Abstract
Objective To identify the determinants of the intention of physicians to screen for decisional conflict in clinical practice.
Background Screening for decisional conflict is one of the key competencies when educating health professionals about shared decision making. Theory‐based knowledge about variables predicting their intention to screen for decisional conflict in clinical practice would help design effective implementation interventions in this area.
Design Data of two cross‐sectional surveys embedded within a large implementation study of the Ottawa Decision Support Framework (ODSF) in primary care.
Setting and participants In total, 122 health professionals from five family practice teaching units.
Methods Intention to screen for decisional conflict in clinical practice was defined as the intention to use the clinical version of the Decisional Conflict Scale (DCS) with patients at the end of the clinical encounter. It was assessed at the entry and the exit from this study. Both intentions were entered as a dependent variable in multivariate analyses.
Main results At entry, the intention was influenced by: attitude (P < 0.001), subjective norm (P < 0.001), perceived behavioural control (P < 0.001) and clinical site (P < 0.05). On exit, it was influenced by: subjective norm (P < 0.001), perceived behavioural control (P < 0.001), clinical site (P < 0.05), international Continuing Medical Education (CME) (P < 0.05), other diplomas (P < 0.05) and intervention (P < 0.05). In post hoc analyses, there was a statistically significant difference between entry and exit in the impact of the level of exposure to the multifaceted implementation intervention on the intention (P = 0.003).
Conclusions Variables predicting the intention of health professionals to screen for decisional conflict in clinical practice using the DCS change over time suggesting that effective implementation interventions in this area will need to be modified longitudinally.
Keywords: decisional conflict, health professionals behaviour, health services research, shared decision making, theory of planned behaviour
Introduction
Patients’ involvement in decisions
In recent years, interest in having patients participate actively in decision making has increased. 1 In this context, the process by which patients are engaged to share their preferences and become involved in primary health‐care decisions is changing. 2 Shared decision making is defined as a process by which a health‐care choice is made by practitioners together with the patient 3 and is said to be the crux of patient‐centred care. 4 It includes the following components: establishing a context in which patients’ views about treatment options are valued and deemed necessary; reviewing the patient’s preferences for role in decision making and the existence and nature of any uncertainty about the course of action to take (i.e. decisional conflict); 3 , 5 transferring technical information; making sure patients understand this information; helping patients base their preference on the best evidence; eliciting patients’ preferences; sharing treatment recommendations; and making explicit the component of uncertainty in the clinical decision‐making process. 6 Therefore, one of the key competencies for shared decision making encompasses the identification of decisional conflict in patients facing difficult decisions. 5
Decisional conflict can be expressed as a state of uncertainty about which course of action to take when choices among competing actions involve risk, loss, regret, or challenge to personal life values. 7 The short clinical version of the Decisional Conflict Scale (DCS) that is provided by the Ottawa Decision Support Framework (ODSF) helps to quickly assess decisional conflict in patients, identify the areas that need to be addressed in order to provide decision support. 8 From a theoretical perspective, as pointed out by Towle and Godolphin (1999), reviewing in patient the existence and nature of any uncertainty about the course of action to take (i.e. decisional conflict) is one of the key competencies of informed shared decision making. 3 , 5 In other words, use of the short clinical version of the DCS by health professionals in clinical practice is an important step towards shared decision making. Based on the ODSF, the DCS is a multidimensional scale that assesses five dimensions: uncertainty and its determinants (knowledge, value, support and effective choice). 8 Therefore, one could argue that systematically assessing decisional conflict in patients using the DCS would encompass many of the key competencies of informed shared decision making. Indeed, from an empirical perspective, decision aids are known for reducing decisional conflict and fostering shared decision making between patients and their physicians. 9 , 10
Existing data suggest that health professionals have not yet adopted shared decision making. 11 , 12 , 13 If shared decision making is desirable, more will need to be performed to understand what factors hinder or facilitate its implementation in clinical practices. 14 We completed a systematic review that found 31 publications of 28 unique studies in 15 countries that reported on barriers and facilitators to implementing shared decision making in clinical practice as perceived by health professionals. 15 However, only two studies were explicit in their use of a conceptual framework or a theory pertaining to the assessment of barriers and/or facilitators to the implementation of best practices in clinical practice. 16 , 17
Thus, at the time this study was conducted, most of the studies that had been conducted to improve our understanding of the implementation of shared decision making in clinical practices had no clear theoretical basis. This is of some concern as it has been acknowledged that more attention needs to be given to the combination of different theories that could help us understand professional behaviours 18 , 19 and design effective implementation strategies. 20 This call for action is congruent with specific recommendations by Briss and colleagues (2000) who argued that research for interventions to improve informed decision making about cancer screening would need to include the application of theoretical models, standardization of outcomes and diverse ethnic groups. 21
The theoretical basis of changing health professionals’ behaviour
The theory of planned behaviour (TPB) is well known through its previous applications to the study of health professionals’ behaviours. 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 The theory of planned behaviour provides a theoretical account of the way in which attitude, subjective norm and perceived behavioural control combine to predict a given behavioural intention. 30 This theory postulates that when the individual has some control over a situation, intention is the immediate determinant of behaviour. 22 Attitude is conceptualized as a disposition to respond favourably or not to an object, person, institution, or event, that is, a personal positive or negative evaluation of the consequences associated with performing the behaviour in question. 22 Subjective norm deals with perceived normative prescriptions and refers to a perceived social pressure to perform the particular behaviour of interest. Perceived behavioural control is a measure of the amount of control the individual perceives he/she has over the behaviour in question. It reflects the individual’s perception of barriers or facilitating factors likely to influence his/her adoption of the behaviour. In the case of non‐volitional behaviour (i.e. when the individual has no control over the behaviour of interest), perception of control may have a direct influence on the behaviour itself and be its sole determinant. For example, in a situation where a physician would like to prescribe a specific drug that is not available in his/her country.
According to the authors of this theory, sociodemographics and other variables will influence behaviour through their influence on the attitude, the subjective norm and the perceived behavioural control. 22 Successful behavioural change will occur only if the underlying determinants of intention (i.e. attitude, social norm and perceived behavioural control) change. Therefore, identification and monitoring of the behavioural intention as well as its determinants over time has the potential to inform as to the nature, content and impact of interventions targeting behavioural change. However, although the theory of planned behaviour has proven useful when studying health related behaviours, a review by Godin and Kok (1996) found that components of this theory explain on average 41% of the variance in intention and 31% of the variance in behaviour. 25 More recently, a systematic review of 422 longitudinal studies found that intentions accounted for 28% of the variance in behaviour, on average. 31 Results from these systematic reviews suggest that other variables must play a direct role on the behavioural intention as well as on the behaviour itself.
