyses examined the reported use of materials by study group, the association between patient characteristics and reported use, and the impact of reported use (adjusting for patient characteristics) on a 10‐item knowledge index.
Results Fifty‐six per cent of those randomized to receive the video and 50% of those randomized to receive the pamphlet reported using the materials. Reported use of the video was higher for patients who had greater than a high school education (OR 1.73), were married (OR 2.20), and reported no prior abnormal prostate cancer screening test results (OR 3.39). Reported use of the pamphlet did not vary by patient characteristics. In intent‐to‐treat analyses (ignoring reported use), individuals randomized to the video and pamphlet groups had significantly higher knowledge scores relative to the control group (7.44, 7.26 and 6.90 respectively). Adjusting for reported use modestly increased the estimated differences across treatment groups but did not substantially change conclusions about the relative effects of these aids on knowledge.
Conclusions Only half of men receiving unsolicited prostate cancer screening decision aids before a visit reported using the aids, and who reported using them varied by type of aid. Efforts to broadly implement decision aids may need to offer a variety of approaches, and incorporate creative strategies to enhance reaching all population subgroups.
Keywords: decision making, education, mass screening, patient, prostate‐specific antigen, prostatic neoplasms
Introduction
A large body of research has examined the efficacy of decision aids for helping patients to make informed decisions about value‐sensitive health‐care issues. This literature has documented favourable effects of decision aids on patient knowledge, expectations, decision‐making participation and decisional conflict for a variety of medical decision‐making issues. 1
With the growing evidence demonstrating the efficacy of decision aids for enhancing patient‐centred outcomes, there is an increasing need for effectiveness studies which focus on evaluating solutions for the practical issues involved in effectively implementing these aids in practice. 2 , 3 One important practical issue is identifying feasible and sustainable approaches to ensuring that those who could benefit from decision aids actually receive and report using them. Unfortunately, however, previous decision aid research does not provide much guidance on this matter. This is because most prior evaluation studies of decision aids typically have made participation in the study conditional on the patients’ willingness to examine the decision aid and participate in its evaluation. In other words, most prior studies of decision aids have been efficacy studies (i.e. conducted under ideal vs. real‐world conditions), and have assumed that all participants were ‘exposed’ to the aid in two senses: (i) access, (i.e. all participating patients received the materials) and (ii) use (i.e. all or virtually all read, watched or listened to the presentation of the decision aids).
In real‐world settings, however, exposure to decision aids – both access and use– will undoubtedly be highly variable. For instance, use of proffered decision supports may vary by patient characteristics including age, education, ethnicity, gender, marital status, insurance coverage and relationship with their provider. Additionally, both the level of exposure to decision aids and the distribution of exposure levels across population subgroups may vary depending on: (i) the provider's practice and the patient's insurance; (ii) the usual timing for the decision to be made; (iii) the usual site for the service to be delivered (e.g. whether the provider discussing the decision would also perform the service or would refer to another such as a surgeon, a medical sub‐specialist or laboratory or diagnostic unit); (iv) the nature of the decision to be facilitated (e.g. screening for disease, diagnostic testing, or treatment); (v) or the type of decision aid offered (e.g. print, audio‐visual, group discussion, web‐based). Information on who uses and benefits from decision aids in real‐world settings is needed to inform the development of strategies for integrating efficacious decision aids into practice.
One of the most common subjects in decision aid research is how to best facilitate decisions about the controversial prostate‐specific antigen (PSA) screening test for prostate cancer. Use of this screening test is controversial because, although it is the most sensitive and specific prostate cancer screening test available, the efficacy of the PSA test for reducing prostate cancer mortality is currently uncertain. Given this uncertainty, most organizations in the United States that have published guidelines about prostate cancer screening currently recommend that providers inform and involve their patients in decisions about whether or not to be screened for prostate cancer. 4 , 5 , 6 , 7
This study provides insights into the important issue of who uses and benefits from decision aids in real‐world settings by examining whether patients will use decision aids about prostate cancer screening if offered at a clinically ‘appropriate’ time (prior to a clinic visit when a screening decision could be considered). Specifically, the objectives of this study were to: (i) describe who reported using two different types of prostate cancer screening decision aids distributed as part of a randomized controlled trial, (ii) determine whether reported use of the aids varied by type of aid (video vs. pamphlet), and finally (iii) assess what effect reported decision aid use had on estimates of prostate cancer screening knowledge. Knowledge was selected as the primary outcome for the trial because both interventions were designed primarily to inform patients about the risks and benefits of prostate cancer screening.
