Abstract
Background If research addresses the questions of relevance to patients and clinicians, decision‐makers will be better equipped to design and deliver health services which meet their needs. To this end, a number of initiatives have engaged patients and clinicians in setting research agendas. This paper aimed to scope the research literature addressing such efforts.
Methods A systematic search strategy combined electronic searches of bibliographic databases with handsearching and contacting key authors. Two researchers, initially working independently, described the relevant reports.
Findings Over 250 studies addressed patients’ or clinicians’ priorities for research and outcomes for assessment. This literature described different routes for patients and clinicians to contribute to research agendas. Two‐thirds of the studies addressing patients’ or clinicians’ research questions were applicable across health care, with the remainder focussed on specific health conditions. The 27 formal studies of patient involvement revealed a literature that has grown in the last decade. Although only nine studies engaged patients and clinicians in identifying research questions together, they show that methods have advanced over time, with all of them engaging participants directly and repeatedly in facilitated debate and most employing formal decision‐making procedures.
Conclusion A sizeable literature is available to inform priorities for research and the methods for setting research agendas with patients and clinicians. We recommend that research funders and researchers draw on this literature to provide relevant research for health service decision‐makers.
Keywords: collaboration, patient involvement, Research agenda, systematic review
Background
If research addresses questions of relevance to patients and clinicians, decision‐makers will be better equipped to design and deliver health services which meet their needs. To this end, a number of initiatives have engaged patients and the public in setting and prioritizing research agendas as evident in policy documents and, to a lesser extent, academic journals internationally. 1 The complementary clinician involvement often has a lower policy profile, possibly because health professionals and their organizations are perceived to be part of the research community and assumed to need no special outreach. No such assumption was held at a recent international workshop on Priority Setting Methodologies in Health Research. 2 Between them, 22 participants bringing experience of national priority setting in 12 countries, joined by experts in priority setting methodologies and 16 WHO staff, identified non‐scientist clinicians, health managers, patients and the wider public (civil society) amongst the stakeholders who should play a part in research priority setting to ensure ‘legitimacy and fairness’, key domains of good practice. Workshop discussion led to a list of principles to guide priority setting, but no recommendations for specific methods of involvement.
Explicit clinician and patient involvement in UK health research began in the early 1990s with the launch of the NHS Research and Development strategy. This heralded the introduction of a ‘systematic approach to identifying and setting R&D priorities in which NHS staff and the users of the Service are being asked to identify important issues which confront them and, in partnership with the research community, to characterise and prioritise these problems as the basis for seeking solutions’. 3 This approach has evolved through a series of agenda setting exercises by mixed groups, some of which have involved patients, carers, service users, the public or representatives of these groups. * Current policy includes a 5‐year programme for ensuring ‘more patients and health professionals participating in health research’. 4 Particular effort is focused on clinical trials, 5 and identifying research priorities addressing uncertainties about the effects of treatments. The Medical Research Council and the Department of Health funded the James Lind Initiative to promote public and professional knowledge about, and engagement with, clinical trials. As one of the activities under the aegis of the James Lind Initiative, the James Lind Alliance (JLA) was launched in 2004 to foster collaboration between patients and clinicians in priority setting partnerships (originally known as ‘working partnerships’) to identify research priorities addressing uncertainties about the effects of treatments. The first of these priority setting partnerships was between Asthma UK and the British Thoracic Society who have a shared objective in seeking to improve the health of people with respiratory disease. The second priority setting partnership brought together patients, carers and clinicians from 22 organizations concerned with urinary incontinence. These activities elicited details of patients’ and clinicians’ research priorities using consensus development methods for health services research. 6 , 7 , 8 Their success contrasts with the apprehension felt by many researchers who feel that the current policy climate requires them to involve patients despite their concerns about roles and values. 9 There is a need to seek examples of clinicians and patients working in partnership to identify research priorities.
Objectives
This paper aims to ascertain whether there is a research literature to inform how patients and clinicians can work in partnership to identify and prioritize suggestions for research. The objectives were to (i) design a conceptual framework for methods to address patients’ and clinicians’ research priorities based on what was already known, current research policy and concepts that emerged from the literature in the course of this work; (ii) identify studies that describe the involvement of clinicians and patients in setting priorities for research; and (iii) apply the conceptual framework to analyse the literature about involving clinicians and patients in setting clinical research agendas. This work was guided by the JLA Strategy and Development Group, which includes clinicians, service users, research funders and managers, and academics. They refined the focus of the work, directed us to relevant reports and discussed emerging findings.
