Abstract
Introduction Shared decision making (SDM) is now considered a desirable goal in health care, yet little is known about current practice in cancer care, and its impact on patient outcomes. This study aimed to develop an oncology‐specific coding system for SDM, explore variations in SDM according to patient and disease characteristics, determine the relationship between SDM and patient satisfaction with the consultation, and explore the impact of SDM on patient anxiety.
Methods Sixty‐three medical and radiation oncology consultations with patients with primary cancer involving consideration of adjuvant therapy after surgery were audio‐taped, transcribed and coded. Intra and inter‐rater reliability of the coding system was 95 and 90% respectively. Patients completed questionnaires before and after the consultation.
Results Construct validity of the SDM coding system was successfully conducted. Oncologists demonstrated on average under 11 of 18 SDM behaviours. Behaviours seeking patient preferences were particularly rare. SDM behaviours were more apparent in consultations involving female breast cancer patients. SDM behaviour scores in combination with patient involvement preference could predict achievement of patient involvement preference but not overall patient satisfaction. Although there was no overall relationship between patient anxiety and SDM scores, it did appear that physicians may change SDM behaviour according to patient factors including anxiety.
Conclusion Our findings reinforce the importance of the doctor in facilitating shared decision making in oncology consultations.
Keywords: patient physician communication, shared decision making
Background
Decision making after cancer surgery with curative intent can be very complex, involving the consideration of combinations of chemotherapy, endocrine and radiation therapy. Furthermore, optimal treatment is increasingly individualized as multidimensional factors including medical evidence, patient values and priorities must be taken into account.
The spectrum of decision‐making models range from a traditional paternalistic view where the physician makes decisions in what he/she deems the best interests of the patient, to a patient‐centred model where all the responsibility of decision making is left to the patient alone. The latter approach may not be desirable as patients may not understand or retain all information given to them by a physician during an often tense and emotional consultation. 1
Shared decision making, where both the patient and physician have a role in medical decision making, avoids the pitfalls of both the paternalistic physician‐based approach and the exclusive patient‐centred model. In a shared decision‐making model, the patient is given information regarding their disease and possible treatments and is a participant along with the physician in medical decision making. Generally in the Western Hemisphere, most patients now prefer full information about their disease although there are studies reporting a significant proportion of patients who prefer to be given little information and/or who prefer the physician to be in charge of medical decision making. 2 , 3 , 4 Interestingly for some colorectal patients, participation in the decision‐making process was more about being involved in the consultation process and being informed rather than actually making the decisions. 4
Many oncologists treating breast cancer patients prefer a shared decision model to a paternalistic model when asked. 5 Studies have shown that patients who engage in shared decision making are most satisfied and have better psychological outcomes. 6 Multiple studies have demonstrated that cancer patients who believed they were more responsible for treatment decisions had a higher quality of life than those who perceived themselves to have had less decisional control. 7 , 8 , 9 , 10 Although SDM has not yet been clearly linked to physical health outcomes, the available evidence suggest that some level of involvement in decision making in oncology is desirable.
Unfortunately, patients often report not having achieved their desired level of participation in the decision‐making process. In one study, although the majority of patients desired a shared decision‐making model, less than half of them felt it was achieved. 11 Several strategies have been proposed to facilitate patient involvement in the decision‐making process, including multimedia programs, brochures, question prompt lists and decision aids. 12 Patient decisions can be facilitated with decision aids and cancer patients using decision aids are more satisfied with the decision‐making process while experiencing less decisional conflict. 13 Most of these interventions target the patient alone. However, recent evidence suggests that the doctor plays a critical role in facilitating patient participation in the medical consultation.
Brown et al. 12 have shown that providing cancer patients with a question prompt sheet immediately before the first oncology consultation increases patient involvement in the interview, decreases patient anxiety, improves patient recall and shortens consultation time, but only if the doctor endorses the prompt list. Subsequently, this research group provided cancer patients with a cancer consultation preparation package (CCPP) or a control booklet 14 before they saw their oncologist for the first time. The package included a booklet on the decision‐making process and medical evidence, a brochure on the rights and responsibilities of patients and a question prompt sheet. While patients who received the CCPP asked more questions, interrupted the physician more often, and challenged the information more often, they were less likely to achieve their preferred decision‐making style than the controls. The authors concluded that even though the CCPP changes patient behaviour, this alone is not enough to achieve shared decision making. It appears that physician behaviour also needs to be targeted and that a shared decision‐making approach cannot be achieved without the co‐operation of both parties.
If the doctor’s behaviour is critical, we need to understand how doctors are currently promoting shared decision making, how this varies according to patient and disease characteristics and whether specific doctor behaviours impact on patient outcomes. There are very few audits of doctor behaviour in this area. Detailed coding systems for shared decision making have been developed by two groups. 15 , 16 Both systems were used to describe general practice consultations, in contrast to consultations with cancer specialists which have some specific characteristics requiring a targeted approach.
Purpose of this study
The goal of this study was to: (i) develop a coding system to code shared decision‐making (SDM) behaviours used by cancer specialists in their consultations, (ii) explore whether SDM behaviours systematically vary according to patient and disease characteristics, (iii) explore whether patient perceptions and satisfaction with the consultation are related to the actual behaviour of the oncologist, and (iv) explore the impact of SDM on patient anxiety. It is hoped that this tool will facilitate the development of interventions which assist doctors to achieve SDM. As the study was largely exploratory, formal hypotheses are not stated.