Methods
Assignment
Population and setting
Study participants were selected from the population of male veterans aged 50 and older who had no prostate cancer and were scheduled to have a general internal medicine appointment at one of four Veterans Affairs (VA) medical facilities in the Midwestern region of the United States between April and June 2001.
Design
The study design was a randomized, controlled trial. This trial was designed as an effectiveness study (i.e. to evaluate the impact of the decision aids in the real‐world context of routine care) rather than an efficacy study (i.e. to evaluate the impact of the decision aids under ideal, controlled circumstances where exposure to the decision aids is, by design, virtually universal). There was no protocol for providers to follow, and providers were unaware which patients had been assigned to receive an aid or usual care.
The unit of randomization was the patient. Using a computer‐implemented algorithm, 1152 eligible patients, stratified by age (50–69, 70+), having had a PSA screening test in the past year (yes, no), and facility (any of the four study sites), were randomly assigned to receive: (i) a mailed pamphlet, (ii) a mailed video or (iii) usual care (control). Two weeks prior to the scheduled general internal medicine appointment that qualified them for this study (referred to hereafter as the ‘target appointment’), participants randomized to the pamphlet group were mailed an educational pamphlet developed by the study team, 8 and participants randomized to the video group were mailed an educational video evaluated in many previous studies. 9 , 10 , 11 Approximately 1 week after their target appointment, all participants were asked to complete a phone survey which assessed knowledge, demographics and other factors related to decision making. A total of 42 of the 1152 participants were later dropped from the analysis because they were found to be ineligible, i.e. deceased (n = 8), female (n = 5) or diagnosed with prostate cancer (n = 29). A total of 893 (80%) of the remaining 1110 participants completed the survey and were included in the analysis.
Protocol
Intervention
Because this was an effectiveness rather than an efficacy study, the protocol was specifically designed to be as efficient and convenient as possible for clinical systems to administer and for patients to use, while not compromising effectiveness. Therefore, the information used to identify eligible patients was easily obtained from administrative records, and the protocol for mailing the interventions was designed with input from clinic staff. Additionally, the intervention content and delivery timing were designed to permit patients to use the materials, reflect and review them again or share them, and still be close enough to an appointment to be able to follow up with questions or a discussion with a clinician.
Participants randomized to the control group received only usual care and whatever decision‐making support was provided in routine appointments. Participants in the Pamphlet group received a mailed pamphlet, written at the 6th grade level that was designed to provide a balanced representation of the potential risks and benefits of screening. Details about the pamphlet content are available elsewhere. 8 , 12 Participants in the Video group received a mailed 23‐min video developed by the Foundation for Informed Medical Decision Making (FIMDM) entitled ‘The PSA Decision: What YOU Need to Know’. The FIMDM video was designed to enable 100% comprehension at the 10th grade level, and, like the pamphlet, sought to provide a balanced representation of the risks and benefits of screening. Additional details about the video content are available elsewhere. 9 The interventions contained the same factual content assessed in the knowledge measure used to evaluate their effectiveness, but used different approaches to convey key points. The most obvious difference (other than the written vs. audiovisual approach) was in how the concept of expert disagreement about the uncertain benefit of prostate cancer screening was conveyed. In the video this message was conveyed by two provider patients (a urologist and a general internist) separately describing their very different views about the value of the PSA test. This use of providers‐as‐patients was done to emphasize that having medical training did not preclude their having different views. In the pamphlet this concept was conveyed in a more subtle approach, with the statement ‘not all doctors agree that men should have the PSA test done regularly’ and an explanation for why (‘because nobody knows whether finding prostate cancer early through the PSA test will help men live longer’).
Data collection and measures
The dependent and independent measures used for the analyses are described below. Most measures were assessed by phone survey approximately 1 week after the target appointment, but information on chronic disease diagnoses and medications was collected from VA outpatient databases.
Decision aid use was assessed from two questions on the post‐visit phone survey. The first question asked whether the respondent recalled receiving any educational materials about prostate cancer in the mail recently. Those who said ‘yes’ to this question were then asked if they ‘had a chance to look at’ the materials. Patients from the pamphlet and video groups who stated both – that they recalled receiving educational materials in the mail and that they had looked at the materials – were coded as having reported using the materials mailed to them.
