Abstract
Background User engagement has become a central tenet of health‐care policy. This paper reports on a case study in progress that highlights user engagement in the research process in relation to medical device development.
Objectives To work with a specific group of medical device users to uncover unmet needs, translating these into design concepts, novel technologies and products. To validate a knowledge transfer model that may be replicated for a range of medical device applications and user groups.
Methods In depth qualitative case study to elicit and analyse user needs. The focus is on identifying design concepts for medical device applications from unmet needs, and validating these in an iterative feedback loop to the users.
Results The case study has highlighted three interrelated challenges: ensuring unmet needs drive new design concepts and technology development; managing user expectations and managing the research process.
Conclusion Despite the challenges, active participation of users is crucial to developing usable and clinically effective devices.
Keywords: ethics, medical device development, user engagement, user expectations, user participation
Introduction
This article reports on a case study in progress, which comprises adults in the UK with epidermolysis bullosa (EB). EB is a rare, lifelong, genetic condition in which the skin and mucosal linings of the body are fragile and blister with ease. Wound management is part and parcel of everyday life for this group. Although there are a large number of wound care products on the market, they fail to manage the problems experienced by those with EB in relation to both materials used and product design. The case study has a dual purpose. First, to turn unmet needs in the EB group into design solutions and wound care products. Secondly, to test a knowledge transfer model that can be replicated for a range of medical device applications.
The case study is throwing up a number of challenges in relation to user engagement including managing and funding a staged research and development process, which is needs‐driven, not technology‐driven, and takes account of participant expectations. It is being conducted within an Engineering and Physical Sciences Research Council (EPSRC) funded project, MATCH (Multidisciplinary Assessment of Technologies Centre for Healthcare). Our remit in MATCH was to develop methods of user engagement in the medical device technology cycle.
Previous research and current trends in the UK highlight the importance of putting the users of health‐care services at the centre of these services through a range of policy initiatives. 1 , 2 , 3 , 4 , 5 , 6 This current emphasis on users can be seen in the context of research processes in several ways. At strategic level, the establishment of INVOLVE (http://www.invo.org.uk) demonstrates a commitment to involving users as active participants in, rather than simply ‘subjects’ of, NHS, public health and social care research. At organizational level, high profile cases of misconduct in relation to research data at Alder Hey 7 and the Bristol Royal Infirmary 8 have resulted in tighter governance procedures and an increased public awareness of issues relating to health services research and evidence‐based practice.
Overall, there is an increasing interest in engaging users, and recognition that service users’ experiential knowledge is a valid evidence source that serves to complement the input of health professionals and researchers. Indeed, it might be argued that without this ‘inside view’ the research picture is incomplete. 9 However, it is also recognized that academics and professionals can have difficulty perceiving the benefits of including users, particularly if it is felt that they lack the necessary knowledge to understand or inform the research process. 9 , 10 , 11 , 12
Background
User engagement
The literature indicates certain imperatives when trying to engage users in health research. Shalowitz and Miller 13 note that researchers have a fundamental obligation to ensure that participants are treated with respect throughout the process rather than treating them as a means to an end. Central to this is ensuring that clear, understandable information is available to individuals so that they can make informed decisions around participation. The researcher must consider how much information individuals can absorb and whether it should be delivered face‐to‐face or in written format. Those giving information must have the expertise to answer questions raised by those invited to participate.
There is a growing consensus towards giving participants information and feedback throughout the research process. This has the benefit of maintaining interest and reducing attrition rates. Moreover, as Dixon‐Woods et al. 14 report, keeping people informed is an ethically responsible stance, though one that is not necessarily straightforward and requires planning, particularly when reporting results.
The importance of giving information about the progress and on‐going findings of a research project can also help manage the expectations of participants. Societal expectations of health‐care and services are increasing but are rarely clearly defined and understood. This contributes to the negative perceptions around expectations. 15 Another dimension is the gap between the expectations and priorities of participants and those of health professionals and researchers. 16 Both sides need to define as far as possible what they expect from a project to limit any unrealistic expectations. If researchers recognize early on the potential for differences around expectations, measures can be taken to manage these.