Uncertainty is a key component of shared decision making. 5 , 32 Gerrity has provided a conceptual model of the influence of physicians’ reactions to uncertainty (PRU) on decision outcomes. 33 , 34 Briefly, in this model, the medical problem and characteristics of patients create the uncertainty inherent in the clinical encounter. 34 Characteristics of physicians influence their reaction to uncertainty. In turn, the decision‐making process occurring during the clinical encounter between a patient and a physician is under the influence of the uncertainty inherent in the clinical encounter and the PRU. Patients and physicians interact to produce a set of decisions 33 that in some cases will be translated into physicians’ behaviour. 34 The decision outcome and, on some occasions, the physician’s behaviour, may be modified by external sources such as source of payment, practice settings, etc. 33, , 34 Physicians’ reaction to uncertainty is composed of four main constructs: anxiety due to uncertainty, concern about bad outcomes, reluctance to disclose uncertainty to patients and reluctance to disclose mistakes to physicians. 34 Previous research showed that physicians’ reactions towards uncertainty were significantly associated with disclosure of uncertainty to patients during clinical encounters 35 as well as with resource use and costs. 36 This is interesting, given the possible influence of physicians’ attitudes toward uncertainty on the adoption of shared decision making. First, if disclosing uncertainty to patients is a key component of shared decision making, it is possible that physicians who are reluctant to do so and who are more anxious when facing uncertainty will be less likely to adopt this model of decision making.
Second, in line with the diffusion of innovation theory, it is also possible that physicians’ reactions toward uncertainty might influence their adoption of shared decision making. 37 In this framework, the innovation is defined from the perspective of the potential adopters as an idea, technology, or process that is perceived as new to them. 37 New ideas carry some degree of uncertainty for the potential adopters. 37 Earlier adopters are better able to cope with uncertainty. 37 In other words, physicians who are more anxious about uncertainty may be less inclined to adopt a new process of decision making. According to the diffusion of innovation theory, other characteristics of physicians such as number of years in formal education, social participation and a cosmopolitan perspective may also influence adoption of shared decision making. A cosmopolitan perspective is defined by the degree to which the physician looks beyond his local situation for guidance and satisfaction related to his work. 38 It was found to be associated with a more rapid adoption of innovation among physicians. 38 , 39
Consequently, embedded within a large implementation study of the ODSF in primary care, we used the theory of planned behaviour to conduct two cross‐sectional surveys of participating health professionals. 40 These surveys aimed at identifying the determinants of these health professionals’ intention to screen for decisional conflict using the short clinical version of the DCS in clinical practice. This would in turn provide an enhanced theoretical foundation for future implementation studies of shared decision making in clinical practice most specifically for those targeting the implementation of screening for decisional conflict, one of the key competencies of shared decision making. We hypothesized that attending an interactive workshop about the ODSF, 41 receiving feedback 42 and being reminded at the point of care about the ODSF 43 would positively influence the intention to use the DCS afterwards. We also hypothesized that PRU would be associated with this behavioural intention and would be responsible for explaining a portion of its variance above those attributed to attitude, social norm and perceived behavioural control. Last, we hypothesized that number of years in formal education, social participation and a cosmopolitan perspective would also be associated with the intention to use the DCS in clinical practice.
Methods
Main study design
A before‐and‐after study design was used to assess the impact of implementing the ODSF in clinical practice on the agreement between patients’ decisional conflict scores and those of their primary care provider. 40 The entire study was conducted in French. Briefly, health professionals were invited to participate in this study to assess the impact of the process offered by this framework on the agreement between their patients’ decisional conflict and their own. At entry into the study, they signed a consent form and completed a questionnaire. During the phase 1 recruitment period, they recruited five patients from their clinics for whom they felt a decision had been made. Both the health professional and the patient completed a post‐clinical encounter questionnaire that assessed their respective level of decisional conflict. Health professionals then participated in an interactive workshop during which they shared their views on decision support and on barriers and facilitators to implementing the ODSF in their practice. 44 , 45 They also received feedback on the agreement between their patients’ decisional conflict and their own. During the phase 2 recruitment period, they also received a reminder about the framework. At the end of the trial, they completed a last questionnaire. However, building on the main study trial, we, a priori, planned for assessing and monitoring the intention of health professionals to screen for decisional conflict in clinical practice as well as its determinants using the theory of planned behaviour. Therefore, this paper reports on data collected during the two cross‐sectional surveys that relate to this assessment: at entry into and exit from the study.
Health professionals’ sample
Clinical teachers and residents from five urban family practice teaching units (FPTUs) were the target participants. Each of these urban FPTUs has a roster of patient‐visits a year that ranges from about 15 000 to 25 000. The inclusion criterion for clinical teachers and residents in family medicine participating in this before‐and‐after study was to be involved in outpatient clinical activity at one of these FPTUs during the time the study was conducted. There was no financial compensation for participants.
Data collection and procedure
Data were collected by means of a self‐administered questionnaire that was comprised of two sections based on the theoretical frameworks that were identified for this study. 22 , 33 , 34 , 37 Items derived from the theory of planned behaviour were generated based on previous work on health professionals’ behavioural intention. 23 , 25 , 26 , 28 , 29 The behaviour under study was defined according to three elements: the target at which the behavioural disposition is directed (a short clinical version of the DCS); the particular action involved (the use of the DCS with patients); and the context in which the action occurs (at the end of the clinical encounter). 22 The time of its occurrence was not defined. In the present study, only the main constructs of the theory of planned behaviour were assessed. The entry questionnaire was pilot‐tested at 2‐week interval with a group of 19 physicians in private practice who did not participate in the main study.
Outcome variable
Intention to use the DCS (INT) was assessed by means of three items. After the general comment ‘At the end of a clinical encounter in which a decision has been made, …’, physicians were asked to answer the following questions on a 7‐point scale: ‘Using the DCS appears’ (‘very unlikely’ to ‘very likely’); ‘I have the intention to use the DCS’ (‘strongly disagree’ to ‘strongly agree’); and ‘I estimated my chance of using the DCS to be’ (‘very low’ to ‘very high’). The mean of the composite score was computed (Cronbach alpha = 0.79).
