Abstract
Background Patients and clinicians report difficulties with the process of informed consent to clinical trials and audiotape audits show that critical information is often omitted or poorly presented. Decision aids (DAs) may assist in improving consent.
Aims This study piloted a DA booklet for a high priority breast cancer prevention trial, IBIS‐II DCIS, which compares the efficacy of an aromatase inhibitor (anastrozole) with tamoxifen in women who have had surgery for ductal carcinoma in situ (DCIS).
Method Thirty‐one Australian women participating in the IBIS‐I breast cancer prevention trial and who are currently in follow‐up agreed to read the IBIS‐II DCIS participant information sheet and the DCIS DA booklet, complete a set of standardized questionnaires, and provide feedback on the DA via a semi‐structured phone interview.
Results Women found the DA helpful in deciding about trial participation, reporting that it aided their understanding over and above the approved IBIS‐II DCIS participant information sheet and was not anxiety provoking. Women’s understanding of the rationale and methods of clinical trials and the IBIS‐II DCIS trial was very good; with more than 80% of items answered correctly. The only areas that were not understood well were the concepts of randomization and blinding.
Conclusions This study suggests that the DA will be acceptable to and valued by potential participants in the IBIS‐II DCIS study. The revised DA is currently being evaluated prospectively in a randomized controlled trial. If successful, such DAs could transform the consent process to large clinical trials and may also reduce dropout rates.
Keywords: breast cancer prevention trial (IBIS‐II DCIS), clinical trials, decision aid, decision making, informed consent
Introduction
Randomized clinical trials are the gold standard for evaluation of medical interventions. 1 , 2 However, small proportions of cancer patients are recruited into clinical trials, 3 , 4 and slow trial accrual delays the assessment and introduction of new treatments and the abandonment of less effective or dangerous ones. Selective refusal can also raise concerns about generalizability of the trial findings to the broader population. 5 , 6 Loss to follow‐up is also a concern, considering that two new participants are required for every patient who does not complete their allocated treatment and follow up. 7
Poor trial recruitment and retention may be a consequence of sub‐optimal consent procedures. Patients commonly fail to understand the rationale and design of clinical trials, which can lead to non‐participation or compromised informed consent. 8 , 9 Information delivered to patients is often unclear. 10 , 11 As clinical trials are increasing in complexity, participant information sheets and consent forms have become longer, more complex and difficult to understand. 12 The complex language and excessive detail of some trial participant information sheets and consent forms may confuse rather than enhance patient understanding of what is proposed. 13 , 14 Although there is now more awareness about language and presentation of information in participant information and consent materials, recent findings indicate that clinical trial consent materials are frequently written at an eleventh grade reading level rather than the recommended grade eight or lower. 12 Clinicians often report difficulty explaining trials to eligible patients and audio‐tape audits have shown that during many consent interviews critical information is omitted or poorly presented. 15 , 16 With increasingly large trials required to ensure adequate statistical power, a single trial may involve hundreds of investigators in many countries. Training recruiters is feasible 17 but its effectiveness may be undermined by sub‐optimal participant information and consent materials.
Decision aids (DAs) have been widely and successfully used in the standard treatment setting to improve consent. 18 DAs typically contain relevant evidence‐based information presented in a simple, clear, graphical form, and lead patients through a process of clarifying their values and weighing the advantages and disadvantages of their options prior to decision making. A systematic review of DA trials has shown that patients receiving DAs have a higher knowledge of options and outcomes, more realistic expectations, less difficulty in reaching a decision, more active participation in decision making, and no differences in anxiety levels, or satisfaction with decisions or the decision making process, compared to controls. 18 In that review, no DAs designed to improve decision making about participation in a clinical trial were identified.
In order to address these deficits, we have developed a DA to assist potential participants who are faced with the decision whether or not to participate in a new breast cancer prevention trial (IBIS‐II). IBIS‐II is a multi‐centre randomized trial being conducted internationally by Cancer Research UK (CRUK), and in Australia and New Zealand by the Australian New Zealand Breast Cancer Trials Group (ANZ BCTG). Post‐menopausal women are being recruited for: (i) IBIS‐II Prevention which involves women at increased risk of breast cancer who have not had a previous breast cancer unless it was ductal carcinoma in situ (DCIS) treated by unilateral mastectomy; and (ii) IBIS‐II DCIS which involves women who have previously been diagnosed with DCIS which has been treated by local excision (with or without radiotherapy). In IBIS‐II DCIS, women receive 5 years of either 1 mg daily of anastrozole or 20 mg daily of tamoxifen (double blind). In IBIS‐II Prevention, women receive either anastrozole, 1 mg daily for 5 years, or a placebo (double blind). The specific aim of this study was to pilot the IBIS‐II DCIS DA prior to commencing a randomized controlled trial (RCT) of the DA.
