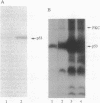
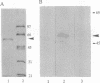
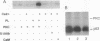Abstract
We report here that the negative cell cycle regulator protein p53 is an in vivo and in vitro substrate for protein kinase C, a cellular receptor for the tumor-promoter phorbol esters. We also demonstrate that p53 interacts in a calcium-dependent manner with S100b, a member of the S100 protein family involved in cell cycle progression and cell differentiation, and that such an interaction inhibits in vitro p53 phosphorylation by protein kinase C. The interaction between p53 and S100b was utilized for the purification of cellular and recombinant murine p53 by affinity chromatography with S100b-Sepharose. Furthermore, and of particular interest, we have shown that purified p53 undergoes temperature-dependent oligomerization and that the interaction between S100b and p53 not only induces total inhibition of p53 oligomerization but also promotes disassembly of the p53 oligomers. We suggest that these effects result from the binding of S100b to the multifunctional basic C-terminal domain of p53 and propose that p53 may be a cellular target for the S100 protein family members involved in the control of the cell cycle at the G0-G1/S boundary.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison C., Jenkins J. R., Stürzbecher H. W. The p53 nuclear localisation signal is structurally linked to a p34cdc2 kinase motif. Oncogene. 1990 Mar;5(3):423–426. [PubMed] [Google Scholar]
- Albert K. A., Wu W. C., Nairn A. C., Greengard P. Inhibition by calmodulin of calcium/phospholipid-dependent protein phosphorylation. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3622–3625. doi: 10.1073/pnas.81.12.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi I. C., Huang Q. H., Means A. R. Identification of amino acids essential for calmodulin binding and activation of smooth muscle myosin light chain kinase. J Biol Chem. 1992 Feb 15;267(5):3024–3029. [PubMed] [Google Scholar]
- Baudier J., Holtzscherer C., Gerard D. Zinc-dependent affinity chromatography of the S100b protein on phenyl-Sepharose. A rapid purification method. FEBS Lett. 1982 Nov 8;148(2):231–234. doi: 10.1016/0014-5793(82)80813-2. [DOI] [PubMed] [Google Scholar]
- Baudier J., Mochly-Rosen D., Newton A., Lee S. H., Koshland D. E., Jr, Cole R. D. Comparison of S100b protein with calmodulin: interactions with melittin and microtubule-associated tau proteins and inhibition of phosphorylation of tau proteins by protein kinase C. Biochemistry. 1987 May 19;26(10):2886–2893. doi: 10.1021/bi00384a033. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Tan T. H., Eliyahu D., Oren M., Levine A. J. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988 Feb;8(2):531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon J. V., Lane D. P. Protein synthesis required to anchor a mutant p53 protein which is temperature-sensitive for nuclear transport. Nature. 1991 Feb 28;349(6312):802–806. doi: 10.1038/349802a0. [DOI] [PubMed] [Google Scholar]
- Graff J. M., Young T. N., Johnson J. D., Blackshear P. J. Phosphorylation-regulated calmodulin binding to a prominent cellular substrate for protein kinase C. J Biol Chem. 1989 Dec 25;264(36):21818–21823. [PubMed] [Google Scholar]
- Hagiwara M., Ochiai M., Owada K., Tanaka T., Hidaka H. Modulation of tyrosine phosphorylation of p36 and other substrates by the S-100 protein. J Biol Chem. 1988 May 5;263(13):6438–6441. [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring B. P. Basic residues are important for Ca2+/calmodulin binding and activation but not autoinhibition of rabbit skeletal muscle myosin light chain kinase. J Biol Chem. 1991 Jun 25;266(18):11838–11841. [PubMed] [Google Scholar]