Explanatory variables
The direct measure of attitude (Aact) was assessed by means of six items using a semantic differential 7‐point scale. Six pairs of adjectives were used to assess Aact: ‘not very useful/very useful’, ‘not very responsible/very responsible’, ‘not very satisfying/very satisfying’, ‘not gratifying/gratifying’, ‘not very pleasurable/very pleasurable’ and ‘not very image‐enhancing/very image‐enhancing’. Each pair of adjectives appeared after the sentence: ‘At the end of a clinical encounter in which a decision has been made, for me, using the DCS would be …’. The mean composite score of six items was computed (Cronbach alpha = 0.85). Direct measure of the subjective norm (SN) was assessed by means of three items, each assessed on a 7‐point scale. Physicians were invited to indicate their level of agreement with the following statements: ‘Most of the persons who are important for me in the profession would recommend that I use the DCS at the end of a clinical encounter’, (‘strongly disagree’ to ‘strongly agree’); ‘Most of the persons who are important for me in the profession think it is preferable that I use the DCS at the end of a clinical encounter’, (‘strongly disagree’ to ‘strongly agree’); ‘Most of the persons who are important for me in the profession would be favourable to my using the DCS at the end of a clinical encounter’, (‘strongly disagree’ to ‘strongly agree’). These three items were used to compute a mean composite score (Cronbach alpha = 0.79). Three items were included to assess a direct measure of the perceived behavioural control (PBC), each on a 7‐point response scale. The items were: ‘At the end of a clinical encounter in which a decision has been made, I see no barriers to using the DCS’ (‘strongly disagree’ to ‘strongly agree’); ‘For me, at the end of a clinical encounter in which a decision has been made, using the DCS would be…’ (‘very difficult’ to ‘very easy’). ‘At the end of a clinical encounter in which a decision has been made, I feel I am in control of using the DCS.’ (‘strongly disagree’ to ‘strongly agree’). These three items were used to compute a mean composite score (Cronbach alpha = 0.78). For all above variables, a positive score indicated that the physician expressed a positive evaluation of the construct. After obtaining permission from the authors, two subscales from the PRU 33 , 34 were translated into French and included in the questionnaire: anxiety about uncertainty (i.e. five items, Cronbach alpha = 0.86) and disclosing uncertainty to patients (i.e. five items, Cronbach alpha = 0.79).
Number of years of formal education was assessed by asking participants to indicate if they had completed degrees other than their medical degree and if so, to indicate the type of degree. Social participation was assessed by asking participants to indicate if they were participating on committees and in continuing professional education activities. A cosmopolitan perspective, the degree to which an individual is oriented outside a social system, was assessed by asking participants to indicate the nature of the committees and of the continuing professional education activities they were involved with: institutional, local, national or international. Awareness of the ODSF was assessed by asking physicians if they knew of the DCS before entering the study (i.e. ‘yes’ or ‘no’). For those who reported knowing the DCS before entering the study, they were asked to estimate the percentage of clinical situations in which they had used it. For those who reported not knowing about the DCS before entering the study, they were asked to estimate the percentage of clinical situations in which it could be useful. On the exit questionnaire, participants were asked to estimate the percentage of clinical situations in which the DCS could be useful, their potential use of the DCS outside the study context and their satisfaction with having been introduced to the DCS (on a scale from ‘0’‘very low’ to 10 ‘very high’). Sociodemographics (i.e. age, number of years in practice, gender and status), number of patients seen in a week 46 and number of hours spent in professional activities 46 were also assessed. With in mind the other objective of the overall project, to evaluate the impact of the ODSF on the agreement of physicians and their patients on the decision‐making process, 40 one further item, preferred role in decision making, 47 was added to the entry questionnaire of the health‐care professionals.
Data processing and analysis
Using chi‐square analysis and Fisher’s exact test when appropriate, characteristics of participants were compared with those of non‐participants. Descriptive analyses of participants were computed. Given the pragmatic nature of the study design, not all participants were exposed to all components of the multifaceted implementation intervention. Therefore, a posteriori, a new variable was created to quantify the different levels of exposure to the implementation intervention and explore the influence of the level of exposure to the intervention on the main outcome (intention to use the DCS). This variable was composed of three categories: no exposure (no workshop, no feedback and no reminder at the point of care), incomplete exposure (workshop plus feedback on phase 1 recruitment period) and complete exposure (workshop, feedback on phase 1 recruitment period plus reminder at the point of care during phase 2 recruitment period).
Multiple regression analysis was used to test the relationship between the intention to use the DCS in clinical practice and the explanatory variables. First, intention at entry was regressed on attitude, subjective norm and perceived behavioural control as measured at entry. Second, intention at exit was regressed on these variables as measured at exit. Third, the intention at exit was regressed on attitude, subjective norm and perceived behavioural control as measured at entry and on the other variables in the same stepwise approach as the one presented above. However, in this last model, the intention at entry was entered as a covariate. In all three models, additional explanatory variables were entered as follows: physicians' reaction to uncertainty, sociodemographics including variables based on the diffusion of innovation theory, previous knowledge of the DCS, exposure to the intervention (‘no intervention’ served as the referent category), clinical site (the clinical site where the principal investigator was located served as the referent category). In all three regression models, variables that were not significant at the level of P = 0.05 were removed. Unstandardized estimates of the beta coefficients as well as the adjusted R 2 are presented. To explore the effects of level of exposure to the implementation intervention on the change in intention, attitude, subjective norm and perceived behavioural control, repeated measures analysis of variance was performed using mixed models for unbalanced data followed by post hoc comparisons between intervention groups. The Statistical Analysis System version 8.2 (SAS Institute, Cary, NC, USA) was used for data analysis. This study was approved by the Ethics Committee of the University of Ottawa and the Ethics Committees of the five health‐care institutions in which the study took place.
Results
The eligible population was composed of 76 physicians and 84 residents. A total of 67 clinical teachers and 53 residents in family medicine entered this study (response rate = 75%). Nine physicians and 31 residents did not participate. At one site, clinical teachers did not give permission to contact the residents (n = 17). When compared with the 120 physicians and residents participating in the study, the other 23 who did not participate were more likely to be male (P = 0.02). There was no difference between the status of those who participated and those who did not (P = 0.06). When compared with the clinical teachers who participated (n = 67), those who did not participate (n = 9) were more likely to have been in practice for 30 years or more than for less than 30 years (P = 0.02). Figure 1 presents the flow of participants for those who completed all phases of the trial for the sample frame of physicians and residents. In one clinical site, one nurse practitioner and one nutritionist enrolled in the study and were included in the final analyses, thus providing a total of 122 cases at entry into the study. One individual did not provide the exit questionnaire, leaving 121 participants who provided entry and exit data.
Figure 1.