Materials and method
Stage 1: Development of the DCIS DA
The content of the DCIS DA (see Table 1 and Appendix) was based on a systematic literature review and interviews with cancer patients that generated 40 information topics relating to clinical trial participation of importance to cancer patients. The DA was developed in accordance with the National Health and Medical Research Council (NH&MRC) guidelines on consumer education materials, 19 and was based on the Ottawa DA framework. 20 The presentation of risk information was based on related literature 21 , 22 and an Australian study of 160 women at high risk of breast cancer eliciting preferences for risk format. 23 The DA was reviewed by members of the ANZ BCTG, the ANZ BCTG Consumer Advisory Panel (CAP), breast cancer clinicians and DA experts.
Table 1.
Content of the IBIS‐II DCIS decision aid
| Lay explanation of the ‘breast cancer risk’ concept and risk factors contributing to the increased risk of breast cancer |
| Numerical (e.g. eight out of 100) and graphic representations of breast cancer risk following treatment for DCIS and for the general population |
| Diagrammatic representation of management options: standard care versus clinical trial |
| Advantages and disadvantages of each management option |
| Lay description of the physical properties and mechanisms and potential risks and benefits, of anastrozole and tamoxifen (shown graphically) |
| Two 1000 dot diagrams illustrating the risks of breast cancer and side effects for women with DCIS, not treated vs. treated with tamoxifen for 5 years. |
| The rationale for conducting clinical trials in general and the IBIS‐II trial in particular, including explanations of key terms (e.g. blind, randomized study, placebo), followed by examples of reasons for joining/not joining a clinical trial |
| A table summarizing procedures and schedules of tests whilst on the trial |
| Personalized worksheets (value clarification exercises) to help participants (i) weigh up how much each risk and benefit is important to them, (ii) facilitate discussions within the family regarding the trial participation and (iii) enable the clinician to see at a glance how the patient has personalized relevant information. Before completing their own worksheet, participants were presented with examples of how other women in a similar position deliberated about available options. |
| A list of ‘Further Contacts’ detailing reliable websites for additional information |
| A reference list |
| Glossary of medical/clinical trial terms |
| ‘Your notes’ pages for patients to write questions or comments. |
Stage 2: PILOT of the DA
Participants
The DA was piloted in a consecutive sample of post‐menopausal women who are currently in follow‐up for the IBIS‐I breast cancer prevention trial 24 at the ANZ BCTG clinic at the Newcastle Mater Hospital in Newcastle, Australia. These are women aged between 35 and 70 years with an elevated risk of developing breast cancer who had been randomized to receive between 5 years of tamoxifen or placebo. Women participating in IBIS‐I were considered an appropriate pilot sample, since although they had not been treated for DCIS, they have experience in making a similar decision, and at the time of the pilot, the IBIS‐II trial had not commenced recruitment in Australia. Ethics approval for this study was obtained from the Hunter New England Area Health Service and University of Sydney Human Ethics Committees.
Procedure
Eligible women were invited to participate in the study whilst attending the ANZ BCTG clinic for their routine IBIS‐I follow‐up visit. Consenting participants were given the IBIS‐II DCIS participant information sheet, the DCIS DA booklet, and a questionnaire to take home and return in the prepaid self‐addressed envelope provided. Participants were asked to read the information sheet first, followed by the DA booklet. The hypothetical nature of the study was emphasized. Semi‐structured telephone interviews, lasting 60–90 min, were conducted with all willing participants following the receipt of the questionnaire. Interview questions were designed to elicit feedback about the clarity and utility of the DA booklet and to establish women’s knowledge about, and attitudes towards, the IBIS‐II DCIS trial.
Measures
We conducted this pilot study in order to assess the feasibility of the assessment process planned for the next RCT stage. We asked participants to complete the following standardized measures used in previous DA research, as well as two purpose‐designed scales:
Anxiety levels were assessed using a 6‐item short‐form of the state scale of the State‐Trait Anxiety Inventory (STAI). 25 The 6‐item STAI produces similar scores to the full 20‐item form with acceptable reliability and validity and sensitivity to fluctuations in state anxiety. 26
Knowledge about clinical trials was assessed using an adapted form of a 7‐item scale used previously in a breast cancer population. 27 The scale assesses understanding of the rationale and methods of randomized controlled trials. This scale has been proven to be sensitive to variability in knowledge during a randomized trial of the impact of an education booklet explaining clinical trials. 3
Perceived understanding of the IBIS‐II DCIS trial was assessed by the 14‐item quality of informed consent (QuIC) scale – Part B which has good test–retest reliability (r = 0.77) and face and content validity. 28 Items relate to general features of the trial, such as treatments and procedures, risks and benefits.