Prostate cancer screening knowledge was assessed in the phone survey using a previously validated 10‐item index. 13 The index score is calculated as the sum of correct responses to 10 knowledge questions. ‘Don't know’ responses are treated as incorrect. Index scores range from zero to 10. Additional details about how the index was developed and its psychometric properties are available elsewhere. 13
Patient characteristics collected from the phone survey that were used as explanatory variables included overall health status, prior PSA testing, prior abnormal PSA, family history of prostate cancer, prostate problems, the American Urological Association's Urological Symptom Index 14 and demographics (i.e. age, race/ethnicity, employment status, marital status and education level achieved). Information on major chronic diseases (coronary heart disease, congestive heart failure, chronic obstructive pulmonary disease, diabetes, asthma, substance abuse and depression) and medications that are typically used to treat or may cause urinary symptoms (alpha blockers, diuretics) were assessed from VA administrative databases. These patient characteristics were collected and included as explanatory variables in this study because they may moderate the effect of the interventions; either through positive effects on knowledge (as we suspected might be the case for education, personal history, family history and prior PSA), or negative effects on how thoroughly patients examine the materials (as we suspected would be the case for comorbidities, personal history and prior PSA).
Analysis
Chi‐squared statistics were used to examine the comparability of study groups on patient characteristics and to test for significant differences across study groups on reported decision aid use. Multivariate logistic regression models were used to examine the effects of patient characteristics on reported decision aid use.
We then employed three different methods for estimating the effect of the interventions on knowledge index scores. The first used an intent‐to‐treat approach (i.e. patients were retained in the groups to which they were randomized in the analyses, whether or not they actually received/reported using the decision aids when randomized to an intervention group). These analyses used unadjusted linear regression models to estimate the effect of intervention materials on knowledge index scores ignoring reported use.
The second method we employed estimated the effect of using the intervention materials on knowledge index scores. In these latter unadjusted linear regression models, we used an exposure‐to‐aid model (i.e. the video and pamphlet intervention groups were restricted to participants who reported using the intervention materials; participants randomized to these groups who reported not using the materials were combined with the control group). These models made no adjustment for patient characteristics associated with reported use.
The third method we employed used linear regression models to adjust the estimates of knowledge obtained using the exposure‐to‐aid method to take into account patient characteristics associated with reported use (see Table 1 for specific measures). These models used propensity scores 15 to balance groups by patient characteristics. The propensity score method involved several steps. In the first step, separate logistic regression analyses were conducted for each intervention group (pamphlet vs. video) to estimate the odds of using the materials, as a function of the characteristics in Table 1. In the second step, the two estimated regression functions from these analyses were used to calculate a probability (or ‘propensity’) of viewing each of the interventions for each member of the study population, had they been sent the intervention. In the final step, the sample was categorized according to the quartiles of these two propensities and this categorization was added as a blocking covariate to the exposure‐to‐aid linear regression models described above. These propensity‐adjusted models allowed us to estimate the effect of the interventions on knowledge among those who reported using the aids, while adjusting for patient characteristics that predicted who would reported using a video vs. a pamphlet. In these models, education is categorized as greater than a high school education vs. less than a high school education; overall health is categorized as good to excellent vs. fair to poor; the various chronic disease indicators are collapsed into a summary measure of one or more chronic diseases present vs. none; and urological symptoms are categorized as moderate to severe vs. mild or none.
Table 1.