Methods
Development of coding system
The coding system was based on a review of the literature concerning SDM in oncology. Two studies were identified which are particularly relevant. Ford et al. 17 conducted semi‐structured interviews with relevant informants and stakeholders (general practitioners, hospital doctors, practice nurses, academics and lay people) to identify key components of an evidence‐based patient choice consultation. The second influential paper was by Gattellari et al. 18 who developed a coding system to determine if patients with metastatic cancer were adequately prepared to make informed decisions. This coding system consisted of 12 elements including informational components and doctor facilitation.
A list of 23 physician behaviours and actions in consultations encompassing these concepts 18 and the six themes elucidated by Ford et al. 17 was created and reviewed by a team of medical oncologists, oncology nurses and health psychologists. This was applied to five consultations, reviewed, with appropriate adjustments made (three categories were combined, leaving 20 behaviours). The coding system is shown in Table 1, and definitions and examples are shown in Appendix 1. The coding system differs from that developed by Guimond et al., 15 in that it focuses more specifically on a doctor‐patient interaction, rather than decisional support in general, and from that developed by Elwyn et al. 16 in that more emphasis is placed on discussion of evidence, and on individualizing options to the patient’s clinical and social situation. The coding system focuses on the doctor’s behaviour, rather than the patient’s, because we were particularly concerned with the impact of doctor behaviour on patient outcomes.
Table 1.
Coding frame for shared decision making
| Establishing problem |
| 1. Reason for consultation established |
| 2. History reviewed |
| 3. Social circumstances reviewed |
| Doctor–patient relationship |
| 4. Interruptions |
| 5. Rapport building |
| Research evidence |
| 6. Evidence presented |
| 7. Quality of research discussed |
| 8. Physician appraisal of data |
| 9. Research relevant to patient |
| Patient perspective |
| 10. Patient views enquired upon |
| 11. Asked regarding amount of information wanted |
| 12. Asked regarding decision‐making preference |
| 13. Physician ensured patient understanding |
| Decision making |
| 14. Treatment option presented |
| 15. Multiple options presented |
| 16. Treatment process described |
| 17. Side‐effects discussed |
| 18. Possibilities and outcomes discussed |
| 19. Patient values in decision considered |
| Time issues |
| 20. Option given to defer treatment decision to next visit |
Scoring guide: ‘1’ for present, ‘0’ for absent (N/A scored as ‘1’). Maximum possible score = 20.
N/A, not applicable.
Sample
In a previous study 14 , 164 consecutive patients with heterogeneous cancers attending their initial appointment with oncologists (at a single Australian tertiary cancer centre) were recruited into a study of a consultation preparation package. All consultations were audio‐taped and transcribed, with patients completing questionnaires before and immediately after the consultation. From this database, we selected patients with a solid tumour primary cancer where the consultation involved consideration of adjuvant therapy after surgery. This was performed to reduce heterogeneity in doctor behaviour because of the clinical context. Decision making within the consultation concerned whether to proceed with adjuvant treatment (chemotherapy and/or radiation) and if so, what type(s). A total of 63 consultations were included in the study.
Coding
The coding system developed as described above (Table 1) was applied to each transcribed consultation. For each identified behaviour a score of ‘present’ = 1 or ‘absent’ = 0 was assigned. If the behaviour was non‐applicable the item was scored 1. A total score was calculated by summing the scores within each consultation. A medical oncology fellow (SS) coded all 63 transcripts. Twenty percentages of transcripts were re‐scored by the same coder with an intra‐rater agreement of 95% on the total scores. These consultations were further re‐scored by another oncologist (MT). Inter‐rater agreement on the total scores was 90%.
Measures
Preferences for information were assessed at baseline using two items derived from the Cassileth Information Styles Questionnaire. 3 The first item measured preference for greater or lesser detail. Respondents indicated their preference on a 5‐point scale anchored on each end by ‘prefer as few details as possible’ and ‘prefer as many details as possible’. The second item elicited type of information preferred. Respondents chose one of three options: (i) only information needed to care for myself properly, (2) additional information only if it is good news or (3) as much information as possible, good or bad.
Preferences for decisional control were assessed using a validated question from previous studies in cancer patients. 4 Patients chose between five options:
-
1
The doctor makes the decision using all that’s known about the treatments;
-
2
The doctor makes the decisions but strongly considers my needs and priorities;
-
3
The doctor and I make the decision together on an equal basis;
-
4
I make the decisions, but strongly consider the doctor’s opinion;
-
5
I make the decisions using all I know or learn about the treatments.
These were later collapsed into a passive (1, 2) or shared/active (3, 4, 5) preference for analysis purposes.
Anxiety before and after the consultation was measured using the state version of the Spielberger State Trait Anxiety Scale 19 (20 items). A total score is calculated. Scores range from 20 to 80 with a high score indicating greater anxiety.
Patient satisfaction with the consultation was assessed using a 25‐item Likert scale, adapted from Rote 20 and Korsch et al., 21 that has been used extensively in our work, 12 , 22 , 23 This scale assessed satisfaction with (i) the amount and quality of information presented, (ii) the communication skills demonstrated by the doctor, and (iii) the level of patient participation in the consultation. A total score is calculated. Scores can range from 25 to 125, with high scores indicating greater satisfaction.