Actual understanding of the IBIS‐II DCIS trial was assessed using a purpose‐designed 13‐item scale developed by our group, assessing specific issues such as tamoxifen and anastrozole effectiveness and side effects, and test and follow‐up schedules on the IBIS‐II DCIS trial. Response options were either true/false, or in multiple‐choice format where women chose between four possible answers (one of which was ‘unsure’).
Attitudes towards participating in the IBIS‐II DCIS trial were assessed using an adapted 9‐item attitude scale developed by Marteau et al. (2001) in which women rated the trial on 7 point semantic differential scales. 29 Women also indicated on a 7‐point scale whether or not they would be inclined to participate in the IBIS‐II DCIS trial if it was offered to them.
Difficulties and satisfaction with the (hypothetical) decision‐making process were assessed using the Decisional Conflict Scale (DCS). 30 The 16‐item scale has three subscales: decision uncertainty, factors contributing to uncertainty, and perceived effective decision making. The DCS has good discriminant and construct validity, and internal consistency (Cronbach’s Alpha = 0.78–0.92) and confirmed test–retest reliability (r = 0.81).
Decision aid feedback was assessed using a 19‐item purpose‐designed measure eliciting general views on the presentation and content of information in the DA compared to the IBIS‐II DCIS participant information sheet.
Demographic information gathered included age, marital status, education, occupation, nationality, medical/allied health training and chronic medical conditions.
Data analysis
This study predominantly employed qualitative research methods. Sampling continued until theoretical saturation (no new themes) was reached. Interviews were content‐analysed into discrete themes. Data from standardized measures and the percentage of participants endorsing the DA were analysed using descriptive statistics.
Results
Of 37 eligible women, 31 (84%) agreed to participate. Women were on average 60 years of age and 29 were married. Two women had postgraduate qualifications, and 15 were in professional occupations. All but one woman was born in Australia or the UK, 11 had medical training and 11 had a chronic medical condition, including osteoporosis (n = 3). The sample was representative of women participating in the IBIS‐I trial. 31 The average length of the women’s participation since consent to the IBIS‐I trial was 8 years.
Anxiety
The DA did not appear to be anxiety provoking; mean reported anxiety was equivalent to that of age‐matched healthy women (mean = 32, SD = 3.73). 25
Understanding
Women’s understanding of the rationale and methods of trials in general was good (see Table 2). On 4/7 items, more than 80% of women answered correctly. The least understood items concerned randomization. Many women (n = 9–10) thought their doctor would know the best treatment, and would make sure they received it, although the correct information was clearly stated in the IBIS‐II information sheet and the DA booklet as follows: ‘The treatment each participant receives (either the standard or new treatment) is determined ‘randomly’ (using a computer).’ and ‘In a randomized ‘double‐blind’ study (such as IBIS‐II DCIS), neither the doctors nor the participants know who is receiving the standard treatment or the newer treatment’.
Table 2.
Women’s general knowledge about clinical trials (n = 31)
| Items | Incorrect (%) | Don’t Know (%) | Correct (%) |
|---|---|---|---|
| My doctor would know which treatment in a trial is better | 6 (20) | 4 (13) | 20 (67) |
| My doctor would make sure I got the better treatment in a clinical trial | 5 (17) | 4 (13) | 21 (70) |
| Trials test treatments nobody knows anything about | 4 (14) | 2 (7) | 23 (79) |
| Trials are only appropriate for serious diseases like cancer | 3 (10) | 3 (10) | 24 (80) |
| RCTs are the best way to find out whether treatment is better than no treatment | 3 (10) | 1 (3) | 25 (86) |
| In a RCT the treatment you get is selected by chance (using a computer) | 1 (3) | 0 (0) | 29 (97) |
| RCTs are the best way to find out whether one treatment is better than another | 0 (0) | 0 (0) | 31 (100) |
Not all participants answered all the items. Some percentages do not add up to 100 due to unavoidable inaccuracies caused by rounding.