Medical device development
Medical device development describes an iterative process of concept generation through to production and use of a device. Medical devices cover a wide range of products, from bandages, to monitors, to cardiac implants and they are classified according to the level of risk attached to their use. The case study focuses on external devices for wounds arising from an underlying medical condition. These devices are in intimate contact with the individuals concerned. Access to these individuals by non clinicians, industry in particular, for the purpose of identifying device related needs, raises a number of issues. Close user involvement in product development with early feedback mechanisms, are factors that are perceived to lead to a faster ‘time‐to‐market’ (the length of time it takes to get a product from idea to the marketplace) with fewer modifications. 17 However, several factors mitigate against embedding users in medical device development.
From a manufacturing perspective, innovation can be constrained by concerns for the financial viability of new products, fast paced technological change and the time‐to‐market. A protracted time‐to‐market phase can result in products that quickly become obsolete, whilst users can become disinterested or cynical about participating in device development particularly when there is an urgent need for new and better products.
Overall, pursuing the ideal of an iterative process of user engagement and product refinement is challenging in terms of both accessing vulnerable users and resourcing the process in the face of commercial pressures to get products into the market place.
Models of user engagement
The findings from a literature survey, locating the user in medical device development and evaluation processes, provided us with the starting point for our case study. 18 Two findings in particular, highlighted the need for a different approach to device development and evaluation. First, it became apparent that users were generally perceived as health‐care professionals who used devices on behalf of patients, rather than the patients themselves as ultimate end‐users of devices. Secondly, user involvement through the cycle of device development (concept, design, manufacture, testing and trials and production) increases the likelihood of producing devices that are safe, usable, clinically effective and appropriate to cultural context.
A few papers that highlighted this dedicated user engagement came from the lower risk devices and assistive technologies. 19 , 20 , 21 , 22 , 23 , 24 , 25 Alam 26 and Magnusson et al. 27 make the point that while the literature is rich with the descriptions and benefits of user involvement, research concerning the process of involving users in medical device development remains relatively under‐developed and poorly defined. Even with the previously mentioned issue of the push towards user engagement initiatives, in many instances, they are passive participants in research. 18
One of the papers from the literature survey described a model of consumer‐driven assistive technologies adopted by the US Veterans Association. 20 This model embedded users throughout the stages of medical device development, including commercialization of new technologies arising. The process begins with a clinically defined need and sets in action an iterative process for engaging patients and their carers. This begins at the earliest stages of concept generation and involves clinical evaluations of the devices in real life settings.
Given the range and nature of medical devices, there is no likelyhood of a single model that fits the development of all devices. The Sheredos and Cupo model appears, however, to be well suited to developing wound care devices and has thus been drawn on for the case study on the wound care needs of adults with EB. To meet the dual purpose of the case study, three interrelated challenges need to be addressed: ensuring unmet needs, drive new design concepts and technologies; managing expectations and managing the staged research process.
Case study: WEB (wound care for EB)
The case study population was identified through our involvement, as clinical researchers, in the EPSRC‐funded WRAP (Woundcare Research for Appropriate Products) project. WRAP highlighted the difficulties faced by adults with EB, their carers and specialist nurses in the management of complex wounds. 28 The WRAP project gave us close links with the EB specialist nurses from DebRA (Dystrophic Epidermolysis Bullosa Research Association), the charitable foundation who advocates for those with EB through the provision of care, support and research. Through them we have access to the user group, EB is a lifelong, rare genetic condition in which the skin is easily damaged resulting in painful wounds that are prone to infection and hard‐to‐heal: mucosal linings of the body are fragile and friable and consequently blister with extreme ease. Consequently, wound management is part and parcel of everyday life for this group. It is distressing, can be painful and is extremely time‐consuming, impacting on independence and quality of life. This situation is compounded by the fact that whilst there are a large number of wound care products on the market, they fail to manage adequately the problems experienced by those with EB in relation to both materials used and product design.
Research design: user needs‐driven process
The stages of the research process are highlighted in Fig. 1. A main feature of this case study is that it is problem‐led rather than product‐led with individuals with EB involved throughout device development to provide invaluable feedback. The initial assessment of benefits and flaws of currently used products, as gleaned from our access to the user group, provides an essential base of information for the identification of new product designs and specifications. 29 Their information has also reinforced our prior knowledge and assumptions that current products failed to manage the severe problems faced by this population.
Figure 1.