Achievement of involvement preference was assessed by asking patients to complete a modified version of the preferences for decisional control scale described above, with reference to their experience during the actual consultation. For example, the first option read ‘The doctor made the decision using all that’s known about the treatments’. Exact matches to pre‐consultation preferences were coded 1, otherwise 0.
Statistics
Factor analysis was carried out to investigate the validity of the SDM Scale and to determine whether there were meaningful subscales of SDM behaviours. To explore univariate relationships between SDM scores (total and subscales) and patient characteristics, independent samples t‐tests were conducted to test differences in SDM between groups (e.g. men and women), and Pearson’s correlation were used to test relationships between SDM and continuous variables (e.g. anxiety). Multiple linear regression was used to examine the simultaneous and independent effects of patient characteristics on total SDM scores. Regression was used to explore the impact of doctor SDM behaviour, initial involvement preference and their interaction on patient satisfaction with the consultation and change in anxiety scores (hierarchical linear regression) and on achievement of involvement preference (binary logistic regression). The 5% significance level was used throughout.
Ethics
All activity was carried out with Ethics Committee approval.
Results
Patient characteristics are outlined in Table 2. Patient ages ranged from 24 to 84, with a mean of 54.9 years (SD = 13.1). The largest proportion of patients had breast cancer, followed by testicular cancer, prostate cancer, lymphoma and lung cancer.
Table 2.
Patient characteristics
| n (%) | |
|---|---|
| Gender | |
| Male | 23 (36) |
| Female | 40 (64) |
| Place of birth | |
| Australia | 36 (57) |
| Other | 27 (43) |
| Primary site of cancer | |
| Breast | 30 (48) |
| Other | 33 (52) |
| Education | |
| Up to high school | 8 (60) |
| Above high school | 25 (40) |
| Age (years) | |
| <40 | 7 (11) |
| 41–50 | 15 (24) |
| 51–60 | 22 (35) |
| 61–70 | 10 (16) |
| >70 | 9 (14) |
| Pre‐consultation anxiety, mean (SD) | 38.6 (12.2) |
Psychometric properties of the Shared Decision‐Making Scale
The frequency with which each item occurred across consultations is shown in Fig. 1. Item 12, eliciting patient’s decision‐making preferences, occurred in only three consultations. Items regarding establishing reason for the consultation were seen in >50% of the consultations as were items related to decision making. Items associated with obtaining patient perspective s to shared decision making were particularly uncommon. None of the items occurred in every consultation.
Figure 1.
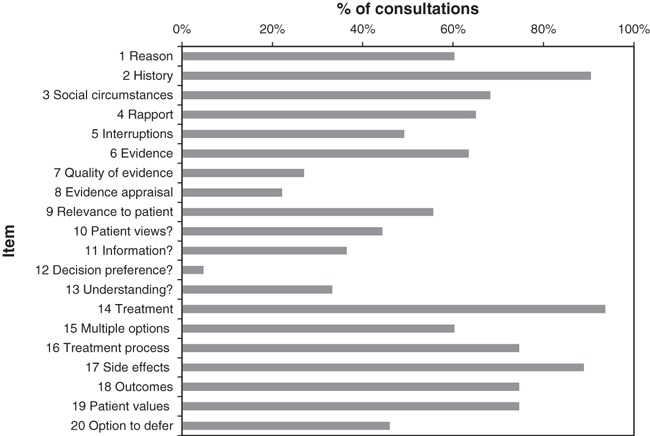
Frequency of shared decision‐making items in consultations.
Validity
Principal components analysis with orthogonal rotation was conducted to investigate construct validity of the SDM Scale. Items 4 and 5 (interruptions and rapport building) formed a separate factor which was deemed to reflect general consultation skills, not related specifically to SDM in oncology, and were therefore omitted from further analyses. The analysis was then repeated with 18 items. Inspection of the scree plot suggested a three factor solution. This accounted for 44.6% of the variance, and the factors could be meaningfully characterized as SDM subscales relating to discussions of treatment (items 15, 20, 16, 19, 10, 14 and 13), of evidence (items 7, 8, 9, 6, 2, 11, 1 and 12) and of patient challenges (items 18, 17 and 3). The final factor solution and Cronbach alphas for the three subscales based on the items with bolded loadings are shown in Table 3. The fact that some items cross‐loaded on more than one subscale, and that the Cronbach’s alpha for the 18 item total scale is satisfactory at 0.77, indicates that the total score on the SDM Scale is reliable and internally consistent. It is interesting to note, however, that the items initially conceptualized as ‘Patient Perspective’ (Table 1; items 10–13) did not fall neatly into the one factor, rather they separated out into discussions of treatment and treatment options (component I), where the physician checked for patient understanding of the treatment options and process, and discussions of evidence (component II) where the physician asked about the amount of information wanted by patients. Patient challenges (component III) include discussion of side‐effects, possibilities and outcomes considered within the context of the patient’s social circumstances. The lower internal consistency for this factor reflects the smaller number of items.
Table 3.