Women reported above average levels of perceived (subjective) understanding of key components of the IBIS‐II DCIS clinical trial, with summary scores ranging from 84 to 100 (mean = 96/100; SD = 5.1). This is well above the mean of 88 reported in the normative sample of 286 cancer patients being offered a range of clinical trials. 28
Overall, women’s actual understanding of specific aspects of the IBIS‐II DCIS trial was also good (see Table 3). On 12/13 items, more than 80% of women gave the correct response. Seventeen women incorrectly thought that anastrozole had been previously studied in women with DCIS and a further four were unsure.
Table 3.
Women’s specific knowledge about the IBIS‐II DCIS trial (n = 31)*
| Items | Incorrect (%) | Unsure (%) | Correct (%) |
|---|---|---|---|
| Anastrozole has been previously studied in women with DCIS | 17 (55) | 4 (13) | 10 (32) |
| Participating in the IBIS‐II DCIS study is my only management option | 4 (13) | 2 (7) | 24 (80) |
| I cannot take HRT (Hormone Replacement Therapy) whilst on the study | 5 (16) | 1 (3) | 25 (81) |
| The estimated risk of developing new or recurrent breast cancer in women with early stage breast cancer taking anastrozole for 5 years is: (same or less than that of women who take tamoxifen)† | 2 (6) | 3 (10) | 26 (84) |
| Whilst on the IBIS‐II DCIS study, I will receive a treatment that may prevent or slow the growth of breast cancer cells | 3 (10) | 1 (3) | 27 (87) |
| Even if a woman has gone through the menopause, tamoxifen could cause hot flushes | 2 (6) | 1 (3) | 28 (90) |
| Studies indicate that anastrozole may increase the risk of fractures and osteoporosis | 2 (6) | 1 (3) | 28 (90) |
| A potential rare side‐effect of taking tamoxifen for 5 years is that it increases the risk of developing endometrial cancer: (yes, but this is rare)† | 2 (6) | 0 (0) | 29 (94) |
| I will have to remain in the IBIS‐II DCIS study even if I decide I want to withdraw | 2 (6) | 0 (0) | 29 (94) |
| Which of the following is not involved in participation in the IBIS‐II DCIS study? (staying overnight in hospital)† | 1 (3) | 1 (3) | 29 (94) |
| Studies indicate that anastrozole has more serious side effects than tamoxifen | 1 (3) | 1 (3) | 29 (94) |
| A placebo is: (a dummy tablet such as a sugar pill)† | 0 (0) | 0 (0) | 31 (100) |
| Whilst on the IBIS‐II DCIS study, I will not know whether I’m given a new treatment (anastrozole) or a standard treatment (tamoxifen) | 0 (0) | 0 (0) | 31 (100) |
Not all participants answered all the items. Some percentages do not add up to 100 due to unavoidable inaccuracies caused by rounding.
*Items without an asterisk were worded in true/false format.
†Multiple‐choice question with correct answer in brackets.
Attitudes
Participants’ quantitative evaluation of the DA is shown in Table 4. The vast majority of participants found the DA helpful in: deciding about the trial participation (97%), understanding the information sheet (87%) and providing useful additional information to the information sheet (97%). Participants found the DA presented the risk management options in a balanced way (97%) and was not anxiety provoking (100%). Receiving both the DA booklet and the information sheet was preferable to receiving only the latter (90%). The women’s attitudes to the IBIS‐II trial were overwhelmingly positive with 97% reporting leaning toward hypothetical participation in the trial.
Table 4.
Women’s attitudes towards the IBIS‐II DCIS trial (n = 31)
| Items | Positive (5–7)* | Negative (1–3)* |
|---|---|---|
| Beneficial–harmful | 31 (100) | 0 (0) |
| A good thing–a bad thing | 26 (84) | 5 (16) |
| Rewarding–unrewarding | 29 (94) | 2 (6) |
| Easy–inconvenient | 26 (84) | 5 (16) |
| Important–unimportant | 30 (97) | 1 (3) |
| Wise–foolish | 30 (97) | 1 (3) |
| Safe–risky | 23 (74) | 8 (26) |
| In my control–out of my control | 29 (94) | 2 (6) |
| Worthwhile–insignificant | 29 (94) | 2 (6) |
| Leaning towards participation | 30 (97) | 1 (3) |
Values in parenthesis are in percentage.
*No participant reported a ‘neutral’ (4) response.
The quantitative data were supported by interview responses. Women’s first impressions of the booklet were very positive: ‘Would give women the confidence to join the trial without being coercive’; ‘It would clear up any questions’; ‘It induced deep‐thinking’; and ‘It helps you to make your own decision.’ Five women found the DA too long, yet could not identify information that could be omitted: ‘when thinking of going on the trial, you would want all the information in it’. The majority of women (n = 22) stated that the DA was compact, with the optimal amount of information: ‘You get a lot of different information pamphlets from the doctor – it’s good that all the information is contained in one booklet’.