Methods
Search strategy
The search strategy is described briefly here; details are available from the authors. Studies were sought in June 2006 and supplemented with additional searches in January 2008. We handsearched all issues of Health Expectations since its first publication in 1998 and contacted members of the JLA and related networks requesting relevant literature. Citation searching was conducted for 30 relevant papers and for eight key authors identified from earlier work in this area (Chalmers, I; Chard, J; Cohen CI; Cream J; Dieppe P; Kirwan J; Oliver S; and Tallon D). Citation searches were run using the Science Citation Index Expanded‐1970‐present, Social Sciences Citation Index‐1970‐present and Arts & Humanities Citation Index (A&HCI)‐1975‐present. We developed an electronic search strategy drawing on keywords for 16 key papers about patients and clinicians research priorities identified by the authors from the JLA. A highly specific search combined terms for patients (e.g. patients, consumers, clients), clinicians (e.g. clinicians, nurses, doctors), priorities and research. The results were screened for relevant studies to provide further keywords and frequently occurring descriptors which were used to build a more sensitive search strategy. This was employed in the MEDLINE database (from 1996), and adapted for: EMBASE (from 1974); PsycINFO (from 1806); CINAHL (R) (from 1982); AMED from 1985) and the Cochrane Methodology Register.
Selection criteria
Studies eligible for analysis were those describing the process of eliciting patients’ or clinicians’ research priorities, either separately or together.
Data collection and analysis
Two researchers independently screened potentially relevant abstracts and, subsequently, full‐text reports, to identify those which described patients’ or clinicians’ priorities for research. Discrepancies between researchers regarding which reports were relevant were resolved by discussion.
Each eligible study was described in terms of: who authored the report(s); whose priorities were identified; whether participants identified full research questions or broader topics, and the health condition of interest. The literature as a whole was described in terms of the number of studies addressing clinicians’ and patients’ research priorities, how much of this literature was directly relevant to treatment uncertainties and outcomes for assessing effects of treatment, whether patients and clinicians were involved separately or together, and the conditions considered.
We did not consider the quality of the engagement methods within this review. We did however examine those activities which led to published research priorities and analysed qualitatively the involvement methods for those studies that addressed research questions identified by both patients and clinicians. Where full questions were identified, we explored who had taken part in these processes in more detail and identified whether they provided contributory (topic) expertise or interactional (group working) expertise. 10 Lastly, we analysed whether the influence of different groups on the final prioritized questions was analysed.
Results
Electronic searching yielded 6838 references, whilst handsearching and citation searching produced a further 85 potentially relevant references. After screening, full‐text reports of 258 studies of patients’ and/or clinicians’ priorities for research were included in this review. These studies contributed to our conceptual framework and were subsequently described in terms of their authorship, the degree to which participants engaged with research, the outputs of their efforts, the types of participants, the focus of research questions identified and the studies that addressed patients’ and clinicians’ questions simultaneously (see Fig. 1).
Figure 1.
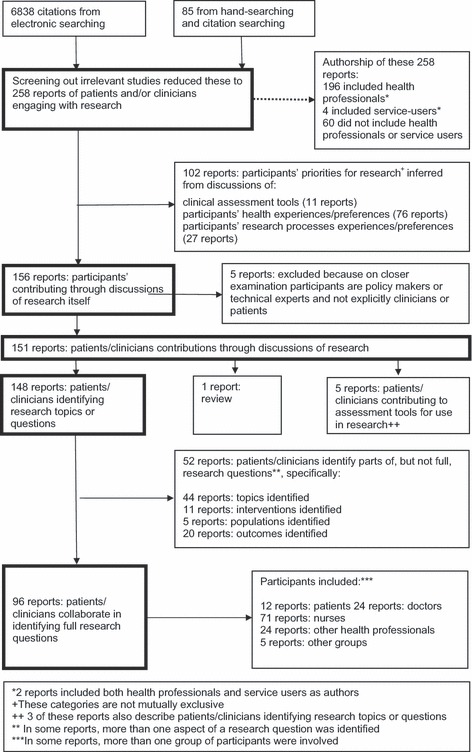
Flow chart showing how we identified the 96 reports in which clinicians and/or patients collaborated in identifying full research questions.