Principal components analysis of the shared decision‐making items
| Aspect of consultation | Subscales | ||
|---|---|---|---|
| I | II | III | |
| Treatment | Evidence | Patient challenges | |
| Item 15: multiple options presented | 0.78 | 0.27 | −0.03 |
| Item 20: option to defer decision | 0.73 | 0.06 | 0.21 |
| Item 16: treatment process described | 0.68 | 0.21 | −0.16 |
| Item 19: patient values considered | 0.61 | 0.13 | 0.01 |
| Item 10: patient views enquired upon | 0.55 | 0.05 | 0.11 |
| Item 14: treatment option presented | 0.45 | −0.07 | −0.04 |
| Item 13: ensure patient understanding | 0.37 | −0.05 | 0.22 |
| Item 7: quality of research discussed | 0.02 | 0.83 | 0.05 |
| Item 8: physician appraisal of data | −0.09 | 0.82 | 0.06 |
| Item 9: research relevant to patient | 0.22 | 0.73 | 0.35 |
| Item 6: evidence presented | 0.35 | 0.61 | 0.45 |
| Item 2: history reviewed | −0.05 | 0.43 | −0.14 |
| Item 11: asked re amount of information | 0.15 | 0.37 | −0.07 |
| Item 1: reason for consultation established | 0.36 | 0.37 | −0.21 |
| Item 12: asked re decision preference | 0.20 | 0.30 | −0.09 |
| Item 18: possibilities & outcomes discussed | 0.04 | −0.06 | 0.82 |
| Item 17: side‐effects discussed | 0.13 | −0.11 | 0.76 |
| Item 3: social circumstances reviewed | −0.24 | 0.27 | 0.35 |
| Cronbach’s alpha | 0.74 | 0.75 | 0.50 |
The largest Cronbach’s alpha values are given in bold.
The mean score on total SDM behaviours was 10.2 out of a maximum of 18 (SD = 3.6) (Table 4). To facilitate comparisons across the subscales that were based on different numbers of items, scores were calculated by summing the items and converting the total into a score out of 10. Mean scores on the treatment, evidence and patient challenge subscales were 6.1, 4.5 and 7.7 respectively (maximum score = 10). These means differed significantly, with higher scores on the discussion of patient challenges than of treatment, and higher scores on discussions of treatment than of evidence (all P < 0.01).
Table 4.
Doctor shared decision‐making (SDM) behaviour
| Range | Mean (SD) | |
|---|---|---|
| Total SDM (out of 18) | 3–16 | 10.2 (3.6) |
| Subscales (out of 10) | ||
| Subscale 1: treatment | 0–10 | 6.1 (2.8) |
| Subscale 2: evidence | 0–8.8 | 4.5 (2.6) |
| Subscale 3: patient challenges | 0–10 | 7.7 (2.9) |
Achievement of involvement preference
To best assess after the consultation if patients had achieved their involvement preference patients were asked to complete a modified version of the preferences for decisional control scale described above with reference to their experience during the actual consultation. Of the total sample, 21 (33.3%) responded that they had achieved their preference, and this proportion was similar across initial involvement preferences (8/23 for patients preferring a more passive role, 13/40 for patients preferring a more active role). Logistic regression explored the impact of total SDM and initial involvement preference (passive/active) on achievement of involvement preference. If the relationship between SDM and preference achievement differs for patients who prefer a more passive role from that found for patients who prefer a more active role, i.e. if there is interaction between these variables in their relationship with achievement of involvement preference, this would serve as preliminary construct validation of the SDM Scale.
Neither involvement preference nor SDM alone predicted achievement of involvement preference, but their interaction was a statistically significant predictor of the probability of patient achievement of the desired level of involvement in the decision making [odds ratio = 1.45, 95% confidence interval (CI) = 1.03–2.04]. As can be seen in Fig. 2, for those who preferred a passive role, the probability of achieving their involvement preference tended to be highest when the doctor exhibited fewer shared decision‐making behaviours and to decrease as SDM increased; on the contrary, for patients who preferred a more active role, the probability of achieving their preference increased as doctor’s SDM behaviours increased. This is a preliminary validation of the SDM Scale.
Figure 2.
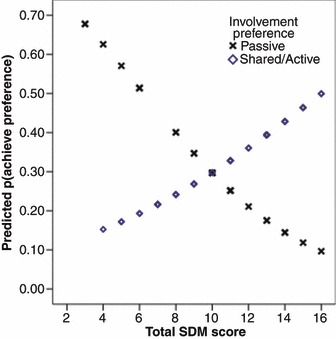
Predicted probability of achieving involvement preference.
Disease and patient predictors of physician behaviour
Tests were conducted to examine whether patient and disease characteristics were related to aspects of physician SDM behaviour. Qualitative variables of interest were patient education (high school or below vs. any tertiary degree), gender, English as a first language (yes or no), and site of primary cancer (breast cancer vs. others). Patients’ preferred level of involvement in the decision making was classified as passive (n = 23) or active/shared (n = 40). Independent samples t‐tests were conducted to test for differences on the three subscales (treatment, evidence and challenges), as well as the total SDM scores. Scores on the evidence subscale were significantly higher with female patients (mean = 5.06, SD = 2.62) than with male patients [mean = 3.53, SD = 2.22; t(61) = 2.35, P = 0.02], and tended to be higher with breast cancer patients [mean = 5.17, SD = 2.52] than with those with other cancers (mean = 3.90, SD = 2.51; t(61) = 1.99, P = 0.05]. Given that all the breast cancer patients were female, it is not surprising that the results should be similar for those variables. There was a trend towards more discussion of evidence with patients who preferred a more active role (mean = 4.97, SD = 2.75) than those who preferred a more passive role [mean = 3.70, SD = 2.05; t(61) = 1.93, P = 0.06]. There were no other differences in subscales or total SDM scores as a function of these patient characteristics.