Clear and balanced presentation of advantages and disadvantages of each management option was particularly valued: ‘the DA stated pros and cons truthfully, in a very balanced way’. Diagrams and graphs were perceived as helpful, ‘consolidating the information in the text’. Dot diagrams proved somewhat controversial, with five women finding them confusing and hard to interpret, preferring the pie chart. When asked directly, 20 women preferred the pie chart format, six liked pie charts and dot diagrams equally and three preferred the dot diagrams.
When asked what the DA adds on top of the information sheet, women commented on the friendly format and graphical/visual presentation of the booklet as well as the worksheets: ‘The information was broken down more, colour and point form was helpful’, ‘Diagrams make it easier to understand’, ‘Helped me to work through my thoughts and make a decision’.
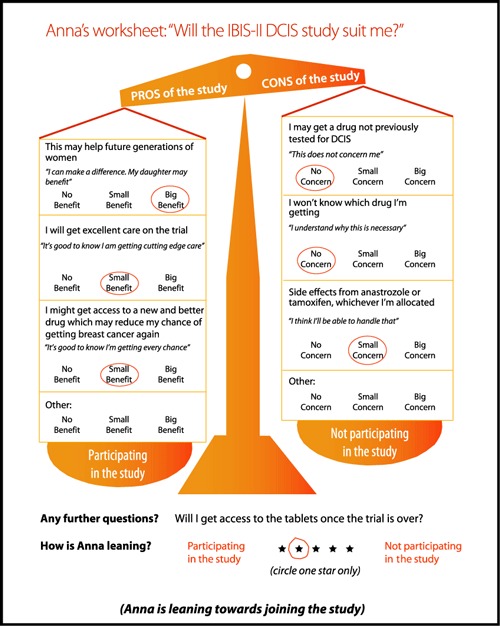
The worksheets were favoured by 26 participants: ‘They make women aware of their concerns – how they feel about certain aspects of the trial and base their decision on that’.
Almost half the sample (n = 16) would have found it helpful if the doctor/study co‐ordinator went through some of the pages with them before the booklet was taken home: ‘May be helpful to bring their attention to important points’. Yet, 11 women did not perceive this necessary: ‘because the book is very precise and clear; there is no need to explain its content’. All women stated that the DA would be helpful and reassuring later on, when the woman is on the trial: ‘Good reference for going back and looking at side‐effects as you progress through the trial’. All women recognized the DA as helpful for discussion of the trial with their partner and family: some women commented on the positive psychological effect of the booklet: ‘Takes away any fears (about the trial)’ and ‘It is nice to know you are not alone in this situation’. No consistent specific changes to the DA were recommended.
Discussion
This study piloted a decision aid for women with DCIS who will be invited to participate in the IBIS‐II DCIS trial. All women found the decision aid useful and would recommend it highly to others. Women did not report heightened anxiety after reading the decision aid and they reported that it aided their understanding over and above the IBIS‐II DCIS participant information sheet. They preferred to receive both. Further, women’s understanding of the general rationale and methods of clinical trials was substantially higher than that reported in other studies using the same measure, suggesting that the decision aid had indeed improved understanding. Thus this intervention has the potential not only to improve the process of informed consent for prospective participants, but also to enhance the quality of, and the satisfaction with, that decision.
Interestingly the only areas less well understood centred around the concepts of randomization and blinding. One‐third of women believed (or were unsure) their doctor knew which treatment was the best and would ensure they received that treatment on the trial. There are two possible explanations for this finding. First, women may simply not understand the randomization process. Alternatively, they may wish to believe that their doctor will ensure they get the best treatment, whether they receive standard care or enter a trial and regardless of which randomized treatment they receive if they enter a trial. Other studies in the standard treatment setting have shown that cancer patients rely heavily on the recommendation of their doctor in choosing treatments, and that if they feel they can trust and have faith in their doctor, they will happily accept that recommendation. 32 , 33 Thus they place a high value on their relationship with the doctor in making treatment decisions. This may be a source of some distress for patients when confronted with the issues of randomization and blinding in a clinical trial and the need for them to make their own decision whether to join a trial. Further, given the weight likely placed on the doctor’s words, the ethical pressure on the doctor to offer and facilitate choice is all the greater. However, many doctors find it difficult to discuss or acknowledge medical uncertainty, 34 , 35 possibly for fear of upsetting the ‘trust’ in the doctor–patient relationship. Indeed, explicit admissions of uncertainty by clinicians have been shown to sometimes undermine patients’ confidence 36 or even reduce the therapeutic effectiveness of individual encounters between doctor and patient. 37 This provides an additional justification for the use of objective tools to support patients’ decision making, such as the decision aid reported here.