- Houbre D., Duportail G., Deloulme J. C., Baudier J. The interactions of the brain-specific calmodulin-binding protein kinase C substrate, neuromodulin (GAP 43), with membrane phospholipids. J Biol Chem. 1991 Apr 15;266(11):7121–7131. [PubMed] [Google Scholar]
- Jindal H. K., Chaney W. G., Anderson C. W., Davis R. G., Vishwanatha J. K. The protein-tyrosine kinase substrate, calpactin I heavy chain (p36), is part of the primer recognition protein complex that interacts with DNA polymerase alpha. J Biol Chem. 1991 Mar 15;266(8):5169–5176. [PubMed] [Google Scholar]
- Kennelly P. J., Krebs E. G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991 Aug 25;266(24):15555–15558. [PubMed] [Google Scholar]
- Kern S. E., Kinzler K. W., Baker S. J., Nigro J. M., Rotter V., Levine A. J., Friedman P., Prives C., Vogelstein B. Mutant p53 proteins bind DNA abnormally in vitro. Oncogene. 1991 Jan;6(1):131–136. [PubMed] [Google Scholar]
- Kligman D., Hilt D. C. The S100 protein family. Trends Biochem Sci. 1988 Nov;13(11):437–443. doi: 10.1016/0968-0004(88)90218-6. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Benchimol S. p53: oncogene or anti-oncogene? Genes Dev. 1990 Jan;4(1):1–8. doi: 10.1101/gad.4.1.1. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Marks A., Petsche D., O'Hanlon D., Kwong P. C., Stead R., Dunn R., Baumal R., Liao S. K. S100 protein expression in human melanoma cells: comparison of levels of expression among different cell lines and individual cells in different phases of the cell cycle. Exp Cell Res. 1990 Mar;187(1):59–64. doi: 10.1016/0014-4827(90)90116-r. [DOI] [PubMed] [Google Scholar]
- Meek D. W., Simon S., Kikkawa U., Eckhart W. The p53 tumour suppressor protein is phosphorylated at serine 389 by casein kinase II. EMBO J. 1990 Oct;9(10):3253–3260. doi: 10.1002/j.1460-2075.1990.tb07524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J., Medcalf E. A. Cotranslation of activated mutant p53 with wild type drives the wild-type p53 protein into the mutant conformation. Cell. 1991 May 31;65(5):765–774. doi: 10.1016/0092-8674(91)90384-b. [DOI] [PubMed] [Google Scholar]
- O'Reilly D. R., Miller L. K. Expression and complex formation of simian virus 40 large T antigen and mouse p53 in insect cells. J Virol. 1988 Sep;62(9):3109–3119. doi: 10.1128/jvi.62.9.3109-3119.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosin J. M., Vilgrain I., Chambaz E. M. A single form of protein kinase C is expressed in bovine adrenocortical tissue, as compared to four chromatographically resolved isozymes in rat brain. Biochem Biophys Res Commun. 1987 Aug 31;147(1):382–391. doi: 10.1016/s0006-291x(87)80133-x. [DOI] [PubMed] [Google Scholar]
- Rosen A., Nairn A. C., Greengard P., Cohn Z. A., Aderem A. Bacterial lipopolysaccharide regulates the phosphorylation of the 68K protein kinase C substrate in macrophages. J Biol Chem. 1989 Jun 5;264(16):9118–9121. [PubMed] [Google Scholar]
- Shaulsky G., Ben-Ze'ev A., Rotter V. Subcellular distribution of the p53 protein during the cell cycle of Balb/c 3T3 cells. Oncogene. 1990 Nov;5(11):1707–1711. [PubMed] [Google Scholar]
- Stumpo D. J., Graff J. M., Albert K. A., Greengard P., Blackshear P. J. Molecular cloning, characterization, and expression of a cDNA encoding the "80- to 87-kDa" myristoylated alanine-rich C kinase substrate: a major cellular substrate for protein kinase C. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4012–4016. doi: 10.1073/pnas.86.11.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Gannon J. V., Lane D. P. Monoclonal antibody analysis of p53 expression in normal and transformed cells. J Virol. 1986 Aug;59(2):444–452. doi: 10.1128/jvi.59.2.444-452.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer D. B., Van Eldik L. J. Analysis of the calcium-modulated proteins, S100 and calmodulin, and their target proteins during C6 glioma cell differentiation. J Cell Biol. 1989 Jan;108(1):141–151. doi: 10.1083/jcb.108.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]