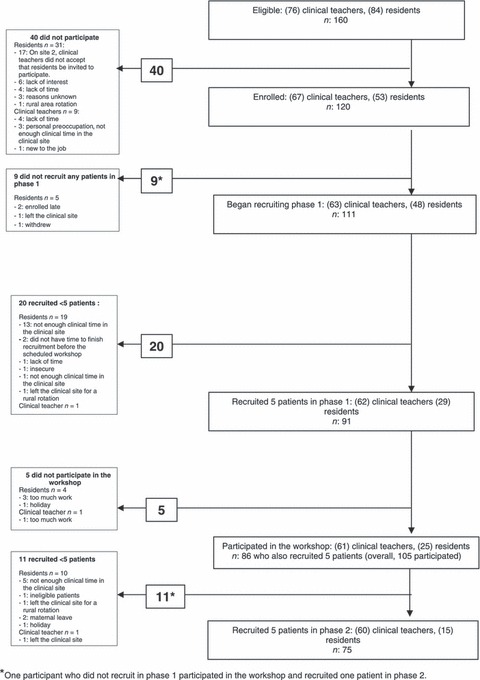
Flow of the study participants.
Table 1 presents sociodemographics of participants. Fifteen participants did not attend the workshop and did not recruit any patients in phase 2. Among those, 11 had recruited at least one patient in phase 1. These 15 participants comprised the no exposure group. Eighteen participants recruited patients in Phase 1 and attended the workshop where they received feedback. However, they did not recruit any patients in phase 2. One participant did not recruit any patients in Phase 1 but attended the workshop and recruited one patient in phase 2. These 19 participants comprise the incomplete exposure group. The largest proportion of participants (n = 88) recruited at least one patient in phase 1 and attended the workshop where they received feedback. They also recruited at least one patient in phase 2 where they received a reminder at the point of care about the ODSF. This group comprised the complete exposure group.
Table 1.
Characteristics of participants (n = 122*)
| Characteristics | Mean ± SD |
|---|---|
| Age | 35.5 ± 9.4 |
| Number of years in practice for clinical teachers (n = 67)* | 15.0 ± 8.6 |
| Hours per week spent in professional activities | 43.5 ± 12.6 |
| Number of patients per week seen in consultation† | 37.1 ± 26.1 |
| Physicians’ reactions to uncertainty (Gerrity, 1995)34 | |
| Stress | 15.0 ± 4.7 |
| Disclosing Uncertainty to Patients | 12.1 ± 4.4 |
| Frequency (%) | |
| Status | |
| Residents 1st year | 32 (26.2%) |
| Residents 2nd year | 21 (17.2%) |
| Clinical teachers in family medicine | 67 (54.9%) |
| Nurse | 1 (0.8%) |
| Nutritionist | 1 (0.8%) |
| Female | 83 (68%) |
| Diplomas (other than the medical degree)‡ | |
| Baccalaureate | 32 (26.2%) |
| Master’s degree (MSc) | 17 (13.9%) |
| Doctoral degree (PhD) | 13 (10.7%) |
| Others | 5 (4.1%) |
| Committee participation in the last year‡ | |
| Institution | 41 (33.6%) |
| University | 45 (36.9%) |
| Regional | 8 (6.6%) |
| Provincial | 6 (4.9%) |
| National | 7 (5.7%) |
| Others | 5 (4.1%) |
| Continuing medical education activities attended in the past year‡ | |
| Institution | 93 (76.2%) |
| Regional | 68 (55.7%) |
| Provincial | 63 (51.6%) |
| National | 21 (17.2%) |
| International | 9 (7.4%) |
| Others | 3 (2.5%) |
| Preference in decision‐making style (Strull, 1984) | |
| Patient alone | 12 (9.8%) |
| Patient after considering my opinion | 40 (32.8%) |
| Patient and myself | 49 (40.2%) |
| Myself after considering the patient’s opinion | 18 (14.8%) |
| Myself alone | 0 (0%) |
| Did not answer | 3 (2.5%) |
*Except if stated otherwise, data is provided for the 122 participants who provided an entry questionnaire.
†Excluding patients seen when on call. For clinical teachers (n = 62): 45 ± 28, and for residents and other physicians (n = 44): 26 ± 19.
‡Categories are not mutually exclusive.
On entry to the study, only 12 participants reported knowing the DCS (9.8%). Seven of those were from the clinical site where the principal investigator was located. Eleven of those who knew the DCS before entering the study estimated having used it in only 9.6% ± 20.0% of clinical encounters. At entry into the study, the 122 participants estimated that the DCS could be useful in 61.5% ± 29.2% of clinical encounters. However, those who provided an exit questionnaire (n = 121) estimated that the DCS could be useful in 53.3% ± 29.1% of clinical encounters. Less than half of the participants (44.3%) reported using the DCS outside of the study context. On exit, satisfaction with having been introduced to the DCS was rated 7.6 ± 2.0 (on a scale from ‘0’‘very low’ to ‘10’‘very high’).
Table 2 summarizes the means and standard deviation (SD) of the variables at baseline and at the exit from the study. In bivariate analyses, stress due to uncertainty and reluctance to share uncertainty with patients were not associated with the behavioural intention of interest at entry (P = 0.64 and 0.42 respectively). However, there was a weak negative association between reluctance to share uncertainty with patients and the behavioural intention at exit (r = −0.184, P = 0.04).
Table 2.
Effects of the level of exposure to the intervention over time: mixed models for unbalanced repeated measure of analysis of variance
| At the entry in the study | At the exit from the study | F‐ratio | |||||
|---|---|---|---|---|---|---|---|
| No intervention | Incomplete exposure | Complete exposure | No intervention | Incomplete exposure | Complete exposure | ||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD | Mean (SD) | Mean (SD) | Group × Time | |
| Intention to use DCS (range −3 to +3) | 0.31 (1.60) | 0.52 (1.20) | 0.68 (1.24) | −0.62 (1.60) | 0.74 (1.15) | 0.92 (1.29) | F 2, 118 = 6.13** |
| Attitude (range −3 to +3) | 1.10 (1.30) | 1.18 (0.70) | 1.19 (0.92) | 0.72 (0.94) | 1.35 (0.82) | 1.36 (0.88) | F 2, 118 = 1.69 |
| Perceived behavioural control (range −3 to +3) | 0.95 (1.35) | 0.63 (1.21) | 0.83 (1.17) | −0.07 (1.21) | 0.89 (1.13) | 1.11 (1.01) | F 2, 118 = 6.72** |
| Subjective norm (range −3 to +3) | 0.67 (1.26) | 0.91 (1.33) | 0.64 (1.39) | 0.33 (1.27) | 1.36 (0.81) | 0.84 (1.06) | F 2, 118 = 2.05 |
*P < 0.05, **P < 0.01.