Sixty‐eight per cent of women reported that the statement ‘anastrozole has been previously studied in women with DCIS’ was correct, (or they were unsure). This occurred despite an explicit statement to the converse in both the information sheet and DA. The DA will be amended in an attempt to further clarify this point and ensure that women understand this issue.
One of the few contentious components of the decision aid was the dot diagrams. These are traditionally included in decision aids because of research evidence that this format is most easily understood when processing complex probabilities. 38 Both dot diagrams and pie charts were included in the DA. While 2/3 of the women reported liking the dot diagrams, when asked directly, 2/3 reported preferring the pie chart over the dot diagram because it was easier to process and more straight‐forward. Other studies have found that cancer patients prefer pie charts over dot diagrams, 39 which presents a dilemma: do we provide information to patients in formats they prefer, or dot‐diagrams which have been shown to better facilitate their understanding? We have chosen to retain the dot diagrams since they allow simultaneous presentation of benefits and costs, however this issue requires further research.
Limitations
Pilot participants had already been on a trial, and were likely to be more knowledgeable and positive about trial participation than women who are currently deciding on trial participation. By the same token, the participants were likely to have expert knowledge of information needs of people on a trial and therefore be able to provide superior feedback about the DA. Further, the IBIS‐II protocol is different to that of IBIS‐I, and therefore some of the information tested was certainly new to these women. Finally, the participants were considering a hypothetical scenario and their views might not be the same if they (or others) were actually considering participation.
Conclusion
Based on the current findings, DAs would be well received by potential participants of clinical trials, and potentially foster participation, a vital element in advancing evidence‐based medicine. The final IBIS‐II DA is currently being evaluated prospectively in a randomized controlled trial. If successful, such DAs could transform the consent process to large clinical trials and may also reduce dropout rates. As many clinical trials require compliance with therapy and assessment regimes over long time periods it is advantageous if informed and motivated participants are randomized.
It is, however, important to recognize and accept that participants may have other motivations and interpretations for research participation, such as altruism. Altruism is usually based on trust and a good doctor–patient relationship and motivation to participate in research for the benefit of the community – sometimes over and above the individual’s own good. Altruism, therefore, may or may not be coupled with a desire for information, or with a desire for better understanding of information provided. It is important that future research attempts to explore these other meanings, motivations and interpretations that potential trial participants have, in order to appreciate and understand all components of the consent process to clinical trials.
Acknowledgements
We would like to thank all the participants and the staff at the Australian New Zealand Breast Cancer Trials Group centre in Newcastle, Australia, for their contribution and assistance. Support for this study was provided by the ANZ BCTG and a Susan G KOMEN Breast Cancer Foundation grant.
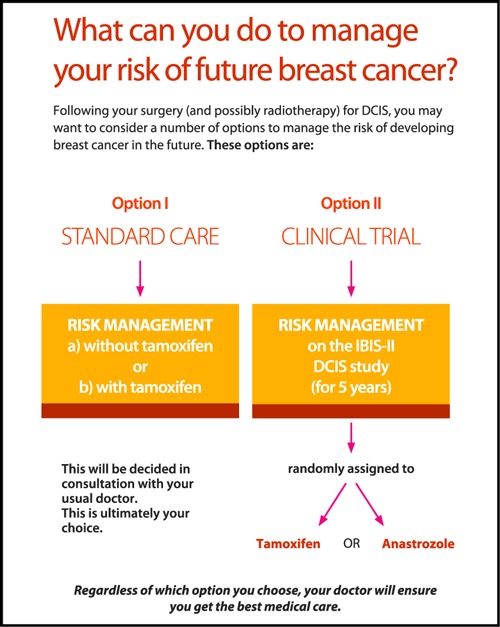
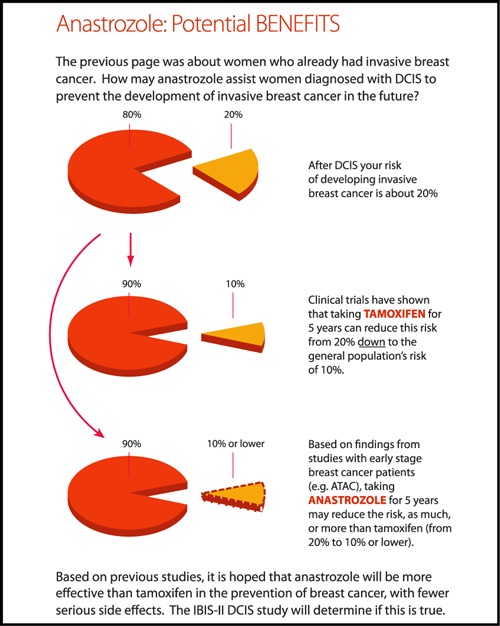
Examples of pages from the DCIS DA illustrating:
(a) Risk management options for women with DCIS.