Sample characteristics
| Characteristic | All groups | Video | Pamphlet | Control | Group difference (P‐value) |
|---|---|---|---|---|---|
| Age | |||||
| 50–69 | 468 (52.4) | 162 (52.6) | 150 (50.9) | 156 (53.8) | 0.77 |
| 70+ | 425 (47.6) | 146 (47.4) | 145 (49.2) | 134 (46.2) | |
| Married | 617 (69.6) | 206 (67.3) | 215 (73.4) | 196 (68.3) | 0.23 |
| Education | |||||
| <High school | 188 (22.3) | 67 (22.9) | 54 (19.4) | 67 (24.3) | 0.19 |
| High school | 319 (37.6) | 117 (39.8) | 96 (34.5) | 106 (38.4) | |
| >High school | 341 (40.2) | 110 (37.4) | 128 (46.0) | 103 (37.3) | |
| Non‐Caucasian | 44 (5.0) | 14 (4.7) | 15 (5.2) | 15 (5.2) | 0.93 |
| Overall health | |||||
| Excellent | 40 (4.5) | 14 (4.6) | 19 (6.5) | 7 (2.4) | 0.11 |
| Very good | 192 (21.7) | 64 (21.0) | 74 (25.2) | 54 (18.8) | |
| Good | 328 (37.0) | 122 (40.0) | 99 (33.7) | 107 (37.2) | |
| Fair | 226 (25.5) | 76 (24.9) | 69 (23.5) | 81 (28.1) | |
| Poor | 101 (11.4) | 29 (9.5) | 33 (11.2) | 39 (13.5) | |
| CHD | 275 (30.8) | 95 (30.8) | 96 (32.5) | 84 (29.0) | 0.64 |
| CHF | 84 (9.4) | 25 (8.1) | 28 (9.5) | 31 (10.7) | 0.56 |
| COPD | 185 (20.7) | 58 (18.8) | 70 (23.7) | 57 (19.7) | 0.29 |
| Diabetes | 223 (25.0) | 82 (26.6) | 79 (26.8) | 62 (21.4) | 0.23 |
| Asthma | 33 (3.7) | 10 (3.3) | 12 (4.1) | 11 (3.8) | 0.86 |
| Substance abuse | 62 (6.9) | 18 (5.8) | 25 (8.5) | 19 (6.6) | 0.42 |
| Depression | 139 (15.6) | 49 (15.9) | 44 (14.9) | 46 (15.9) | 0.93 |
| Ever had PSA | 623 (70.2) | 209 (68.5) | 207 (70.4) | 207 (71.9) | 0.67 |
| Ever abnormal PSA | 97 (10.9) | 26 (8.5) | 33 (11.2) | 38 (13.2) | 0.19 |
| Prostate problems | 182 (20.5) | 62 (20.3) | 61 (20.8) | 59 (20.5) | 0.99 |
| Family history | 132 (14.9) | 44 (14.4) | 35 (11.9) | 53 (18.4) | 0.09 |
| Urological symptoms | |||||
| None | 141 (16.6) | 55 (18.8) | 44 (15.8) | 42 (15.2) | 0.38 |
| Mild | 410 (48.3) | 147 (50.2) | 129 (46.2) | 134 (48.4) | |
| Moderate | 252 (29.7) | 76 (26.0) | 94 (33.7) | 82 (29.6) | |
| Severe | 46 (5.4) | 15 (5.1) | 12 (4.3) | 19 (6.9) | |
| Using alpha blocker | 158 (17.7) | 43 (14.0) | 62 (21.0) | 53 (18.3) | 0.07 |
| Using diuretic | 267 (30.0) | 87 (28.3) | 86 (29.2) | 94 (32.4) | 0.51 |
Values are given as n (%). CHD, coronary heart disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; PSA, prostate‐specific antigen.
Masking and disclosures
The Study Coordinator distributed all study mailings but did not have direct contact with participants. Providers were blinded to the fact that their patients were participating in a trial. Follow‐up interviewers were blinded from intervention assignment, but the statisticians conducting the analyses were not. The pamphlet was developed by the study team but the video was not.
Ethical approval
The protocol was reviewed and approved by the Institutional Review Board at the four participating facilities, the University of Minnesota and Dartmouth College.
Results
Sample characteristics
The mean age of the sample was 68 years (standard deviation 9.38) and did not vary significantly across study groups. Table 1 presents data on other characteristics of the study sample and the comparability of study groups. As shown in the last column of Table 1 and described in greater detail in a prior publication from this trial, 12 the study groups are comparable on all descriptive measures presented in Table 1.
Reported use of materials by type of intervention
Figure 1 displays the proportion of study participants that recalled receiving and reported using materials about prostate cancer screening. Roughly 64% (n = 185) of pamphlet participants and 78% (n = 235) of video participants recalled receiving the materials sent to them. The 14% difference between pamphlet and video participants on recall was statistically significant (P = 0.0003). Despite differences in recalling receipt, however, the groups did not appear to differ in actual reported use of the materials. The proportion reporting using the materials was 50% (n = 143) among pamphlet participants and 56% (n = 170) among video participants, and did not vary significantly by type of intervention (P = 0.12).
Figure 1.
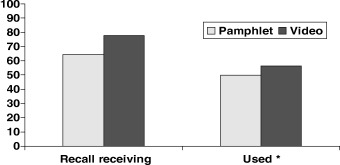
Proportion who recall receiving/using decision aids. *Those who both recalled receiving and reporting looking at the materials.