Conceptual framework
An earlier review of public involvement in setting research agendas distinguished purposeful research agenda setting from opportunistic agenda setting (when research priorities were identified in the course of planning services). 1 An additional distinction with purposeful approaches was between researchers listening to patients or clinicians and then making decisions informed by their views (consultation), and researchers and patients or clinicians making decisions between them about priorities (collaboration).
In the course of the work, we developed these distinctions further into a framework. This framework distinguished between consultative and collaborative approaches and highlighted those collaborative approaches that led to the identification of prioritized research questions. It was in examining the literature reported here we recognized that in some studies researchers engaged clinicians or patients in discussions about research (either consulting them or collaborating with them), while in others researchers’ inferred patients’ or clinicians’ research priorities after consulting them about their experiences, preferences, values or ‘measures’ of success as they talked about: services or interventions (for example treatments or therapies) or health conditions (for example disability or illness). Although this route did not involve patients or clinicians in considering research priorities, they did draw on patients’ or clinicians’ perspectives more than if the researchers drew conclusions about research priorities from their observations alone. This last approach is typical of most research reports which draw out implications for further research supported with references to research knowledge, whether or not this has been selected systematically, to identify research gaps. In contrast, by engaging patients and clinicians in discussions about research itself, recommendations for research could be drawn from the interpretations of research by patients and clinicians as well as by researchers. This may entail patients and clinicians themselves identifying or prioritizing topics deserving research, research questions or measures for use in research.
Another distinction within this literature is the extent to which patients’ and clinicians’ priorities lead to subsequent research. Some individual studies ask for their views, but the resulting priorities are not explicitly linked to subsequent research. Some agenda setting exercises do provide a link between patients’ or clinicians’ views and research conducted in the light of these. Others linked their views with funded research programmes. These differences prompted us to consider how patients’ and clinicians’ views expressed in this literature as a whole might inform subsequent research. Health or intervention topics that patients or clinicians considered deserving of research may be useful to funders of responsive programmes in setting the scope of their programmes, or priorities within them; research questions from patients or clinicians that are yet to be addressed may be useful to funders of commissioning programmes and to research teams seeking funds from responsive programmes; and measures for use in research endorsed by patients or clinicians may be useful to research teams.
Scoping the literature and authorship
We found 258 relevant studies. Research authors were also qualified health professionals for 196 and, in four, authors were also described as service users. † Sixty of the 258 studies included authors who were neither health professionals nor service users.
Inferring research priorities
Of the 258 studies, many drew on patients’ or clinicians’ experiences of health or services to infer priorities for research topics, questions or measures. Seventy‐six were about participants’ experiences or preferences for health, where researchers’ interpretations informed the recommendations for research. For instance, Garland and colleagues identified desired outcomes for adolescent mental health services according to various stakeholders—adolescents, parents and therapists. 11 A further 27 were about participants describing their experiences of, or preferences for, research methods such as recruitment or consent for trials.
Eleven described clinicians and/or patients contributing to the development of assessment tools for use in clinical settings. Examples include identifying patient‐defined endpoints for remission and clinical improvement in ulcerative colitis 12 and developing a utility function for multiple outcome measurements in mental health evaluation. 13
Engagement with research
Of the 258 studies, 156 described participants engaging with research rather than only considering their experiences or perceptions of health from which researchers inferred their priorities for research. Five of these studies specified groups other than clinicians or patients, such as ‘policy makers’ or ‘technical experts’.
Of the 151 studies about patients’ or clinicians’ research priorities, one was a review. This considered the published literature on mental health users’ involvement in setting research priorities and identified five priority topic areas: social and welfare issues, involvement in services, medication, alternative treatments and ethnicity.
Outputs of engagement
Of the 150 remaining studies in which patients or clinicians engaged with research, 148 described participants identifying important research topics or questions and five described participants contributing to research measurements for assessment tools.