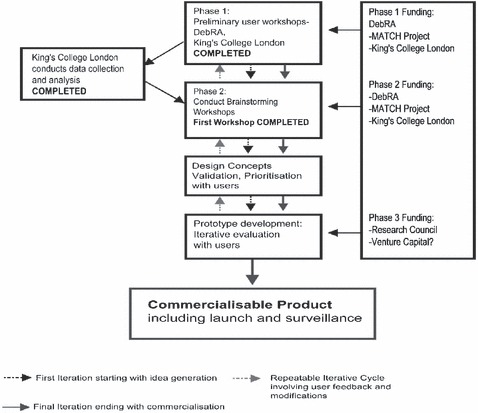
The research process.
Our approach is not only to elicit data on current products, but also to go back to the drawing board to come up with totally fresh ideas. This is especially pertinent in the field of wound care when we consider that although new materials have been developed, there has been little corresponding innovation in relation to product design. We are using a multidisciplinary approach to foster the alignment of technology, science and design. Along with the EB nurses, the multidisciplinary team comprises designers, materials scientists, engineers and a pharmacist.
Ethical issues
Gaining ethical approval for this research is necessarily an on‐going process because it is developmental and cannot be defined in total at the outset. The first application was for a series of informal workshops with patients, their carers and nurses together with the brainstorming sessions. There has been an on‐going exchange with the ethics committee updating them on progress and asking them for permission, for example, for home visits to witness dressing changes. Invitations to observe dressing changes were given out by workshop participants. Further applications to the ethics committee will be required for the proof of concept and the clinical and cost‐effectiveness studies.
Recruitment of potential workshop participants was carried out by the EB specialist nurses, which was important for several reasons. Their knowledge of the EB population and their long‐standing relationships with them meant that they were well placed to recruit individuals, taking into account the severity of their condition, their level of need and their type of wounds. It was also important to involve the carers as they are in many instances the main providers of daily care, and have in‐depth experience of the practical difficulties faced.
The workshop format was the principal method of data collection for both practical and methodological reasons. Individuals with EB comprise a small widely dispersed population whose medical care is managed through a few dedicated hospitals. Running workshops in conjunction with hospital EB clinics meant that data could be gathered from a larger number of people in a shorter period of time. The workshop format has allowed the gathering of rich data from varying perspectives, allowing participants to voice their own ideas and reflect on the responses of others. 30 As previously mentioned, it also resulted in invitations to patients’ homes to observe dressing changes and take photographs where appropriate.
The construction of the workshops had to be carefully thought out and the process was facilitated by the EB nurses. Participants were informed about the workshop format, including audio recording the proceedings and a request for one person speaking at any one time in order not to lose vital information. In addition to prior distribution of patient information sheets and consent forms, the project aims were outlined verbally, and displayed on A3 sheets, and included how we anticipated the project would proceed. Particular emphasis was given to explaining the process of analysing and anonymizing their information, before taking it to the design team to brainstorm new concepts and bringing these concepts back to the workshops. The type of information needed to guide new product development, in particular their experiences of past and current wound care products, was also explained. On the advice of the design team, it was also important to convey to the users that they were not expected to come up with actual solutions to their problems, although their thoughts on the subject were welcomed. 31
In practice, almost 4 months elapsed since receiving ethical approval until the first workshop was held. It quickly became clear that flexibility was required in relation to running workshops and that this might have a knock on effect on the project as a whole. Individuals with EB have periods when they are unwell and not able to attend workshops. For example, the first workshop had to be postponed twice because of participants being unavailable for a number of reasons including hospitalization. We also had to be sensitive to the fact that EB nurses are primarily clinicians and not researchers. Their input in the project was voluntary and on top of their already intensive work schedule. Patience and flexibility paid off and the workshops together with observations of dressing changes have yielded rich data, capturing experiences of dressing changes and obvious limitations to current dressing products.
These data have been presented to a panel of experts in a brainstorming session. Two design solutions were identified, which have been taken back to the workshop participants for discussion and approval. For commercial reasons, we are not able to describe these, but they are going forward to ‘proof of concept’ studies, subject to securing funding.
Managing the research process: including managing participant expectations
A number of factors can contribute to the successful engagement of users in the research process. 31 It is vital to get participants on board from the beginning. To this end, we held meetings with medical and nursing staff from DebRA to put forward our research proposal. We anticipated that the EB specialist nurses would receive the proposal favourably as they are only too well aware of the practical problems associated with wound management on a daily basis. For DebRA, and particularly the medical team, a research resource issue surfaced. Substantive research funds from DebRA are dedicated to understanding the genetic causes of EB and ways of preventing skin damage at the tissue level. A case had to be made for an additional research stream, with extra resources, to address problems affecting the immediate day‐to‐day lives of the EB community from a skin management perspective.