Association of patient characteristics with reported material use
Table 2 displays the results from the multivariate logistic regression models used to examine the association of patient characteristics with reported decision aid use within each intervention group. Reported pamphlet use did not vary significantly by any of the patient characteristics examined. Reported video use was significantly higher for patients with greater than a high school education, married patients, and patients reporting no prior abnormal prostate cancer screening test results. Reported video use was also marginally higher (P ≤ 0.06) among patients reporting prior prostate problems and those with a family history of prostate cancer.
Table 2.
Association of aid use with patient characteristics, by intervention group
| Patient characteristics | Odds ratio (95% CI) | |
|---|---|---|
| Pamphlet | Video | |
| Age 70+ | 0.71 (0.42–1.21) | 0.82 (0.49–1.38) |
| Married | 1.10 (0.62–1.95) | 1.73* (1.02–2.94) |
| >High school education | 0.82 (0.48–1.43) | 2.20* (1.25–3.85) |
| Caucasian race | 1.70 (0.53–5.47) | 0.87 (0.27–2.81) |
| Overall health good to excellent | 1.20 (0.70–2.06) | 1.24 (0.71–2.16) |
| 1+ chronic conditions | 0.78 (0.45–1.37) | 1.15 (0.67–1.99) |
| Ever had PSA | 1.21 (0.68–2.13) | 1.18 (0.68–2.02) |
| Never had abnormal PSA | 1.21 (0.52–2.81) | 3.39* (1.30–8.83) |
| Prostate problems | 1.17 (0.57–2.39) | 1.92** (0.97–3.81) |
| Equally or more likely to get prostate cancer | 1.23 (0.74–2.04) | 0.81 (0.48–1.36) |
| Family history | 0.74 (0.35–1.56) | 2.03** (0.96–4.28) |
| Moderate to severe urological symptoms | 1.85 (0.72–4.73) | 1.20 (0.41–3.15) |
| Using alpha blocker | 1.53 (0.79–2.97) | 1.39 (0.65–2.99) |
| Using diuretic | 0.99 (0.57–1.72) | 0.65 (0.31–1.12) |
PSA, prostate‐specific antigen. *P ≤ 0.05; **P = 0.06.
Effect of materials on knowledge
Figure 2 and table 3 display the knowledge index scores derived from the three different analytic approaches, by study group. In the original intent‐to‐treat analysis (results represented by the first three rows in Table 3 and the first three bars in Figure 2), individuals randomized to both video and pamphlet groups had significantly higher knowledge scores (7.44 and 7.26 respectively) relative to the control group (6.90). After restricting each intervention group to patients who said they used the materials and combining those who said they did not use the materials with controls (results represented by the next rows in Table 3 and the next three bars in Figure 2, labelled ‘exposure to aid’), the knowledge scores were 7.74 for the using video group, 7.52 for the using pamphlet group and 6.83 for the combined non‐user/control group. The effect estimates (or differences between intervention and control groups) resulting from this analysis showed the effect size was 66% higher for the video group and 83% higher for the pamphlet group relative to the intent‐to‐treat results. After adjusting the exposure to aid analyses for variation in propensity to use by patient characteristics (the last three rows of Table 3 and the last three bars in Figure 2, labelled ‘adjusted exposure to aid’), the knowledge scores were 7.75 for the using video group, 7.45 for the using pamphlet group and 6.97 for the combined non‐user/control group. The effect estimates resulting from this analysis were 47% higher for the video group and 33% higher for the pamphlet group relative to the intent‐to‐treat results. The differences in knowledge scores between video and pamphlet groups were not statistically significant in any of the analyses conducted.
Table 3.
Ten‐item knowledge index scores, by study group and analysis approach
| Study group | Index score | 95% CI | Difference from control | P‐value vs. control |
|---|---|---|---|---|
| Intent to treat approach | ||||
| Pamphlet | 7.26 | 7.04–7.49 | 0.36 | 0.001 |
| Video | 7.44 | 7.22–7.65 | 0.54 | 0.03 |
| Control | 6.90 | 6.68–7.13 | – | – |
| Exposure to aid approach | ||||
| Pamphlet | 7.52 | 7.18–7.87 | 0.69 | 0.001 |
| Video | 7.74 | 7.42–8.06 | 0.91 | <0.0001 |
| Control | 6.83 | 6.66–7.01 | – | – |
| Adjusted exposure to aid approach | ||||
| Pamphlet | 7.45 | 7.14–7.77 | 0.48 | 0.008 |
| Video | 7.75 | 7.46–8.04 | 0.78 | <0.0001 |
| Control | 6.97 | 6.81–7.13 | – | – |
Figure 2.