Using all 121 complete cases, there was no change in the intention to use the DCS between baseline and exit (P = 0.375). However, Table 2 shows that there was a statistically significant difference between entry and exit between the groups based on their level of exposure to the implementation intervention on the intention (F 2, 118 = 6.13, P = 0.003) and on the perceived behavioural control (F 2, 118 = 6.72, P = 0.002). We found a significant group by time effect on the intention. This scale measures the intention to use the DCS in clinical practice. Low scores indicate a low level of intention. Results indicate a significant decrease in the no intervention group and a slight increase in the other two groups. In post hoc comparisons, we found that for the complete exposure group, the change from baseline had a P‐value of 0.06, for the no exposure group, the change from baseline had a P‐value of 0.0035. We also found that the difference in score that was observed between the no exposure group and the complete exposure group at exit had a P‐value of 0.0001 and that the difference in score that was observed between the no exposure group and the incomplete exposure group at exit had a P‐value of 0.0032.
Similarly, we found a significant group by time effect on the PBC. This scale measures the perception of control (perception of barriers and facilitators) to use the DCS in clinical practice. Low scores indicate a low level of PBC. Results indicate a significant decrease in the no intervention group and a slight increase in the other two groups; with the complete exposure group experiencing a statistically significant change from baseline (P = 0.03). In post hoc comparisons, we found that for the no exposure group, the change from baseline had a P‐value of 0.003. We also found that the difference in score that was observed between the no exposure group and the complete exposure group at exit had a P‐value of 0.0003 and that the difference in score that was observed between the no exposure group and the incomplete exposure group at exit had a P‐value of 0.01.
The results of the multivariate analyses are presented in Table 3. First, the following variables as assessed at entry into the study were positively associated with the intention to use the DCS at entry: attitude (P < 0.001), subjective norm (P < 0.001) and perceived behavioural control (P < 0.001). Overall, the variable ‘clinical site’ was not statistically significant (P = 0.094). However, when compared to being located in the referent clinical site, being located in the clinical site 1 had a statistically significant influence on the intention at baseline (P < 0.05). No other variables added to the explained variance in the intention at entry into the study.
Table 3.
Regression models
| Intention at the entry regressed on the constructs measured at the entry (n = 122) | Intention at the exit regressed on the constructs measured at the exit (n = 121) | Difference between the intention (intention at the exit – intention at entry) regressed on the constructs measured at entry (n = 121) | ||||
|---|---|---|---|---|---|---|
| β | Standard error | β | Standard error | β | Standard error | |
| Intercept | −0.321* | 0.158 | −0.765*** | 0.217 | −0.430 | 0.317 |
| Variables from the theory of planned behaviour | ||||||
| Attitude | 0.515*** | 0.096 | – | – | −0.456*** | 0.108 |
| Subjective norm | 0.284*** | 0.054 | 0.478*** | 0.061 | – | – |
| Perceived | 0.265*** | 0.075 | 0.552*** | 0.076 | – | – |
| Behavioural | ||||||
| Control | ||||||
| Stress to uncertainty | – | – | – | – | – | – |
| Reluctance to share uncertainty | – | – | – | – | – | – |
| Other variables | ||||||
| Clinical site | ||||||
| Site 1 | −0.652* | −0.615* | 0.279 | – | – | |
| Site 2 | −0.148 | 0.021 | 0.193 | – | – | |
| Site 3 | 0.059 | 0.041 | 0.175 | – | – | |
| Site 4 | 0.104 | 0.013 | 0.181 | – | – | |
| Site 5 | (Referent) | (Referent) | (Referent) | – | – | |
| Other diploma | – | – | 0.263* | 0.131 | – | – |
| CME International | – | – | 0.553* | 0.243 | – | – |
| Intervention | N/A | N/A | ||||
| Complete | 0.560* | 0.215 | 1.208*** | 0.317 | ||
| Incomplete | 0.261 | 0.251 | 1.188 | 0.388 | ||
| No intervention | (Referent) | (Referent) | (Referent) | (Referent) | ||
| Adjusted R 2 | 0.70 (F 7,114 = 37.96, P < 0.0001) | 0.78 (F 10,109 = 38.43, P < 0.0001) | 0.21 (F 3,117 = 10.55, P < 0.0001) | |||
N/A: This variable was not entered in this model.
–: This variable was not kept in the final model.
***P < 0.001, **P < 0.01, *P < 0.05.
Second, the following variables as assessed at exit from the study were associated with the intention to use the DCS in clinical practice at exit: subjective norm (P < 0.001) and perceived behavioural control (P < 0.001). Three other variables were kept in the final model: no exposure compared with complete exposure to intervention (P < 0.05), CME activities at the international level (P < 0.05) and other diplomas (P < 0.05). Overall, the variable ‘clinical site’ was not statistically significant (P > 0.05). However, when compared to being located in the referent clinical site, being located in the clinical site 1 was statistically significantly related to intention at exit (P < 0.05).
When the behavioural intention of interest at exit was regressed on the variables as assessed at entry and the intention to use the DCS at the entry (entered as a covariate), two variables explained 42% of the variance in the dependent variable: intention at entry (P < 0.001) and level of exposure to the implementation intervention [no exposure compared to complete exposure (P < 0.01) and no exposure compared to incomplete exposure (P < 0.001)] (data not shown).
Discussion
This study is important because it addresses three of the most important challenges that have been identified in the existing body of research on informed and shared decision making. 48 , 49 First, it articulates the theoretical model on which it was based, i.e. the theory of planned behaviour, and pushes forward in adding constructs from other theoretical models: diffusion of innovations 37 and the physicians’ reaction to uncertainty. 33 , 34 , 50 Second, it uses reliable measures of the intention, attitude, subjective norm, and perceived behavioural control towards the screening of decision conflict with the DCS in clinical practice 22 as well as stress due to uncertainty and reluctance to disclosing uncertainty to patients. 34 Last, it provides insight on a French‐speaking group of Canadian health professionals thus enlarging the perspective on the application of shared decision‐making in diverse ethnic groups. Therefore, results from this study improve the knowledge base in two areas: implementation of shared decision‐making in clinical practice and health‐care professional behavioural change. Moreover, to the best of our knowledge, this is the first study to report on the intention of health professionals to screen for decisional conflict in clinical practice, one of the key competencies of shared decision making, using the theory of planned behaviour thus laying the grounds for future implementation strategies that would be theoretically‐driven.