Participant expectations
Through the workshops it has become evident that participants with EB and their carers have low expectations of wound care products and of research itself. With regard to research, many of these individuals have participated in a variety of studies through the course of their lives with no apparent benefits to themselves. It is apparent, for example, that individuals with EB have low expectations of new wound care products against their high level of need. They have come to expect to ‘make do’ with ineffective products or to have to adapt existing products to meet their individual needs. In some instances, standard management techniques and dressings have been abandoned by individuals and their carers in favour of self‐devised systems. Preliminary ideas around new products have thus been greeted with a healthy dose of scepticism. This scepticism is due in part to the tendency of medical device manufacturers to design new products based on old, a practice that results in flaws and inadequacies being repeated over and over again. 32
As already indicated, through the workshops and observational work a clear picture has emerged that some wound care products are better than others, but none is effective for independent and ‘normal’ living. This has given us direction and impetus to progress the research. It has also started to raise expectations of what may come from the research and in what time frame. The strategy we are adopting to manage raised expectations is to communicate regularly about the progress and milestones of the project, interjecting reminders about the time it can take to develop novel usable products.
Phased funding process
Our funding applications are necessarily phased (see Fig. 1). This in itself is challenging in relation to keeping a research team together, and maintaining the motivation and focus of both the design team and the user community.
Logically, the higher technology innovations, using advanced materials or manufacturing processes, will take longer and cost more than the lower technology solutions. Following patent searching, we are looking to incorporate existing science, knowledge and materials, into novel applications anticipating that research and development, clinical and regulatory processes may be fast tracked. The problem identification and potential design solution process, through the workshops, has taken less time than anticipated. The expectation is that within 9 months, at least one of the ‘proof of concept’ studies will have been completed.
This remains, however, a complex process of prioritizing according to the participants’ views and planning the funding to support the various phases: prototype development, proof of concept work, clinical and cost‐effectiveness studies.
The case study is dedicated to the DebRA community. However, the new technologies are predicted to benefit a raft of other groups of patients with complex long‐term wounds, for example, patients with advanced malignant wounds. 33 In addition, the case study is potentially a proving ground project for a knowledge transfer model, with a sustainable funding mechanism.
Knowledge transfer model
The components of the model include a research team capable of translating clinical problems into design concepts and prototypes. If successful, the licensing of Intellectual Property (IP) will generate revenue back into the research team. These factors constitute the basis for a sustainable research company to drive R&D for unmet clinical needs, as illustrated in Fig. 2.
Figure 2.
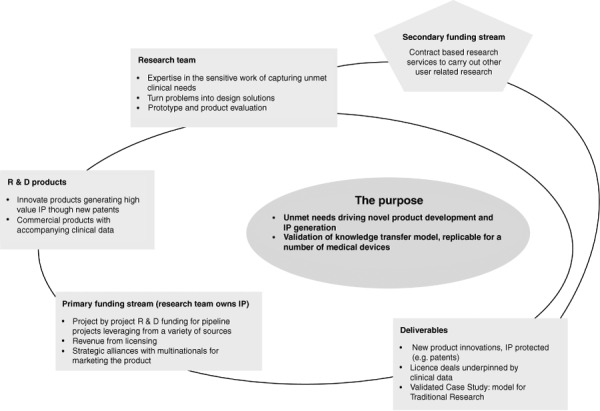
Knowledge transfer model.
This model has the potential to overcome critical difficulties in the exchange of information between manufacturers and user groups. There is no obvious route for users and clinicians to communicate their dissatisfaction about particular failures and concerns to manufacturers. 34 Both groups are consulted when products are a fait accompli and then it is too late for user input to be taken up. Equally there is no open door for wound care manufacturers to access user groups to understand poorly or unmet needs. Key drivers for this case study model are that as clinical researchers we can access user groups and through the research process ensure that their message is not lost or misinterpreted by the manufacturers. The aim here was to have a complete package, to license to the wound care manufacturers, comprising novel technologies and designs backed by clinical and cost‐effectiveness data. The anticipated value to the manufacturers is access to novel products generated from clinical need with the evidence to support tenders to health service supply chains.