In two of the five studies describing participants contributing to research measurements for assessment tools, researchers chose the outcomes and invited participants to contribute to developing tools for assessing them. One study measured the inclusion of consumer and community values in cancer research funding decisions, 14 and another measured asthma symptoms. 15 The other three studies of assessment tools, where participants also chose the outcomes, developed outcome measures for research about midwifery, 16 nursing 17 and arthritis. 18 , 19 , 20 , 21 , 22 , 23 , 24 The development of these assessment tools is not considered further here, but the identification of research priorities by patients and clinicians reported in the same studies is considered below as part of a larger literature.
Of the remaining 148 studies, participants identified research priorities in terms of full research questions (96) or aspects of research questions (52), which included research topics (44), interventions (11), populations (5) and outcomes (20).
Working in homogenous or mixed groups
Within the 148 studies identifying research topics, 38 included doctors, 123 included other health professionals (in 93 studies these were nurses), 27 included patients and 17 included additional groups such as researchers, research funders, national agency staff, local government officials and administrators.
More studies reported patients and clinicians working separately to identify research topics, than together. Furthermore, a fewer studies involved patients than clinicians. In 120 of 148 studies, the participants were homogenous: only doctors (10), only other health professionals (99) or only patients (11). Delphi studies of nurses’ priorities were particularly common (78 studies); examples include identifying oncology nurses’ priorities for cancer research in Canada 25 and the identification of practice issues within a hospice as a means of prioritizing areas for research and development in palliative care in England 26 .
In 28 of 148 studies, people worked together in mixed groups: doctors and other health professionals (10), doctors and patients (4), doctors, other health professionals and patients (6), doctors, other health professionals, patients and other groups (6), or doctors and other groups (2).
Of the 96 studies which described patients’ and/or clinicians’ research questions, participants were patients (12), doctors (24), nurses (71), other health professionals (24), and in some cases included other groups altogether (5), e.g. researchers. Of the 12 which included the views of patients, three included patients alone, nine included patients working alongside doctors and four included both doctors and nurses.
The health focus of research questions
Between them, the 96 studies of patients’ and clinicians’ research questions addressed a wide range of health conditions (see Fig. 2). Many studies (61) included priorities relating to generic health care such as nursing care, or general health services, rather than specific conditions.
Figure 2.
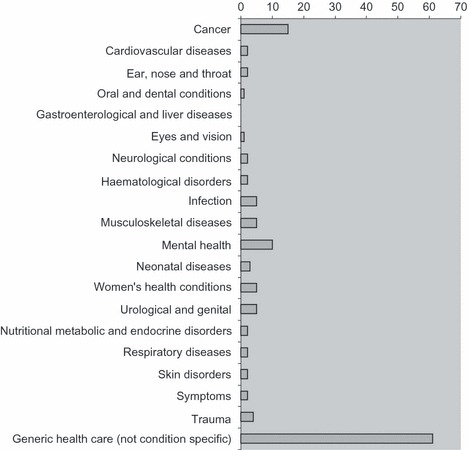
An overview of the health topics included in the 96 studies in which research questions are identified.
Eliciting patients’ research questions
Nine studies engaged both patients and clinicians in identifying research questions. 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 These nine studies varied in the health topics covered including: respiratory diseases (specifically asthma 27 ), nutritional, metabolic and endocrine disorders (specifically diabetes 27 ), urological and genital disorders (specifically incontinence 34 ) and kidney disease; 27 infection, 35 mental health 29 , 33 (including depression 29 ); and general health relevance. 6 , 28 , 30 , 31 One of the nine studies did not report the actual research questions identified, only the process of identifying them. 28 Eight of the nine reported the prioritized research questions. 27 , 29 , 30 , 31 , 32 , 33 , 34 , 35
All these studies engaged patients and others directly and repeatedly with the issues and with each other, either face‐to‐face 27 , 30 , 32 , 33 , 34 , 35 or through Delphi exercises. 29 , 31 One used a two‐step process whereby people were engaged first in homogenous groups before debating within a mixed group of stakeholders. 36 Most studies employed formal methods for reaching decisions about priorities, either a Delphi exercise, 29 , 31 individual rating and applying of criteria, 36 voting, 30 scoring, 32 or a consensus conference. 34 , 35
Exploring the nature of the expertise invited from participants highlighted how the two Delphi studies 29 , 31 gathered only contributory expertise from those taking part selecting them for their knowledge of the topic, with no reference made to the need for, or utilization of, interactional expertise. Two other studies also appear to have valued participants primarily for their contributory expertise. 33 , 34 The other four studies sought participants with a mixture of both contributory and interactional expertise, 27 , 30 , 32 , 35 for example by choosing participants with experience of working on committees, with particular attention given to selecting patients with interactional skills. 27
Lastly, of the eight studies analysed in greater detail, only three consider the influence of different groups of participants on the research questions prioritized. 27 , 29 , 30 All three observed no significant differences in the prioritization of the different stakeholder groups. Patients’ contributions are noted as valuable and constructive, 27 but not necessarily fundamental in changing the substance of the prioritized research agenda.