(b) Potential benefits of anastrozole.
(c) Tamoxifen: side effects and potential risks.

(d) Personalised worksheet.
References
- 1. Sackett D, Rosenberg W, Muir J. Evidence based medicine; what it is and what it isn’t. British Medical Journal, 1996; 312: 71–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fisher B. On clinical trial participation. Journal of Clinical Oncology, 1991; 9: 1927–1930. [DOI] [PubMed] [Google Scholar]
- 3. Ellis PM, Dowsett SM, Butow PN, Tattersall MHN. Attitudes to randomised clinical trials among outpatients attending a medical oncology clinic. Health Expectations, 1999; 2: 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee JY, Breaux SR. Accrual of radiotherapy patients to clinical trials. Cancer, 1983; 52: 1014–1016. [DOI] [PubMed] [Google Scholar]
- 5. Slevin M, Mossman J, Bowling EJ et al. Volunteers or victims: patients’ views of randomised clinical trials. British Journal of Cancer, 1995; 71: 1270–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jenkins V, Fallowfield L. Reasons for accepting or declining to participate in randomised clinical trials for cancer therapy. British Journal of Cancer, 2000; 82: 1783–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simes RJ. Controlled clinical trials In: Kerr C, Taylor R, Heard G. (eds) Handbook of Public Health Methods. Sydney: McGraw Hill, 1997: 130–136. [Google Scholar]
- 8. Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent in cancer clinical trials: a cross‐sectional survey. The Lancet, 2001; 358: 1772–1777. [DOI] [PubMed] [Google Scholar]
- 9. Llewellyn‐Thomas HA, Theil ECX, Semin WC et al. Presenting clinical trial information: a comparison of methods. Patient Education and Counseling, 1995; 25: 97–107. [DOI] [PubMed] [Google Scholar]
- 10. Verheggen FWSM, Jonkers R, Kok G. Patients’ perceptions on informed consent and the quality of information disclosure. Patient Education and Counseling, 1996; 29: 137–153. [DOI] [PubMed] [Google Scholar]
- 11. Edwards SJL, Lilford RJ, Hewison J. The ethics of randomised controlled trials from the perspectives of patients, the public, and healthcare professionals. British Medical Journal, 1998; 317: 1209–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beardsley E, Jefford M, Mileshkin L. Longer consent forms for clinical trials compromise patient understanding: so why are they lengthening? Journal of Clinical Oncology, 2007; 25: e13–e14. [DOI] [PubMed] [Google Scholar]
- 13. Grossman SA, Piantadosi S, Cohavey C. Are informed consent forms that describe clinical oncology research protocols readable by most patients and their families? Journal of Clinical Oncology, 1994; 12: 2211–2215. [DOI] [PubMed] [Google Scholar]
- 14. Stead M, Eadie D, Gordon D, Angus K. “Hello, hello ‐ it’s English I speak!”: a qualitative exploration of patients’ understanding of the science of clinical trials. Journal of Medial Ethics, 2005; 31: 664–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jenkins VA, Fallowfield LJ, Souhami A, Sawtell M. How do doctors explain randomised clinical trials to their patient? European Journal of Cancer, 1999; 35: 1187–1193. [DOI] [PubMed] [Google Scholar]
- 16. Loh W, Butow PN, Brown RF, Boyle F. Ethical communication in clinical trials: issues faced by data managers in obtaining informed consent. Cancer, 2002; 95: 2414–2421. [DOI] [PubMed] [Google Scholar]
- 17. Brown RF, Butow PN, Boyle F, Tattersall MHN. Seeking informed consent to cancer clinical trials; evaluating the efficacy of doctor communication skills training. Psycho-Oncology, 2007; 16: 507–516. [DOI] [PubMed] [Google Scholar]
- 18. O’Connor AM, Roston H, Fiset V et al. Decision aids for patients facing health treatment or screening decisions: systematic review. British Medical Journal, 1999; 319: 731–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Health and Medical Research Council (NHMRC) . How to Present the Evidence for Consumers: Preparation of Consumer Publications. Commonwealth of Australia: NHMRC, 2000. [Google Scholar]