Conclusion
Although there has been academic research on user engagement, there is a lack of commensurate work on the practicalities of such engagement. By conducting this case study, we are exploring many of the theoretical concepts of user engagement and the practical issues and challenges that are raised when undertaking a study of user engagement in medical device development.
The key issues that the case study is addressing centre on gaining access to vulnerable users and ensuring that user needs drive product development, as opposed to existing technologies and commercial pressures, to bring products to the market. The knowledge transfer model is addressing these issues by separating the research and development process from the commercial ones until the new technologies, driven by user needs, are protected and mature. The clinical/academic team can access the vulnerable user groups. With public and charitable funding together with the design team they can, independently and objectively, generate IP that has commercial value because it is grounded in the ultimate users’ needs and will have been validated through clinical and cost‐effectiveness studies. Clearly, this is a case study in progress and some of what is being presented is aspirational. However, it is a faithful account of the challenges and practicalities of research involving users.
Acknowledgement
This project is being conducted within the Engineering and Physical Sciences Research Council funded Multidisciplinary Assessment of Technologies Centre for Healthcare (EPSRC funding ref: GR/S29874/01).
References
- 1. Department of Health . The Expert Patient: a New Approach to Chronic Disease Management for the 21st Century. London: Department of Health, 2001. Available at: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyandGuidance/DH_4006801, accessed on 15/08/05. [Google Scholar]
- 2. Department of Health . Best Research for Best Health: A New National Health Research Strategy. London: Department of Health, 2005. Available at: http://www.dh.gov.uk/assetRoot/04/11/69/35/04116935.pdf Last Accessed 23/08/05. [Google Scholar]
- 3. Department of Health . Creating a Patient led NHS – Delivering the NHS Improvement Plan. London: Department of Health, 2005. Available at: http://www.dh.gov.uk/assetRoot/04/10/65/07/04106507.pdf, accessed on 23/08/05. [Google Scholar]
- 4. Oliver S, Harden A, Rees R. An emerging framework for including different types of evidence in systematic reviews for public policy. Evaluation, 2005; 11: 428–446. [Google Scholar]
- 5. Smith E, Ross F, Masterson A. Developing a service user framework to shape priorities for nursing and midwifery research. Journal of Research in Nursing, 2005; 10: 107–118. [Google Scholar]
- 6. Healthcare Industries Task Force . Better Health Through Partnership: a Programme for Action. London: Healthcare Industries Task Force, 2004. Available at: http://www.advisorybodies.doh.gov.uk/hitf/hitf_final_report_nov2004.pdf, accessed on 15/08/05. [Google Scholar]
- 7. Royal Liverpool Children’s Hospital, Royal Liverpool Children’s Hospital . The Report of the Royal Liverpool Children’s Inquiry, 1999. Available at: http://www.rlcinquiry.org.uk, accessed on 05/03/22. [Google Scholar]
- 8. Parliamentary Report . Learning from Bristol: The Report of the Public Inquiry into children's heart surgery at the Bristol Royal Infirmary 1984–1995. Norwich, Stationary office 2001. Available at: http://www.bristol‐inquiry.org.uk. [Google Scholar]
- 9. Hanley B, Bradburn J, Barnes M et al. Involving the Public in NHS, Public Health, and Social Care Research: Briefing Notes for Researchers. 2003. Available at: http://www.invo.org.uk/pdfs/Briefing%20Note%20final.dat.pdf. [Google Scholar]
- 10. Beresford P. User involvement in research: exploring the challenges. Nursing Times Research, 2003; 8: 1. [Google Scholar]
- 11. Entwistle VA, Renfrew MJ, Yearley S, Forrester J, Lamont T. Lay perspectives: advantages for health research. British Medical Journal, 1998; 316: 463–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liberati A. Editorial: Consumer participation in research and health care. British Medical Journal, 1997; 315: 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shalowitz DI, Miller FG. Disclosing individual results of clinical research – implications of respect for participants. Journal of the American Medical Association, 2005; 294: 737–740. [DOI] [PubMed] [Google Scholar]