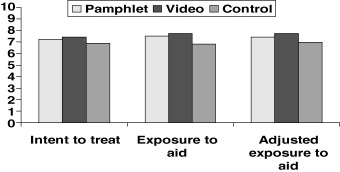
Ten‐item knowledge index scores by study group and analysis approach.
Discussion and conclusions
This study evaluated the impact of two prostate cancer decision aids in a real‐world (effectiveness) setting and found that: (i) reported decision aid use was considerably lower than observed in prior efficacy trials where eligibility was predicated on willingness to use, and (ii) the association of reported use with patient characteristics varied by type of aid. However, the proportion of patients receiving the decision aids that actually reported using them did not differ significantly by the type of aid, suggesting that the video and pamphlet interventions were comparably acceptable to patients. Given the comparable levels of reported use and effects of the interventions on knowledge, the pamphlet may be a more attractive population‐based approach, as it is lower in cost 8 and relatively easier to implement on a large scale.
As the original intent of the research was to examine the impact of patient decision‐aids on knowledge, we did not measure several potentially important predictors of decision‐aid use, such as being undecided or unaware of prostate cancer screening, or preferences regarding how prostate cancer screening information is provided. Consequently, our examination of use was limited to basic demographic characteristics and health‐related factors. Despite these limitations, we found some interesting patterns in regard to who reported using the two different aids distributed. While reported video use varied significantly by several patient characteristics (education, marital status and prior abnormal prostate cancer screening test results), pamphlet use did not. We can only speculate about why we found these results. One explanation could be that the video (which requires special technology and is not easily ‘skimmed’ through) may take a greater amount of effort to use, and hence using it may require some additional motive. For example, married men may have been encouraged to use the video by their spouse and men without an abnormal PSA may have been more interested because they were undecided about the value of screening. The finding that reported pamphlet use did not vary by educational level but that video use was higher among those with greater than a high school education is particularly interesting, as it contradicts the common belief that written materials may not be used by the lowest education groups. One possible explanation for this finding is that those with greater than a high school education may be more likely to own the technology required to view the mailed videos at home. The fact that video use may not be equally appealing to all population subgroups has important implications for broad‐based dissemination of prostate cancer screening decision aids and warrants further study.
To our knowledge, a total of five prior studies of prostate cancer screening decision aids have been designed as effectiveness studies, where exposure to the aids evaluated was voluntary and variable. 10 , 16 , 17 , 18 , 19 However, only three of these studies provided information on the proportions of participants using the decision aids distributed as part of the trial. 16 , 17 , 20 Participation in all of these trials was contingent on agreement to receive the materials being evaluated. Frosch et al. 10 found that, among patients who agreed to enrol, 86%, 84% and 82%, respectively, actually participated in the (i) group discussion alone, (ii) video alone and (ii) discussion plus video decision aid programmes offered. In a later trial comparing Internet and video decision aids, Frosch et al. 16 found that 54% of patients who agreed to receive the Internet materials actually reviewed them, whereas 98% of patients that agreed to view a video prior to their clinic appointment actually viewed the video. Finally, Gattellari and Ward 17 found that greater than 96% of individuals enrolled in a trial comparing leaflet, video and evidence‐based booklet decision aids actually viewed the materials distributed to them. Only one of these studies provided information on the extent to which decision aid use varied by patient characteristics. Frosch et al. 16 found that individuals who completed the Internet decision aid offered as part of their trial were more likely to have a family member or friend who had been diagnosed with prostate cancer. In our study, this measure was marginally associated with exposure to the video intervention. In contrast to our findings, however, Frosch et al. did not find any demographic characteristics that were associated with use of the Internet aid.
As indicated above, most of the prior studies of prostate cancer screening decision aids that have examined use found that at least 80% of those randomized to receive the aids used them. The comparably lower levels of use for similar decision aids observed in our trial may be due to several factors. One key factor is that our materials were unsolicited. All previous trials on prostate cancer screening decision aids enrolled only those who expressed an interest in receiving the aids or at least agreed to receive them. We chose to distribute unsolicited aids because this approach was being proposed by local VA hospital administrators as a means to satisfy a recently implemented performance measure mandating that all male patients age‐eligible for prostate cancer screening be informed annually about the risks and benefits of testing.