Quantitative variables of interest were patient age and pre‐consultation anxiety. SDM behaviours did not vary significantly with patient age, although there was a trend towards higher total SDM scores with younger patients (r = −0.22, P = 0.08). There was significantly more discussion of challenges with patients who were more anxious pre‐consultation (r = 0.33, P = 0.01). No other correlations were significant.
Multiple regression analysis was conducted to determine whether total SDM behaviours might be uniquely related to particular patient or disease characteristics when controlling for the other variables in the model, i.e. for the patient characteristics detailed above. One potential problem with the analysis was the high correlation between patient gender and site of cancer: all of the patients with breast cancer were female. In the analyses for subscale scores, discussion of research evidence was higher with females and with breast cancer patients. To determine if this finding is primarily because of patient gender or site of tumour, patient gender and site of tumour were dummy‐coded into two variables with males as the reference group, females with breast cancer as the comparison group on the first variable, and females with other cancers on the second variable. The overall model was not statistically significant, R 2 = 0.18, P = 0.17, but age and site of cancer made significant independent contributions to variability in total SDM behaviours. All other things being equal, SDM scores were higher in consultations with younger patients (b = −0.08, P = 0.04) and with female breast cancer patients compared with male cancer patients (b = 2.40, P = 0.03). It should be noted that male and female patients did not differ in age (means = 53.9 and 55.4 respectively).
Patient satisfaction with the consultation and shared decision‐making behaviour
Patient satisfaction with the consultation was generally high, with scores ranging from 84 to the maximum of 125 (mean = 112.2, SD = 10.3). Pearson correlations revealed no relationship between total SDM score and satisfaction with the consultation (r = 0.09) when considered over the whole sample, with a correlation of 0.24 for those preferred a more active role, and −0.10 for those who preferred a more passive role. Although in a direction that might be expected, neither correlation was statistically significant. Hierarchical regression analysis was conducted to investigate whether the relationship between total SDM and satisfaction with the consultation varied depending on initial involvement preference. Total SDM (centred) and initial involvement preference were entered as predictors in model one, and these variables plus an interaction term were entered in model two. The R 2 change of 0.03 was not significant (P = 0.23); thus satisfaction with the consultation was not higher if patients initially preferred more involvement and saw a doctor who exhibited strong SDM behaviours. Patient satisfaction with the consultation overall could not be predicted from knowledge of patient involvement preference or level of SDM during the consultation.
Total shared decision‐making scores and anxiety
Mean anxiety scores were 38.7 (SD = 12.4) before consultation and 35.7 (SD = 13.6) after consultation. Hierarchical linear regression showed no significant relationship between total SDM and change in anxiety (mean = 3.0, SD = 11.6), regardless of initial involvement preference. However, the relationship between SDM scores and change in anxiety did approach significance, as seen in Table 5, with the sign of the correlation suggesting that higher SDM scores were associated with an increase in anxiety. Further investigation revealed that the increase in anxiety was systematically related to the treatment subscale (r = −0.35, P = 0.03), in particular offering the patient the option to defer making a decision (r = −0.40), presentation of multiple treatment options (r = −0.27), and consideration of patient values (r = −0.28). Taken together these results could suggest either that the doctor was responding to perceived patient anxiety by offering to defer decision making, or that too much uncertainty tends to increase patient anxiety.
Table 5.
Correlations between shared decision making (SDM) and patient anxiety and satisfaction
| Treatment | Evidence | Patient challenge | Total SDM | |
|---|---|---|---|---|
| Pre‐consultation anxiety | 0.04 | −0.02 | 0.33 | 0.10 |
| Post‐consultation anxiety | 0.32 | 0.17 | 0.26 | 0.34 |
| Change in anxiety | −0.35 | −0.16 | 0.08 | −0.26 |
| Satisfaction with consultation | 0.09 | 0.16 | −0.22 | 0.09 |
Values in bold are statistically significant at the 0.05 level.
Discussion
This study had multiple aims. We endeavoured to (i) develop a coding system for SDM behaviour in oncology consultations where adjuvant therapy was considered after surgery, (ii) determine if oncologists vary their SDM behaviour in response to patient characteristics, (iii) explore the association between SDM behaviour and patient perceptions and finally, and (iv) explore the impact of SDM on patient anxiety.
The coding system we developed will require further validation, but we were able to achieve excellent inter and intra‐rater reliability, satisfactory internal consistency and evidence of construct validity. There was evidence that patients who preferred active involvement were more likely to feel their involvement preference was met when their doctor displayed more SDM behaviours during the consultation. On the other hand, patients who preferred a more passive role had a higher probability of attaining their involvement preference when the doctor engaged in fewer SDM behaviours. This interaction between involvement preference and SDM behaviours is evidence of the construct validity of the coding system developed. No other existing coding system was felt to be adequately specific for this group of patients. Future studies may usefully compare coding systems to establish their relative sensitivity and specificity in capturing behaviours considered important to both doctors and patients in treatment decision making.