In line with the theory of planned behaviour, the intention to use the DCS at entry into the study was largely explained by the attitude, subjective norm and perceived behavioural control. However, following exposure to the intervention, factors influencing the behavioural intention of interest had changed. The exit intention was still influenced by subjective norm and perceived behavioural control but not by attitude as assessed at the exit. This supports findings from other studies in the field of adoption of an innovation 51 and in the field of health related behaviour. 52 Perceived outcomes of adopting a new behaviour are likely to change over time when the potential adopters actually go through the experience. Potential adopters may become disillusioned because the new behaviour does not produce the positive expected outcomes. This might relate to the expectancy disconfirmation model in which the health professional forms beliefs about the performance of the new behaviour upon prior experience and/or communication about it. His actual response will depend on the degree to which performance of the new behaviour is consistent with his expectations. 53 If his expectations are not met, he may be dissatisfied and then reverse his intention to adopt the new behaviour. Moreover, personal experience with the new behaviour is likely to create a better perception of what the barriers and facilitators to implementing it in one’s own clinical practice really are. This emphasizes the importance of monitoring longitudinally the factors influencing the translation and use of evidence in clinical practice so they can be addressed rapidly by modifying the implementation strategy. 54
Compared to no intervention, a combination of an interactive workshop 41 , feedback 42 and a reminder at the point of care 43 was positively associated with the intention to use the DCS in clinical practice. Three explanations are possible. On the one hand, given the pragmatic nature of the study design, self‐selection of enrolled subjects into one of the three groups is possible (i.e. selection bias). This would be congruent with the gradient of intention that is observed across the three groups of health professionals based on their level of exposure to the implementation strategy at the entry into the study. Based on the diffusion of innovation theory, these individuals could be referred to as ‘laggards’ or ‘traditional’ and would represent the group of individuals who are the most resistant to change. 37 However, the lack of gradient in the perceived behavioural control that is observed at the entry into the study would not be congruent with a selection bias. Another possible explanation is that among the 15 physicians who were not exposed to any component of the intervention, 11 had recruited at least one patient in phase 1 and thus, completed one DCS. It is possible that experiencing the DCS produced a better understanding of what this new behaviour was all about by making the barriers to its use clearer to some of the participants. Thus, it may have lowered their perceived behavioural control that is their personal appreciation of the barriers to adopt such behaviour in routine clinical practice. This is congruent with the observation that over time, the perceived behavioural control of this group decreased from the point of entry into this trial. The third possible explanation is that notwithstanding the limitations of this study, the combination of an interactive workshop, feedback and a reminder at the point of care may be more effective at improving the behavioural intention (or avoiding a deterioration of the behavioural intention) than only a combination of two components or no intervention at all. Given the conflicting results on the effectiveness of multiple interventions on health‐care professionals’ behaviour change, 55 more research using experimental designs will need to be performed.
The two constructs from the PRU did not add to the explained variance in the behavioural intention above those of attitude, subjective norm and perceived behavioural control. Therefore, it is possible, in accordance with the theory of planned behaviour, that the influence of this construct occurs through the main constructs of attitude, subjective norm and perceived behavioural control. However, in bivariate analyses, reluctance to disclose uncertainty to patients was found to be weakly associated with the intention at exit in the expected direction. In other words, at exit from this study, the more a physician was reluctant to shared uncertainty with patients, the less likely he or she had the intention to screen for decisional conflict in his or her patients. Given that sharing uncertainty is a key component of shared decision making, 5 , 32 more studies will need to be performed assessing this predisposition of health professionals towards uncertainty in relationship with shared decision making.
In contrast, years of formal education and a cosmopolitan perspective were shown to be positively associated with the intention to use the DCS in clinical practice. Fifty years ago, Coleman and colleagues (1959) found that among 125 physicians, those who attended more specialty meetings that included out‐of‐town meetings, adopted a new antibiotic at a much faster pace than those who did not. 39 Similarly, among a group of 95 health officers, a more cosmopolitan perspective was found to speed the adoption of new programs. 38 Thus, it appears that these characteristics might play a role as effect modifiers in the adoption by health professionals of screening for decisional conflict in clinical practice. Therefore, in future studies targeting health professionals’ behavioural change in this area, they should be assessed.
Limitations of the study
In spite of its interesting findings, this study has a number of limitations. First, from a theoretical perspective, the behaviour under study was not defined as precisely as in other study of health professionals’ behaviour (i.e. principle of compatibility). In other words, we did not specify a clinical situation or a time frame for the behaviour of interest such as within the next 3 weeks. A short paragraph introduced the DCS to health professionals by providing them with a definition of decisional conflict and an example of its short clinical version. Although in the workshop a video was used to provide an example of using the DCS for a specific clinical situation (hormone therapy for relieving symptoms of menopause), the questionnaires did not refer to a specific clinical situation. Perhaps this lowered the perceived clinical relevance of the DCS and affected the determinants of the intention to use the DCS accordingly. Second, we did not address the gap between intention and behaviour. In other words, we did not assess how the intention was related to the behaviour of interest in subsequent clinical practice. However, based on the most recent literature, behavioural intention is recognized as the most important predictor of behaviour. 56 Third, although 75% of the target population participated in this study, we cannot infer that its results can be generalized to other clinical settings. This study was conducted in academically oriented clinical settings. Physicians who act as clinical teachers in these clinical settings are not representative of their colleagues in private practice. 57 On the other hand, these clinical settings represent a unique opportunity to study implementation of shared decision making within an environment that has an influence on practice patterns of future physicians. 58 Lastly, given that the objective of the main study was to assess the impact of implementing the ODSF on the agreement between patients’ decisional conflict scores and those of their primary care provider, 40 we cannot infer that the multifaceted implementation strategy that was used throughout this trial had an impact on the behavioural intention. At the time this study was planned, there was no theoretical basis for designing an effective implementation strategy to implement screening for decisional conflict in clinical practice. Although the implementation strategy that was used in the main study was evidence‐based, it was not theory‐based. Thus, it could not have been expected to impact on the behavioural intention of interest.
Implication of the study results
In summary, results from this study point to the need to monitor over time the implementation process of evidence‐based innovation into health professionals’ practices. In this regard, behavioural intention and its determinants as explained by the theory of planned behaviour could be useful for designing the appropriate interventions that will improve the uptake of the desired change in practice. This study also underscores the importance and challenges of managing outcome expectations of health professionals towards shared decision making in a longitudinal perspective. Therefore, if shared decision making is valued and desirable, then more resources will need to be devoted to its implementation in clinical practice. Results from this study will provide the necessary theoretical basis for future implementation studies in this area. Future trials aimed at changing health professionals behaviour would gain from integrating the theory‐driven measurements that were developed for this study and for designing intervention that would address the determinants of screening for decisional conflict in clinical practice that were identified. Only then will the knowledge base for translating shared decision making into practice will be complete.