Discussion
We found a sizable research literature (258 reports) addressing patients’ or clinicians’ either reflecting on their experiences of health and health services, or engaging with research itself (148), to identify important areas for research, questions for research and tools for assessment. Two‐thirds of the studies addressing patients’ or clinicians’ research questions were not limited to particular conditions but applicable more widely across health care such as nursing care or health services generally. The 27 formal studies of patient involvement identified in this review reveal a literature that has grown since the six formal studies identified in an earlier systematic review. 1 They also reveal a shift since this earlier review towards more initiatives which engage participants directly and repeatedly in facilitated debate and adopt formal methods for decision‐making. Eight studies described patients and clinicians contributing their expert knowledge to prioritize research questions. Half of these also sought participants with interactional skills. In two other studies, using Delphi designs, interactional skills were required more of the researchers than the patients or clinicians participating. A Delphi study requires participants to be able to understand and take into account the views of others in providing written responses, but not the interactional debating skills required by methods such as consensus conferences. Although the two remaining studies incorporated group interactions, there was no acknowledgement of the interactional skills required. This is despite the fact that the need for both interactional expertise and contributory expertise has been recognized as particularly important where the public and scientists work together. 10
Increasing use of formal methods for decision‐making provides growing opportunities not only for participants to exert their influence, but also for formal evaluations to investigate their influence. The two pilot priority setting partnerships of the JLA addressing research agendas for asthma and urinary incontinence also share these features. In the literature we reviewed, we identified three evaluations comparing patients’ and others’ influence on the research agendas, all of which suggested patients’ influence was not significantly different from health professionals. This contrasts with the findings of one more recently published paper which found public contributions have changed decisions and influenced research plans. 37 This issue warrants investigation with further assessment of the methods of involvement and how they might shape the scope for and extent of patients’ influence.
Whilst this map of the literature on patients’ and clinicians’ research priorities is the most comprehensive that we know of, it only describes this literature and does not assess its quality. This literature has yet to be appraised for the legitimacy and fairness of participation methods or the quality of any evaluations. There is also scope for further examination of the content of the questions raised by patients and clinicians to see if there are any trends in their priorities.
Despite policy support for both clinician and patient involvement within health research, we found a few instances where both patients and clinicians were involved in identifying research priorities and their conclusions made available (only 9 out of 258 papers which addressed this topic). This in itself suggests that eliciting patients’ and clinicians’ priorities may be a largely academic exercise, and currently unlikely to lead to the desired improvement in health care and policy. Although our framework accommodated a link between patients’ and clinicians’ views and individual studies or programmes of research, the international literature we found suggests this link is rarely made. Moreover, a UK survey confirmed that research priorities for the public and charitable sector are often set by the research community and rarely restrict what research is funded. 38
One example where a link was evident between patients’ and clinicians’ views and research was instigated by research funders eliciting views about the primary‐secondary care interface as part of the priority setting process for a needs‐led commissioned research programme. 6 This is an example of the rhetoric of research being for, and informed by, patients and clinicians being translated into policy by a government‐led national research programme. Links with programmes that respond to researchers’ proposals, whether in the public or charitable sectors, are less direct. We recommend that research funders and researchers strengthen these links by drawing on the growing literature identified in this paper, to consider the research priorities already identified by patients and clinicians, and the methods available for identifying priorities in their own areas of interest. We recommend that research funders and researchers strengthen these links by stating in their research tenders and applications how their research questions relate to published priorities of patients and clinicians, and where these do not exist, consider eliciting priorities using the approaches outlines in this paper. Funders need to drive this change by demanding an explanation for how research questions have been chosen. In addition, those who have been involved in working with patients and clinicians to develop research priorities need to ensure these are published and available to those who can make use of them.