- 20. O’Connor AM, Tugwell P, Wells G, Elmslie T, Jolly E, Hollingworth G. A decision aid for women considering hormone therapy after menopause: decision support framework and evaluation. Patient Education and Counseling, 1998; 33: 267–279. [DOI] [PubMed] [Google Scholar]
- 21. Feldman‐Stewart D, Kocovski N, McConnell BA, Brundage MD, Mackillop WJ. Perception of quantitative information for treatment decisions. Medical Decision Making, 2000; 20: 228–238. [DOI] [PubMed] [Google Scholar]
- 22. Wills CE, Holmes‐Rovner M. Patient comprehension of information for shared treatment decision making: state of the art and future directions. Patient Education and Counseling, 2003; 50: 285–290. [DOI] [PubMed] [Google Scholar]
- 23. Lobb EA, Butow PN, Meiser B et al. Women’s preferences and consultants’ communication of risk in consultations about familial breast cancer: impact on patient outcomes. Journal of Medical Genetics, 2003; 40: e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cuzick J, Forbes J, Edwards R et al. First results from the International Breast Cancer Intervention Study (IBIS‐I): a randomized prevention trial. The Lancet, 2002; 360: 817–824. [DOI] [PubMed] [Google Scholar]
- 25. Spielberger CD. Manual for the State‐Trait Anxiety. Palo Alto: Consulting Psychologists Press, 1983. [Google Scholar]
- 26. Marteau TM, Bekker H. The development of a six‐item short‐form of the state scale of the Spielberger State‐Trait Anxiety Inventory (STAI). British Journal of Clinical Psychology, 1992; 31: 301–306. [DOI] [PubMed] [Google Scholar]
- 27. Ellis PM, Butow M, Simes J, Tatersall MHN, Dunn SM. Barriers to participation in breast cancer trials: a survey of attitudes to randomised clinical trials among Australian breast cancer specialists. ANZ Journal of Surgery, 1999; 69: 505–510. [DOI] [PubMed] [Google Scholar]
- 28. Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent: a new measure of understanding among research subjects. Journal of the National Cancer Institute, 2001; 93: 139–147. [DOI] [PubMed] [Google Scholar]
- 29. Marteau TM, Dormandy E, Michie S. A measure of informed choice. Health Expectations, 2001; 4: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O’Connor AM. Validation of a decisional conflict scale. Medical Decision Making, 1995; 15: 25–30. [DOI] [PubMed] [Google Scholar]
- 31. IBIS investigators . First results from the International Breast Cancer Intervention Study (IBIS‐I): a randomised prevention trial. The Lancet, 2002; 360: 817–824. [DOI] [PubMed] [Google Scholar]
- 32. Salkeld G, Solomon M, Butow P. A matter of trust – patient’s views on decision making in colorectal cancer. Health Expectations, 2004; 7: 104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Henman MJ, Butow PN, Brown RF, Boyle F, Tattersall MH. Lay constructions of decision‐making in cancer. Psycho-Oncology, 2002; 11: 295–306. [DOI] [PubMed] [Google Scholar]
- 34. Brown RF, Butow PN, Ellis P et al. Seeking informed consent to cancer clinical trials: describing current practice. Social Science and Medicine, 2004; 58: 2445–2457. [DOI] [PubMed] [Google Scholar]
- 35. Henry MS. Uncertainty, responsibility, and the evolution of the physician/patient relationship. Journal of Medical Ethics, 2006; 32: 321–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ogden J, Fuks K, Gardner M et al. Doctors expressions of uncertainty and patient confidence. Patient Education and Counseling, 2002; 48: 71–76. [DOI] [PubMed] [Google Scholar]
- 37. Di Blasi Z, Harkness E, Ernst E, Georgiou A, Kleijnen J. Influence of context effects on health outcomes: a systematic review. Lancet, 2001; 357: 757–762. [DOI] [PubMed] [Google Scholar]
- 38. Elting LS, Martin CG, Cantor SB, Rubenstein EB. Influence of data display formats on physician investigators’ decisions to stop clinical trials: a survey of attitudes to randomised clinical trials among Australian breast cancer specialists. ANZ Journal of Surgery, 1999; 69: 505–570. [Google Scholar]
- 39. Butow PN, Solomon MJ, Young JM et al. Consumer impact of an interactive decision aid for rectal cancer patients offered adjuvant therapy. Colorectal Disease, 2006; 8: 676–682. [DOI] [PubMed] [Google Scholar]