Acknowledgements
We would like to thank the participating health professionals. Dr Légaré is Tier 2 Canada Research Chair in Implementation of Shared decision‐making in Primary Care. Dr O’Connor is Tier 1 Canada Research Chair in Health Related Decision Support. Dr Ian Graham holds a new scientist award from CIHR. This research was supported by the Canada Research Chair in Health Related Decision Support, ICEBERG, Chaire Andrée et Lucie Chagnon pour une approche intégrée en santé and the Centre de recherche du CHUQ, Hôpital St‐François d’Assise.
References
- 1. Hibbard JH. Engaging health care consumers to improve the quality of care. Medical Care, 2003; 41: I61–I70. [DOI] [PubMed] [Google Scholar]
- 2. Coulter A. Paternalism or partnership? Patients have grown‐up and there’s no going back [editorial; comment] [see comments]. BMJ, 1999; 319: 719–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Towle A, Godolphin W. Framework for teaching and learning informed shared decision‐making. BMJ, 1999; 319: 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weston WW. Informed and shared decision‐making: the crux of patient centred care. CMAJ, 2001; 165: 438–439. [PMC free article] [PubMed] [Google Scholar]
- 5. Towle A, Godolphin W. Education and training of health care professionals In: Edwards A, Elwyn G. (eds) Evidence‐Based Patient Choice Inevitable or Impossible? Oxford: Oxford University Press, 2001: 245–270. [Google Scholar]
- 6. Elwyn G, Edwards A, Kinnersley P. Shared decision‐making in primary care: the neglected second half of the consultation. British Journal of General Practice, 1999; 49: 477–482. [PMC free article] [PubMed] [Google Scholar]
- 7. Carpenito LJ. Decisional conflict In: Carpenito LJ. (ed) Nursing Diagnosis: Application to Clinical Practice. Philadelphia, PA: Lippincott, Williams & Wilkins, 2000: 312–321. [Google Scholar]
- 8. O’Connor AM. Validation of a Decisional Conflict Scale. Medical Decision Making, 1995; 15: 25–30. [DOI] [PubMed] [Google Scholar]
- 9. Elwyn G, O’Connor A, Stacey D et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ, 2006; 333: 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O’Connor AM, Stacey D, Entwistle V et al. Decision aids for people facing health treatment or screening decisions (Cochrane Review). The Cochrane Database of Systematic Reviews, 2003; 2: CD001431. [DOI] [PubMed] [Google Scholar]
- 11. Makoul G, Arntson P, Schofield T. Health promotion in primary care: physician‐patient communication and decision making about prescription medications. Social Science and Medicine, 1995; 41: 1241–1254. [DOI] [PubMed] [Google Scholar]
- 12. Holmes‐Rovner M, Valade D, Orlowski C et al. Implementing shared decision‐making in routine practice: barriers and opportunities. Health Expectations, 2000; 3: 182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elwyn G, Edwards A, Wensing M et al. Shared decision‐making: developing the OPTION scale for measuring patient involvement. Quality & Safety in Health Care, 2003; 12: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frosch DL, Kaplan RM. Shared decision‐making in clinical medicine: past research and future directions. American Journal of Preventive Medicine, 1999; 17: 285–294. [DOI] [PubMed] [Google Scholar]
- 15. Gravel K, Legare F, Graham ID. Barriers and facilitators to implementing shared decision‐making in clinical practice: A systematic review of health professionals’ perceptions. Implementation Science, 2006; 1: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Howell JW. Physicians’ Opinions About Patient Involvement in Health and Medical Care Decisions and Telephone‐Based Decision Support. Denver: University of Colorado, 1999. [Google Scholar]
- 17. Stacey D, Graham I, O’Connor AM, Pomey M. Barriers and facilitators influencing call center nurses’ decision support for callers facing values‐sensitive decisions: a mixed methods study. Worldviews on Evidence-Based Nursing, 2005; 2: 184–195. [DOI] [PubMed] [Google Scholar]
- 18. Grol R, Leatherman S. Improving quality in British primary care: seeking the right balance. British Journal of General Practice, 2002; 52(Suppl.): S3–S4. [PMC free article] [PubMed] [Google Scholar]
- 19. Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet, 2003; 362: 1225–1230. [DOI] [PubMed] [Google Scholar]
- 20. Hardeman W, Johnston M, Johnston DW et al. Application of the theory of planned behaviour in behaviour change interventions: a systematic review. Psychology and Health, 2002; 17: 123–158. [Google Scholar]
- 21. Briss PA, Zaza S, Pappaioanou M et al. Developing an evidence‐based guide to community preventive services–methods. The Task Force on Community Preventive Services. American Journal of Preventive Medicine, 2000; 18: 35–43. [DOI] [PubMed] [Google Scholar]
- 22. Ajzen I. Attitudes, Personality and Behavior. Milton Keynes, UK: Open University Press, 1988. [Google Scholar]
- 23. Godin G, Boyer R, Duval B et al. Understanding physicians’ decision to perform a clinical examination on an hiv seropositive patient. Medical Care, 1992; 30: 199–207. [DOI] [PubMed] [Google Scholar]
- 24. Millstein SG. Utility of the theories of reasoned action and planned behavior for predicting physician behavior: a prospective analysis. Health Psychology, 1996; 15: 398–402. [DOI] [PubMed] [Google Scholar]
- 25. Godin G, Kok G. The theory of planned behavior: a review of its applications to health‐related behaviors. American Journal of Health Promotion, 1996; 11: 87–98. [DOI] [PubMed] [Google Scholar]
- 26. Walker AE, Grimshaw JM, Armstrong EM. Salient beliefs and intentions to prescribe antibiotics for patients with a sore throat. British Journal of Health Psychology, 2001; 6: 347–360. [DOI] [PubMed] [Google Scholar]
- 27. Park ER, DePue JD, Goldstein MG et al. Assessing the transtheoretical model of change constructs for physicians counseling smokers. Annals of Behavioral Medicine, 2003; 25: 120–126. [DOI] [PubMed] [Google Scholar]
- 28. Gagnon MP, Godin G, Gagne C et al. An adaptation of the theory of interpersonal behaviour to the study of telemedicine adoption by physicians. International Journal of Medical Informatics, 2003; 71: 103–115. [DOI] [PubMed] [Google Scholar]
- 29. Liabsuetrakul T, Chongsuvivatwong V, Lumbiganon P, Lindmark G. Obstetricians’ attitudes, subjective norms, perceived controls, and intentions on antibiotic prophylaxis in caesarean section. Social Science and Medicine, 2003; 57: 1665–1674. [DOI] [PubMed] [Google Scholar]