Source of funding
This study was funded by the JLA, Oxford, UK.
Conflicts of interest
None.
Acknowledgements
This study was funded by the JLA. The study protocol, emerging findings and a draft report were presented to their Strategy and Development Group who provided guidance and feedback.
Footnotes
Many terms are used to describe people whose principal interest is in their own health and/or that of their families. We use ‘patients’ in this paper to encompass this broad group of people.
Two of the reports authored by service users also include authors who are qualified health professionals.
References
- 1. Oliver S, Clarke‐Jones L, Rees R et al. Involving consumers in research and development agenda setting for the NHS: developing an evidence‐based approach. Health Technology Assessment, 2004; 8: 1–148. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization in Geneva, Switzerland from 10th ‐11th April 2008.
- 3. Oliver S. The progress of lay involvement in the NHS research and development programme. Journal of evaluation in clinical practice, 2009; 2: 273–280. [DOI] [PubMed] [Google Scholar]
- 4. Department of Health . Best Research for Best Health. Research and Development Directorate, London: Department of Health, 2006. [Google Scholar]
- 5. Medical Research Council . Clinical Trials for Tomorrow: An MRC review of randomised control trials. London: MRC, 2003. [Google Scholar]
- 6. Jones J, Hunter D. Consensus methods for medical and health services research. British Medical Journal, 1995; 311: 376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones J, Hunter D. Using the Delphi and nominal group technique in health services research In: Pope C, Mays N. (eds) Qualitative Research in Health Care. London: BMJ Books, 2000: 132–141. [Google Scholar]
- 8. Murphy MK, Black NA, Lamping DL et al. Consensus development methods, and their use in clinical guideline development. Health Technology Assessment, 1998; 2: 1–90. [PubMed] [Google Scholar]
- 9. Thompson J, Barber R, Ward PR et al. Health researchers’ attitudes towards public involvement in health research. Health Expectations, 2009; 12: 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collins HM, Evans R. The third wave of science studies: studies of expertise and experience. Social Studies of Science, 2002; 32: 235–296. [Google Scholar]
- 11. Garland AF. Multiple stakeholder agreement on desired outcomes for adolescents’ mental health services. Psychiatric services (Washington, D.C.), 2004; 55: 671–676. [DOI] [PubMed] [Google Scholar]
- 12. Higgins PDR, Schwartz M, Mapili J, Krokos I, Leung J, Zimmerman E. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut, 2005; 54: 782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clark A, Friedman M. The relative importance of treatment outcomes: a Delphi group weighting in mental health. Evaluation review, 1982; 6: 79–93. [Google Scholar]
- 14. Saunders C, Girgis A, Butow P, Crossing S, Penman A. Beyond scientific rigour: funding cancer research of public value. Health Policy, 2007; 84(2–3): 1–9. [DOI] [PubMed] [Google Scholar]
- 15. Revicki DA, Leidy NK, Brennan‐Diemer F, Sorensen S, Togias A. Integrating patient preferences into health outcomes assessment: the multiattribute Asthma symptom utility index. Chest, 1998; 114: 998–1007. [DOI] [PubMed] [Google Scholar]
- 16. Devane D, Begley CM, Clarke M, Horey D, O’Boyle C. Evaluating maternity care: a core set of outcome measures. Birth, 2007; 34: 164–172. [DOI] [PubMed] [Google Scholar]
- 17. Hagen S, Hunt J. Assessing nurses’ views of research priorities: a pilot study. Managing Clinical Nursing, 1998; 2: 49–53. [Google Scholar]
- 18. Bellamy N, Kirwan J, Boers M et al. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip, and hand osteoarthritis. Consensus development at OMERACT III. Journal of Rheumatology, 1997; 24: 799–802. [PubMed] [Google Scholar]