Oncologists generally exhibited little over half of the behaviours that were considered important in shared decision making. Particularly few consultations displayed evidence of patients being asked their decision‐making preference (item 12) and perspective overall (items 10–14). We were somewhat surprised at this finding, given the wide‐spread acceptance of shared decision making as the preferred model for doctor–patient communication. Some mention of the Hawthorne effect is warranted. 24 The Hawthorne effect refers to the fact that individuals may change their behaviour if they are aware of being observed. If the Hawthorne effect was operating, one would have expected doctors to be performing at their best. In our experience, oncologists and patients quickly forget they are being audio‐taped as they are caught up with the real‐life drama of the consultation. It is also important to note that this is a secondary analysis of consultations recorded for another study 14 and the doctors were not aware of the goal of this current analysis. Thus, it is likely that our findings reflect usual behaviour, or if the Hawthorne effect were operating, to be an under, rather than over estimate of behaviour.
One explanation for the relatively low rate of shared decision‐making behaviour is that oncologists simply proceeded in the consultation without enquiring on patient decision‐making preference strengthening the argument for shared decision‐making interventions at the physician level. However, communication consists of both non‐verbal and verbal components. One of the limitations of our study is that it does not attempt to capture non‐verbal communication such as body language, physical cues, etc. Perhaps oncologists did not need to explicitly ask about decision‐making preference but were able to obtain this information for other cues as the consultation proceeded. Future studies incorporating coding of non‐verbal behaviour may explicate this finding further.
Shared decision‐making behaviours were more apparent in consultations involving female breast cancer patients, notably, for discussion of research evidence. Previous studies have found that female cancer patients are more likely to prefer shared decision making than males 2 , 4 and perhaps oncologists are responding to these preferences. The evidence base for adjuvant breast cancer treatments is also arguably more extensive than for any other cancer site, and there is wide acknowledgement of the option of mastectomy or breast conservation as treatments with similar survival.
The results shown in Fig. 1 demonstrate that patients were more likely to report an experience of SDM which matched their initial preference if the doctor’s shared decision‐making behaviours did in fact match their initial preference, support our previous findings 12 , 14 which suggested that physician intervention is an essential part of the equation to enhance shared decision making. As only one in three patients was found to have achieved their desired decision‐making involvement preference, physician‐ and patient‐based intervention tools may be worthwhile exploring. Patients may need such interventions for a number of reasons. They are often in a vulnerable position in the consultation room and despite previous interventions and intentions, find it difficult to change their behaviour when actually in the high pressure consultation. This is even more difficult in the oncology setting where decisions are often regarded as life and death, making the patient even more fearful of negative consequences if he or she tries to take more responsibility in the decision‐making process.
The coding scores revealed little relationship between doctor behaviours assessed by this scale, and overall patients’ satisfaction with the consultation. Even when the patient’s initial involvement preference was taken into account, this relationship remained non‐significant. Patients who preferred to share decision making, or to take an active role, were not more satisfied if the doctor displayed more SDM behaviours. One possible explanation for this finding is that our coding system, which focused on SDM, failed to capture aspects of the consultation such as physician communication style, body language and mannerisms as well as displays of empathy, which may be more highly related to satisfaction. Patients may also change their involvement preference as the consultation proceeds (an issue which has received very little attention in the literature) or may be looking to the physician for other SDM behaviours we have not captured in this coding system. More likely, a ceiling effect in satisfaction may have limited our ability to detect relationships between this variable and SDM. As in most studies using this outcome, satisfaction in this study was on average very high (mean of 112 out of a possible score of 125). Thus, the power to detect associations was limited.
Previous work has not found an increase in anxiety associated with encouraging patients to ask questions 12 or providing them with decision aids. 25 Our findings were consistent with this as there was no significant relationship between total SDM score and change in anxiety pre‐ and post‐consultation. To our surprise, there was a trend in these data towards higher SDM scores being associated with an increase in anxiety, especially related to the treatment subscale (offering option of deferring decision, offering multiple options and consideration of patient values). Other studies have reported a short‐term increase in anxiety when patients are offered detailed information about treatment options. For example, Simes et al., found a short‐term increase in anxiety in patients who were offered detailed information about a clinical trial, compared with patients provided with standard information. 26 However, anxiety in both groups was reduced and similar a few weeks later. Thus, while the opportunity to consider multiple options and to play a role in decision making may provoke anxiety in the short‐term, this may dissipate quickly, and is arguably a necessary and unavoidable part of responsible decision making. Another explanation for this finding is that oncologists responded to high patient anxiety by offering deferred decision making, thus scoring higher on this component of the SDM Scale; thus the direction of association is unclear.
The fact that younger patients, and women with breast cancer, tended to receive more SDM behaviours from their clinician is not surprising, given the well‐established data that these groups have a stronger preference for sharing decision making 2 and the fact that there are clearly recognized treatment options in breast cancer. Other studies have noted that the context influences doctors’ views on the appropriateness of shared decision making. 27
A limitation of the study is that it audited only consultations about adjuvant therapies after surgical treatment in one cancer centre, and further research involving a wider range of consultations across multiple centres would provide more evidence on this important topic. As mentioned above our study is also limited by only attempting to capture verbal communication.