- 30. Rutter D, Quine L. Social cognition models and changing health behaviours In: Rutter D, Quine L. (eds) Changing Health Behaviour Intervention and Research with Social Cognition Models. Buckingham: Open University Press, 2002: 1–27. [Google Scholar]
- 31. Sheeran P. Intention‐behaviour relations: a conceptual and empirical review In: Stroebe W, Hewstone M. (eds) European review of Social Psychology. Chichester, UK: Wiley, 2002: 1–36. [Google Scholar]
- 32. Elwyn G, Edwards A, Hood K et al. Achieving involvement: process outcomes from a cluster randomized trial of shared decision‐making skill development and use of risk communication aids in general practice. Family Practice, 2004; 21: 337–346. [DOI] [PubMed] [Google Scholar]
- 33. Gerrity MS, DeVellis RF, Earp JA. Physicians’ reactions to uncertainty in patient care. a new measure and new insights. Medical Care, 1990; 28: 724–736. [DOI] [PubMed] [Google Scholar]
- 34. Gerrity MS, White KP, DeVellis RF, Dittus RS. Physicians’ reactions to uncertainty: refining the constructs and scales. Motivation and Emotion, 1995; 19: 175–191. [Google Scholar]
- 35. Gordon GH, Joos SK, Byrne J. Physician expressions of uncertainty during patient encounters. Patient Education and Counseling, 2000; 40: 59–65. [DOI] [PubMed] [Google Scholar]
- 36. Allison JJ, Kiefe CI, Cook EF et al. The association of physician attitudes about uncertainty and risk taking with resource use in a Medicare HMO. Medical Decision Making, 1998; 18: 320–329. [DOI] [PubMed] [Google Scholar]
- 37. Rogers EM. Diffusion of Innovations, 4th edn New York: The Free Press, 1995. [Google Scholar]
- 38. Becker MH. Factors affecting diffusion of innovations among health professionals. American Journal of Public Health and the Nation's Health, 1970; 60: 294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Coleman J, Menzel H, Katz E. Social processes in physicians’ adoption of a new drug. Journal of chronic diseases, 1959; 9: 1–19. [DOI] [PubMed] [Google Scholar]
- 40. Legare F, O’Connor AM, Graham ID et al. Impact of the Ottawa Decision Support Framework on the agreement and the difference between patients’ and physicians’ decisional conflict. Medical Decision Making, 2006; 26: 373–390. [DOI] [PubMed] [Google Scholar]
- 41. Thomson O’Brien MA, Freemantle N, Oxman AD et al. Continuing Education Meetings and Workshops: Effects on Professional Practice and Health Care Outcomes. The Cochrane Database of Systematic Reviews, 2001; 2: CD000260. [DOI] [PubMed] [Google Scholar]
- 42. Thomson O’Brien MA, Oxman AD, Davis DA et al. Audit and feedback versus alternative strategies: effects on professional practice and health care outcomes. The Cochrane Database of Systematic Reviews, 2002; 2: CD000260. [DOI] [PubMed] [Google Scholar]
- 43. Freemantle N, Harvey EL, Wolf F et al. Printed educational materials: effects on professional practice and health care outcomes. The Cochrane Database of Systematic Reviews, 2000; 2: CD000172. [DOI] [PubMed] [Google Scholar]
- 44. Legare F, O’Connor AM, Graham ID et al. Primary health care professionals’ views on barriers and facilitators to the implementation of the Ottawa Decision Support Framework in practice. Patient Education and Counseling, 2006; 63: 380–390. [DOI] [PubMed] [Google Scholar]
- 45. Légaré F, O’Connor A, Graham I et al. Supporting patients facing difficult health care decisions. Use of the Ottawa Decision Support Framework. Canadian Family Physician, 2006; 52: 476–477. [PMC free article] [PubMed] [Google Scholar]
- 46. Le Collège des médecins de famille du Canada . Profile of Family Physicians / General Practitioners (FP’s) in Quebec. Results of the 2001 National Family Physician Workforce Survey. Mississauga, ON, Canada: Le Collège des médecins de famille du Canada, 2002. [Google Scholar]
- 47. Strull WM, Lo B, Charles G. Do patients want to participate in medical decision making? JAMA, 1984; 252: 2990–2994. [PubMed] [Google Scholar]
- 48. Bowen DJ, Allen JD, Vu T et al. Theoretical foundations for interventions designed to promote informed decision making for cancer screening. Annals of Behavioral Medicine, 2006; 32: 202–210. [DOI] [PubMed] [Google Scholar]
- 49. Mullen PD, Allen JD, Glanz K et al. Measures used in studies of informed decision making about cancer screening: a systematic review. Annals of Behavioral Medicine, 2006; 32: 188–201. [DOI] [PubMed] [Google Scholar]
- 50. Gerrity MS, Earp JAL, De Vellis RF, Light DW. Uncertainty and Professional Work: Perceptions of Physicians in Clinical Practice. American Journal of Sociology, 1992; 97: 1022–1051. [Google Scholar]
- 51. Karahanna E, Straub DW, Chervany NL. Information technology adoption across time: a cross‐sectionnal comparison of pre‐adoption and post‐adoption beliefs. MIS Quarterly, 1999; 23: 183–213. [Google Scholar]
- 52. Légaré F, Godin G, Dodin S et al. Adherence to hormone replacement therapy: a longitudinal study using the theory of planned behaviour. Psychology and Health, 2003; 18: 351–371. [Google Scholar]
- 53. Tse DK, Wilton PC. Models of consumer satisfaction formation: an extension. Journal of Marketing Research, 1988; XXV: 204–212. [Google Scholar]
- 54. Logan J, Graham ID. Toward a Comprehensive Interdisciplinary Model of Health Care Research Use. Science Communication, 1998; 20: 227–246. [Google Scholar]
- 55. Grimshaw JM, Thomas RE, MacLennan G et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technology Assessment, 2004; 8: iii–iv, 1–72. [DOI] [PubMed] [Google Scholar]
- 56. Eccles MP, Hrisos S, Francis J et al. Do self‐ reported intentions predict clinicians’ behaviour: a systematic review. Implementation Science, 2006; 1: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Contandriopoulos A, Fournier M, Dassa C et al. Profil de pratique des médecins généralistes du Québec. Rapport de recherche. Montréal: Groupe de recherche interdisciplinaire en santé. Faculté de médecine. Secteur santé publique. Montréal: Université de Montréal, 2001. September. Report No.: R01‐10. [Google Scholar]
- 58. Tamblyn R, Abrahamowicz M, Dauphinee WD, Hanley JA et al. Association between licensure examination scores and practice in primary care. JAMA, 2002; 288: 3019–3026. [DOI] [PubMed] [Google Scholar]