- 19. Clarke M. Standardising outcomes for clinical trials and systematic reviews. Trials, 2007; 8: 39 DOI:10.1186/1745‐6215‐8‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kirwan J, Heiberg T, Hewlett S et al. Outcomes from the patient perspective workshop at OMERACT 6. Journal of Rheumatology, 2003; 30: 868–872. [PubMed] [Google Scholar]
- 21. Kirwan J, Ahlmen M, De Wit M et al. Progress since OMERACT 6 on including patient perspective in rheumatoid arthritis outcome assessment. Journal of Rheumatology, 2005; 32: 2246–2249. [PubMed] [Google Scholar]
- 22. Kirwan J, Hewlett SE, Heiberg T et al. Incorporating the patient perspective into outcome assessment in rheumatoid arthritis–progress at OMERACT 7. Journal of Rheumatology, 2005; 32: 2250–2256. [PubMed] [Google Scholar]
- 23. Saag KG. OMERACT 6 brings new perspectives to rheumatology measurement research. Journal of Rheumatology, 2003; 30: 639–641. [PubMed] [Google Scholar]
- 24. Tugwell P, Boers M, Brooks P, Simon L, Strand V, Idzerda L. OMERACT: an international initiative to improve outcome measurement in rheumatology. Trials, 2007; 8: 38 DOI:10.1186/1745‐6215‐8‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Western Consortium for Cancer Nursing Research . Priorities for cancer nursing research: a Canadian replication. Cancer Nursing, 1987; 6: 319–326. [PubMed] [Google Scholar]
- 26. Daniels L. The way forward: identifying palliative nursing research priorities within a hospice. International Journal of Palliative Nursing, 2001; 7: 442–448. [DOI] [PubMed] [Google Scholar]
- 27. Caron‐Flinterman JF, Broerse JEW, Teerling J et al. Patients influence. Stakeholder participation in health research agenda setting: the case of asthma and COPD research in the Netherlands. Science & Public Policy, 2006; 33: 291–304. [Google Scholar]
- 28. Grant‐Pearce C, Miles I, Hills P. Mismatches in Priorities for Health Research Between Professionals and Consumers. A Report to the Standing Advisory Group on Consumer Involvement in the NHS R&D Programme. Manchester, UK: Policy Research in Engineering, Science and Technology (PREST), University of Manchester, 1998. [Google Scholar]
- 29. James P, Aitken P, Burns T. Research priorities for primary care mental health: a Delphi exercise. International Journal of Psychiatry in Clinical Practice, 2002; 8: 27–30. [Google Scholar]
- 30. Johanson R, Rigby C, Newburn M, Stewart M, Jones P. Suggestions in maternal and child health for the National Technology Assessment Programme: a consideration of consumer and professional priorities. The Journal of the Royal Society for the Promotion of Health, 2002; 122: 50–54. [DOI] [PubMed] [Google Scholar]
- 31. Johnson MA, Wells SJ, Testa MF, McDonald J. Illinois’s child welfare research agenda: an approach to building consensus for practice‐based research. Child Welfare, 2003; 82: 53–75. [PubMed] [Google Scholar]
- 32. Jones R, Lamont T, Haines A. Setting priorities for research and development in the NHS: a case study on the interface between primary and secondary care. British Medical Journal, 1995; 311: 1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Renvoize E, Patel J. Consumer voices steer the course of research. Journal of Dementia Care, 2002; 10: 37–38. [Google Scholar]
- 34. Whitehead WE, Wald A, Norton NJ. Priorities for treatment research from different professional perspectives. Gastroenterology, 2004; 126 (1 Suppl 1): S180–S185. [DOI] [PubMed] [Google Scholar]
- 35. Zulu I, Schuman P, Musonda R et al. Priorities for antiretroviral therapy research in sub‐Saharan Africa: a 2002 consensus conference in Zambia. Journal of Acquired Immune Deficiency Syndromes, 2004; 36: 831–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Caron‐Flinterman JF. A New Voice in Science: Patient Participation in Decision‐making on Biomedical Research. PhD Thesis, Amsterdam: Vrije Universiteit, 2005. [Google Scholar]
- 37. Oliver S, Armes DG, Gyte G. Public involvement in setting a national research agenda. Patient, 2009; 2: 179–190. [DOI] [PubMed] [Google Scholar]
- 38. Staley K, Hanley B. Scoping Research Priority Setting with UK Clinical Research Organisations and Funders. London: James Lind Alliance, 2008. [Google Scholar]