In conclusion, we have developed a coding system for shared decision making in oncology consultations which has demonstrated reliability and construct validity in the setting of discussion of adjuvant therapy. Overall, use of shared decision‐making behaviours was relatively low in these consultations, but was more commonly used with female breast cancer patients and younger patients. The study results show that shared decision‐making behaviours of the oncologist are related to patients’ achievement of their involvement preference in consultations where adjuvant therapy is considered after cancer surgery, without altering patient satisfaction with the consultation. We did not find any significant relationship between anxiety and SDM scores, consistent with previous work. Overall our findings reinforce the importance of the doctor in facilitating shared decision making, and support its use in oncology consultations. Training physicians to better promote/facilitate shared decision making in a manner that is sensitive to patient preferences and developing interventions to better prepare both physicians and patients for these discussions remain a priority.
Conflicts of interest
None declared.
Appendix 1
Coding for shared decision making
| Code | Description | Example |
|---|---|---|
| Establishing a problem | ||
| Reason for consultation established | Doctor explicitly outlines aims of consultation | Now I understand that Dr XXX has sent you to me to discuss whether chemotherapy would be useful for you |
| History reviewed | Doctor goes over recent medical history relevant to the current cancer to ensure mutual understanding | Now my understanding is that you were diagnosed with an early stage breast cancer, and on surgery, 3 nodes were found to have cancer in them. Is that right? |
| Social circumstances reviewed | Doctor establishes the patient’s social and employment circumstances | And are you working? Are you married? |
| Doctor–patient relationship | ||
| Interruptions | Consultation is interrupted by one or more phone calls or the doctor being called out of the room | |
| Rapport Building | Doctor attempts to build rapport through social exchange or empathic responses | That must have been quite a shock.This may feel overwhelming right now, but I will give you some booklets to take home |
| Research evidence | ||
| Evidence presented | Doctor presents evidence underlying treatment options presented | We know from studying 1000s of women like you, that this treatment will reduce the chances of the cancer coming back from 30% to about 20% |
| Quality of research discussed | Doctor comments on the strength of evidence | Only one fairly small study has shown [drug x] is better than the standard treatment |
| Research relevant to the patient | Doctor individualizes the evidence to the patient’s circumstances | The study showed that women who were over 70, like you, had a much better chance of avoiding recurrence if they had chemotherapy, than if they didn’t |
| Physician appraisal of the data | Doctor provides a clear recommendation based on his or her own appraisal of the data | In my view, there is a clear benefit to having the chemotherapy |
| Patient perspective | ||
| Patient asked how much information they wanted | Doctor offers a range of information and determines patient preferences | Now some people like a lot of detail, and others are big picture people. What sort of person are you? |
| Patient asked for decision making preference | Doctor asks how involved patient wants to be in the decision | Would you like to have a think about what I have said and let me know what you decide, or would you rather I choose the treatment for you, based on what I know about you? Or we could decide together now what we both think is the best course |
| Physician ensured patient understanding | Doctor checks that the patient has understood what was discussed. | So did that all make sense? How do you see the options in front of us? |
| Patient views enquired upon | Doctor checks what decisional leaning the patient has | So having heard about the pros and cons of chemo, what are you thinking? |
| Decision making | ||
| Treatment option presented | Doctor explicitly states that there is at least two options (including no treatment) | So the surgery has removed all visible cancer. We could stop there, or you could consider a further treatment to reduce the risk of the cancer coming back |
| Multiple options presented | Doctor presents multiple options if appropriate | We could do nothing, or you could try tamoxifen alone, or you could have chemotherapy followed by tamoxifen. There are a few options here |
| Treatment process described | Doctor clearly outlines the procedures involved with having treatment | If you decide to have chemotherapy, you would be coming in here once every three weeks just for the day; we would… |
| Side effects discussed | Doctor clearly outlines the side effects of each treatment option | There are some downsides of chemo which you may have heard of. They vary depending on the specific drugs used. With this drug, you are likely to have fairly complete hair loss, … |
| Possible benefits discussed | Doctor clearly states likely benefits of each option | If you decide not to have chemotherapy, you avoid the side effects from a toxic therapy that you may not have needed anyway. However, we have no way of knowing whether all the cancer has gone already. So people have chemotherapy to reduce the chances of the cancer coming back, given that uncertainty |
| Patient values in decision considered | Doctor clearly states that the patient’s values play an important role in the optimal treatment choice | So what matters to you is the important thing here–whether you want to feel you have done everything possible to avoid the cancer coming back, or feel that the side effects of chemo are just not worth that |
| Time issues | ||