A second factor that may have contributed to lower observed levels of use is that our ‘practical’ protocol distributed aids to some men who were not clinically ‘appropriate’ to screen at the time of the visit. In particular, some men in our sample had had a PSA or may have already talked to their provider about a PSA decision within the previous 12 months. These men may have concluded appropriately that they need not make a decision about PSA testing at their next visit and may have opted not to examine the materials.
A third factor is that men in a VA population, despite differences in educational level, age and history of prostate cancer screening, may be less likely than comparable populations of men in non‐VA settings to be interested in participating in decision making about prostate cancer screening. Prior studies have found that males 21 , 22 , 23 and elderly 17 , 22 , 24 , 25 , 26 individuals are more likely to defer to their doctors for making medical decisions; we did not collect data about these preferences. Those developing future systems for implementing decision aids should be cognizant of these factors that may limit use of aids, and strive for systems that maximize the degree to which the decision aids reach those receptive to them and are available at the time that they most need them.
A final practical issue warranting discussion is the selection of the method for distributing the decision aids. We chose to distribute our interventions by mail for the following reasons: (i) mailed administration is one of the most feasible approaches for distributing interventions that may need to be repeated over time to large and dispersed groups of people, (ii) the mailed approach allowed patients time to thoroughly digest the materials prior to a discussion with their provider, and (iii) some participating facilities could not garner the appropriate space and other necessary resources for providing opportunities to view the materials on site. Future studies should explore whether distribution strategy affects use and effectiveness.
Because this study examined reported use of decision aids for prostate cancer screening only, the sample selected was exclusively male and primarily Caucasian, and the observed intervention effects were relatively modest in size, the extent to which our findings regarding reported use apply to other types of decisions, outcomes and populations is not clear. However, as one of the few studies to examine how selective and limited exposure may influence the population level impact of preventive health care‐related decisions aids, this study makes an important contribution to the relatively nascent literature on appropriate population level decision aid implementation strategies. Indeed, the low levels and selectivity of reported decision aid use documented in this study suggest that future efforts to broadly implement decision aids may need to offer a variety of approaches, and incorporate creative strategies to enhance use, in order to reach all population subgroups and to enhance decision making on a population level. Additional research examining use of decision aids in real‐world settings is needed to inform the development of programmes for implementing decision aids found to be effective in controlled trials.
Acknowledgement
This study was funded by VA Health Services Research and Development Service Grant No. IIR 99 277‐1 to the Center for Chronic Disease Outcomes Research, Veterans Affairs Medical Center, Minneapolis, MN.
References
- 1. O'Connor AM, Stacey D, Entwistle V et al. Decision Aids for People Facing Health Treatment or Screening Decisions (Cochrane Review). The Cochrane Library [4]. Chichester, UK: John Wiley & Sons, Ltd, 2005. [Google Scholar]
- 2. O'Cathain A, Thomas KJ. Evaluating decision aids – where next? Health Expectations, 2004; 7: 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Connor AM, Llewellyn‐Thomas HA, Flood AB. Modifying unwarranted variations in health care: shared decision making using patient decision aids. Health Affairs (Millwood), 2004. (Suppl. Web Exclusive); VAR63–VAR72. [DOI] [PubMed] [Google Scholar]
- 4. American College of Physicians . Clinical guideline: screening for prostate cancer. Annals of Internal Medicine, 1997; 126: 480–484. [PubMed] [Google Scholar]
- 5. Office of Technology Assessment . Costs and Effectiveness of Prostate Cancer Screening in Elderly Men. OTA‐BP‐H‐145. Washington, DC: US Government Printing Office, 1995. [Google Scholar]