- 14. Dixon‐Woods M, Jackson C, Windridge KC, Kenyon S. Receiving a summary of the results of a trial: qualitative study of participants’ views. British Medical Journal, 2006; 332: 206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Janzen JA, Silvius J, Jacobs S et al. What is a health expectation? Developing a pragmatic conceptual model from psychological theory Health Expectations, 2006; 9: 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Williamson C. Editorial: What does involving consumers in research mean? QJM: Oxford University Press, 2001; 94: 661–664. [DOI] [PubMed] [Google Scholar]
- 17. Tam M. Trends and Outlooks for New Product Development: Is R&D in Danger? CA Research Report, 2006. Redwood City, CA, USA: CA ClarityTM Research Center. Available at http://www.niku.com/research‐center‐513.html. [Google Scholar]
- 18. Bridgelal Ram M, Browne N, Grocott PR, Weir HCM. Methodologies to Capture User Perspectives in Medical Device Development: A Survey of the Healthcare Literature. Multidisciplinary Assessment of Technologies Centre for Healthcare (MATCH) Report 2005. Project 3: Deliverable 6. Available at: http://www.match.ac.uk. [Google Scholar]
- 19. Sethi PK. Appropriate technology for rehabilitation aids in developing countries. Annals of the National Academy of Medical Sciences (India), 1982; 18: 34–42. [Google Scholar]
- 20. Sheredos SJ, Cupo ME. The Department of Veterans Affairs Rehabilitation Research and Development Service’s technology transfer process. Technology & Disability, 1997; 7: 25–29. [Google Scholar]
- 21. Burkitt J, Torrens GE, Kay GH, Sandbach D, Sutherland IA. The development of the Autosip: a hygienic, self‐operated, drinking device for people with minimal sucking ability and/or minimal arm strength. Journal of Rehabilitation Science, 1995; 8: 115–118. [Google Scholar]
- 22. Hefzy MS, Nemunaitis G, Hess M. Design and development of a pressure relief seating apparatus for individuals with quadriplegia. Assistive Technology, 1996; 8: 14–22. [DOI] [PubMed] [Google Scholar]
- 23. Malassigne P, Nelson AL, Cors MW, Amerson TL. Design of the advanced commode‐shower chair for spinal cord‐injured individuals. Journal of Rehabilitation Research and Development, 2000; 37: 373–382. [PubMed] [Google Scholar]
- 24. Malassigne P, Nelson AL, Cors MW, Jensen RP, Amato M, Schnurr ES. Iterative design and evaluation of new prone carts for individuals with SCDs: a technical note. Journal of Rehabilitation Research and Development, 2002; 39: 127–139. [PubMed] [Google Scholar]
- 25. Mulholland SJ, Packer TL, Laschinger SJ, Lysack JT, Wyss UP, Balaram S. Evaluating a new mobility device: feedback from women with disabilities in India. Disability and Rehabilitation, 2000; 22: 111–122. [DOI] [PubMed] [Google Scholar]
- 26. Alam I. An exploratory investigation of user involvement in new service development. Journal of the Academy of Marketing Science, 2002; 30: 250–261. [Google Scholar]
- 27. Magnusson PR, Matthing J, Kristensson P. Managing user involvement in service innovation: experiments with innovating end users. Journal of Service Research, 2003; 6: 111–124. [Google Scholar]
- 28. Cowley SA, Richardson A, Grocott P et al. Woundcare Research for Appropriate Products – Position Paper. 2004. Available at: http://www.kcl.ac.uk/wrap/docs/position.pdf [DOI] [PubMed] [Google Scholar]
- 29. Bruseberg A, McDonagh‐Philp D. New product development by eliciting user experience and aspirations. International Journal of Human-Computer Studies, 2001; 55: 435–452. [Google Scholar]
- 30. Gibbs A. Social research update: focus groups. Online publication available at http://www.sru.soc.surrey.ac.uk.
- 31. Wilson S, Bekker M, Johnson P, Johnson H. Helping and Hindering User Involvement – a Tale of Everyday Design. Association for Computing Machinery 1997. Available at: http://acm.org/sigchi/chi97/proceedings/paper/sw‐obf.htm [Google Scholar]
- 32. Norman AN. The Design of Everyday Things. New York: Basic Books, 2002. [Google Scholar]
- 33. Grocott P, Cowley S. The palliative management of fungating malignant wounds – generalising from multiple‐case study data using a system of reasoning. International Journal of Nursing Studies, 2001; 385: 533–545. [DOI] [PubMed] [Google Scholar]
- 34. Browne N, Grocott P, Cowley S. The wound dressing supply chain within England’s National Health Service: unravelling the context for users. Journal of Nursing Management, 2004; 12: 51–61. [DOI] [PubMed] [Google Scholar]