| Option given to defer treatment decision | Doctor clarifies that there is time to think about the options before making a decision | There’s time for you to go home and talk this over with your husband before making a decision. It won’t make any difference when we start the chemotherapy, as long as it is within about 4 weeks of the surgery–so don’t feel rushed |
References
- 1. Tattersall MH, Gattellari M, Voigt K, Butow PN. When the treatment goal is not cure: are patients informed adequately? Supportive Care in Cancer, 2002; 10: 314–321. [DOI] [PubMed] [Google Scholar]
- 2. Blanchard CG, Labrecque MS, Ruckdeschel JC, Blanchard EB. Information and decision making preferences of hospitalized adult cancer patients. Social Science and Medicine, 1988; 27: 1139–1145. [DOI] [PubMed] [Google Scholar]
- 3. Cassileth BR, Zupkis RV, Sutton‐Smith K, March V. Information and participation preferences among cancer patients. Annals of Internal Medicine, 1980; 92: 832–836. [DOI] [PubMed] [Google Scholar]
- 4. Degner LF, Sloan JA. Decision‐making during serious illness: what role do patients really want to play? Journal of Clinical Epidemiology, 1992; 45: 941–948. [DOI] [PubMed] [Google Scholar]
- 5. Beaver K, Campbell M, Craven O, Jones D, Luker KA, Susnerwala SS. Colorectal cancer patients attitudes towards involvement in decision making. Health Expectations, 2009; 12: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Charles C, Gafni A, Whelan T. Self‐reported use of shared decision‐making among breast cancer specialists and perceived barriers and facilitators to implementing this approach. Health Expectations, 2004; 7: 338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gattellari M, Butow PN, Tattersall MH. Sharing decisions in cancer care. Social Science and Medicine, 2001; 52: 1865–1878. [DOI] [PubMed] [Google Scholar]
- 8. Street RL, Voigt B. Patient participation in deciding breast cancer treatment and subsequent quality of life. Medical Decision Making, 1997; 17: 298–306. [DOI] [PubMed] [Google Scholar]
- 9. Hack TF, Degner LF, Watson P, Sinha L. Do patients benefit from participating in medical decision making? Longitudinal follow‐up of women with breast cancer. Psychooncology, 2006; 15: 9–19. [DOI] [PubMed] [Google Scholar]
- 10. Davison BJ, Goldenberg SL. Decisional regret and quality of life after participating in medical decision‐making for early‐stage prostate cancer. BJU International, 2003; 91: 14–17. [DOI] [PubMed] [Google Scholar]
- 11. Moyer A, Salovery P. Patient participation in treatment decision making and the psychological consequences of breast cancer surgery. Womens Health, 1998; 4: 103–106. [PubMed] [Google Scholar]
- 12. Ford S, Schofield T, Hope T. Are patients’ decision‐making preferences being met? Health Expectations, 2003; 6: 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown RF, Butow PN, Dunn SM, Tattersall MH. Promoting patient participation and shortening cancer consultations: a randomized trial. British Journal of Cancer, 2001; 85: 1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Whelan T, Levin M, Willan A et al. Effect of a decision aid on knowledge and treatment decision making for breast cancer surgery: a randomized trial. The Journal of American Association, 2004; 292: 435–441. [DOI] [PubMed] [Google Scholar]
- 15. Butow P, Devine R, Boyer M, Pendlebury S, Jackson M, Tattersall MH. Cancer consultation preparation package: changing patients but not physicians is not enough. Journal of Clinical Oncology, 2004; 22: 4401–4409. [DOI] [PubMed] [Google Scholar]
- 16. Guimond P, Bunn H, O’Connor AM et al. Validation of a tool to assess health practitioners’ decision support and communication skills. Patient Education and Counseling, 2003; 50: 235–245. [DOI] [PubMed] [Google Scholar]
- 17. Elwyn G, Edwards A, Wesing M, Hood K, Atwell C, Grol R. Shared decision making: developing the OPTION scale for measuring patient involvement. Quality and Safety in Health Care, 2003; 12: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ford S, Schofield T, Hope T. What are the ingredients for a successful evidence‐based patient choice consultation? A qualitative study. Social Science and Medicine, 2003; 56: 589–602. [DOI] [PubMed] [Google Scholar]
- 19. Gattellari M, Voigt KJ, Butow PN, Tattersall MH. When the treatment goal is not cure: are cancer patients equipped to make informed decisions? Journal of Clinical Oncology, 2002; 20: 503–515. [DOI] [PubMed] [Google Scholar]
- 20. Spielberger CD. Manual for the State Trait Anxiety Inventory (Form Y). Palo Alto, CA, USA: Consulting Psychologists Press, 1983. [Google Scholar]
- 21. Roter DL. Patient participation in the patient‐provider interaction: the effects of patient question asking on the quality of interaction, satisfaction and compliance. Health Education Monographs, 1977; 5: 281–315. [DOI] [PubMed] [Google Scholar]
- 22. Korsch BM, Gozzi EK, Francis V. Gaps in doctor‐patient communication. Pediatrics, 1968; 42: 855. [PubMed] [Google Scholar]
- 23. Brown R, Butow PN, Boyer MJ, Tattersall MH. Promoting patient participation in the cancer consultation: evaluation of a prompt sheet and coaching in question asking. British Journal of Cancer, 1999; 80: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mayo E. The Human Problems of an Industrial Civilization. New York: MacMillan, 1933: 55–77. [Google Scholar]
- 25. O’Connor AM, Rostom A, Fiset V et al. Decision aids for patients facing health treatment or screening decisions: systematic review. British Medical Journal, 1999; 319: 731–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simes RJ, Tattersall MH, Coates AS, Raghavan D, Solomon HJ, Smartt H. Randomised comparison of procedures for obtaining informed consent in clinical trials of treatment for cancer. British Medical Journal (Clinical Research Ed.), 1986; 293: 1065–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shepherd H, Tattersall M, Butow P. The context influences doctors’ support of shared decision making in cancer care. British Journal of Cancer, 2007; 97: 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