- 6. Smith RA, Cokkinides V, Eyre HJ. American Cancer Society guidelines for the early detection of cancer, 2003. CA: A Cancer Journal for Clinicians, 2003; 53: 27–43. [DOI] [PubMed] [Google Scholar]
- 7. U.S. Preventive Services Task Force . Screening for prostate cancer: recommendation and rationale. Annals of Internal Medicine, 2002; 137: 915–916. [DOI] [PubMed] [Google Scholar]
- 8. Partin MR, Dillon N, Haas M, Wilt TJ. The PSA Test for Prostate Cancer: Is it Right for ME?. Minneapolis, MN: Minneapolis VA Medical Center, 2002. [Google Scholar]
- 9. Flood AB, Wennberg JE, Nease RF Jr, Fowler FJ Jr, Ding J, Hynes LM. The importance of patient preference in the decision to screen for prostate cancer. Prostate Patient Outcomes Research Team. Journal of General Internal Medicine, 1996; 11: 342–349. [DOI] [PubMed] [Google Scholar]
- 10. Frosch DL, Kaplan RM, Felitti V. The evaluation of two methods to facilitate shared decision making for men considering the prostate‐specific antigen test. Journal of General Internal Medicine, 2001; 16: 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Volk RJ, Cass AR, Spann SJ. A randomized controlled trial of shared decision making for prostate cancer screening. Archives of Family Medicine, 1999; 8: 333–340. [DOI] [PubMed] [Google Scholar]
- 12. Partin MR, Nelson D, Radosevich D et al. Randomized trial examining the effect of two prostate cancer screening educational interventions on patient knowledge, preferences, and behaviors. Journal of General Internal Medicine, 2004; 19: 835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Radosevich DM, Partin MR, Nugent S et al. Measuring patient knowledge of the risks and benefits of prostate cancer screening. Patient Education and Counseling, 2004; 54: 143–152. [DOI] [PubMed] [Google Scholar]
- 14. Barry MJ, Fowler FJ Jr, O'Leary MP et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. The Journal of Urology, 1992; 148: 1549–1557. [DOI] [PubMed] [Google Scholar]
- 15. Rosenbaum PR, Rubin DR. The central role of the propensity score in observational studies for causal effects. Biometrika, 1983; 70: 41–45. [Google Scholar]
- 16. Frosch DL, Kaplan RM, Felitti VJ. A randomized controlled trial comparing internet and video to facilitate patient education for men considering the prostate specific antigen test. Journal of General Internal Medicine, 2003; 18: 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gattellari M, Ward JE. Measuring men's preferences for involvement in medical care: getting the question right. Journal of Evaluation in Clinical Practice, 2005; 11: 237–246. [DOI] [PubMed] [Google Scholar]
- 18. Gattellari M, Ward JE. Does evidence‐based information about screening for prostate cancer enhance consumer decision‐making? A randomised controlled trial. Journal of Medical Screening, 2003; 10: 27–39. [DOI] [PubMed] [Google Scholar]
- 19. Wilt TJ, Paul J, Murdoch M, Nelson D, Nugent S, Rubins HB. Educating men about prostate cancer screening. A randomized trial of a mailed pamphlet. Effective Clinical Practice, 2001; 4: 112–120. [PubMed] [Google Scholar]
- 20. Frosch DL, Kaplan RM, Felitti V. Evaluation of two methods to facilitate shared decision making for men considering the prostate‐specific antigen test. Journal of General Internal Medicine, 2001; 16: 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blanchard CG, Labrecque MS, Ruckdeschel JC, Blanchard EB. Information and decision‐making preferences of hospitalized adult cancer patients. Social Science and Medicine, 1988; 27: 1139–1145. [DOI] [PubMed] [Google Scholar]
- 22. Degner LF, Sloan JA. Decision making during serious illness: what role do patients really want to play? Journal of Clinical Epidemiology, 1992; 45: 941–950. [DOI] [PubMed] [Google Scholar]
- 23. Llewellyn‐Thomas HA, McGreal MJ, Thiel EC. Cancer patients’ decision making and trial‐entry preferences: the effects of ‘framing’ information about short‐term toxicity and long‐term survival. Medical Decision Making, 1995; 15: 4–12. [DOI] [PubMed] [Google Scholar]
- 24. Beisecker AE, Beisecker TD. Patient information‐seeking behaviors when communicating with doctors. Medical Care, 1990; 28: 19–28. [DOI] [PubMed] [Google Scholar]
- 25. Benbassat J, Pilpel D, Tidhar M. Patients’ preferences for participation in clinical decision making: a review of published surveys. Behavioral Medicine, 1998; 24: 81–88. [DOI] [PubMed] [Google Scholar]
- 26. Guadagnoli E, Ward P. Patient participation in decision‐making. Social Science & Medicine, 1998; 47: 329–339. [DOI] [PubMed] [Google Scholar]


