Abstract
Aim The paper aims to develop a model of translational research in which service user and other stakeholder involvement are central to each phase.
Background ‘Translational’ is the current medical buzzword: translational research has been termed ‘bench to bedside’ research and promises to fast‐track biomedical advances in the service of patient benefit. Models usually conceive of translational research as a ‘pipeline’ that is divided into phases: the early phase is characterized as the province of basic scientists and laboratory‐based clinical researchers; the later phases focus on the implementation, dissemination and diffusion of health applications. If service user involvement is mentioned, it is usually restricted to these later phases.
Methods The paper critically reviews existing literature on translational research and medicine. The authors develop a theoretical argument that addresses why a reconceptualization of translational research is required on scientific, ethical and pragmatic grounds.
Results The authors reconceptualize the model of translational research as an interlocking loop rather than as a pipeline, one in which service user and other stakeholder involvement feed into each of its elements. The authors demonstrate that for the ‘interlocking loop’ model of translational research to be materialized in practice will require changes in how health research is structured and organized.
Conclusion The authors demonstrate the scientific, ethical and pragmatic benefits of involving service users in every phase of translational research. The authors’ reconceptualized model of translational research contributes to theoretical and policy debates regarding both translational research and service user involvement.
Keywords: biomarkers, mental health, service user involvement, translational research
The turn to translation
Translational research aims to translate findings from basic research more quickly and efficiently into clinical and health‐care practice. It is frequently given the shorthand ‘from bench to bedside’: in other words, such research is intended to ease the path from laboratory experiments through to clinical trials to patient (and population‐level) interventions and applications. The concept of translational research emerged in the 1990s in oncology, specifically with regard to attempts to find new drugs. In 2003, the National Institutes of Health (NIH) in the United States raised the term to international prominence with the announcement of its new Roadmap, the third stream of which centred on translational research. 1 Since then, other countries have prioritized translational research (e.g. England’s National Institute for Health Research (NIHR) has funded 12 biomedical research centres and 16 research units focused on ‘translat[ing] fundamental biomedical research into clinical research that benefits patients’; 2 the European Commission Seventh Framework health budget of €6bn includes a strong focus on translational research). 3 Translational research is therefore likely to be influential for some time to come – both as a vision and as a way of structuring and funding research and health care.
Why the turn to translational research? While there have been extraordinary advances in the basic sciences in the last few decades (e.g. the mapping of the human genome, the vigorous growth of the neurosciences), there is concern that this progress has not led to many significant cures and that a ‘valley of death’ 4 has opened between basic and clinical research. Contopoulos‐Ioannidis et al., 5 for example, found 101 articles published in basic science journals between 1979 and 1983 that explicitly stated that the technologies studied had novel therapeutic or preventive promise; by 2002, only five of those findings were licensed for clinical use. Translational research hopes to cross the ‘valley of death’ and reduce the frequency of findings being ‘lost in translation’. 6
The core instruments of translational research are biomarkers. Biomarkers are characteristics that are ‘objectively measured and evaluated as … indicator[s] of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention’. 7 In other words, biomarkers are intended to assist in understanding the causal pathways through which particular conditions develop, individuals’ susceptibility to developing particular conditions, and individuals’ responses to treatments (most commonly pharmacological interventions). Biomarkers lie at the heart of the attempt to install a new era of personalized medicine, in which it is hoped that they will be able to predict with much greater precision the development of a disease and allow fine‐tuning of appropriate therapeutic strategies for increasingly specific patient subgroups. Translational research certainly encompasses much research that is not focused around biomarkers (e.g. much translational psychological, social care and public health research). Nonetheless, it is fair to argue that the vision that drives translational research and medicine is a vision in which biomarkers will expedite the development of new pharmacological treatments as they move from animal models through clinical testing through to effective use in humans. As the scientist Wehling has put it:
methods and tools to facilitate the translational process need urgently to be developed. A major aspect in this regard is the description and assessment of key indicators in a translational process, so‐called biomarkers, which are needed for translational prediction. They are the main elements in predicting efficacy and safety from animal to man [sic] and could be seen to be accountable for 80–90% of translational success. 8
The translational pathway was initially understood as unidirectional, moving from the laboratory to the clinic. But researchers and policy makers increasingly argue that the phrase ‘bench to bedside’ erroneously simplifies what is a complex and two‐way process. 9 There is therefore growing recognition that knowledge ‘from the bedside’ must feed back into the laboratory if the translational endeavour is to have any real success. Similarly, there is the growing realization that we need to understand much more about how and why interventions actually reach ‘the bedside’ (and the community): it is far from guaranteed that health applications move into real world practice. 10 , 11 The T1 phase – which encompasses the movement from basic science to clinical research – has therefore been complemented by phases T2, T3 and T4 (see Table 1).
Table 1.
Translational phases (adapted from Khoury et al. 12 )
| Translational phase | Research focus of translational phase |
|---|---|
| T1 | Research that seeks to move a basic discovery into a candidate health application. |
| T2 | Research that seeks to move T1 research into an actual health application, and research that develops evidence‐based guidelines. |
| T3 | Research that seeks to move evidence‐based guidelines into health practice through dissemination, implementation and diffusion research. |
| T4 | Research that seeks to move health practice into population health impact through outcomes research. |
The divisions between phases include those of expertise and personnel. While clinical laboratory‐based scientists such as geneticists and molecular biologists populate T1, phases T2–T4 demand a variety of expertise (including disease/illness‐specific expertise, clinical epidemiology, evidence synthesis and qualitative research). Notably, service user and other stakeholder participation, when mentioned at all, are assigned to these latter phases (and often to the very end of the pipeline). See, for example, the influential models of translational research developed by the President’s Cancer Panel in the US (Fig. 1a) and the Wellcome Trust in the United Kingdom (Fig. 1b). The model from the President’s Cancel Panel in the US indicates that advances will be disseminated to, and then adopted by, patients and the public; the Wellcome model implies that ‘engaging society’ takes place once the products/interventions are ready for the market. Neither indicates that patients and the public might have significant and active roles to play in earlier translational phases. In fact, while there have been multiple suggestions about how exactly to conceptualize translational research, it is virtually unheard of for patients and the public to be positioned anywhere but at the end of the (translational) line (though see van den Hoonaard 13 for a rare exception).
Figure 1.
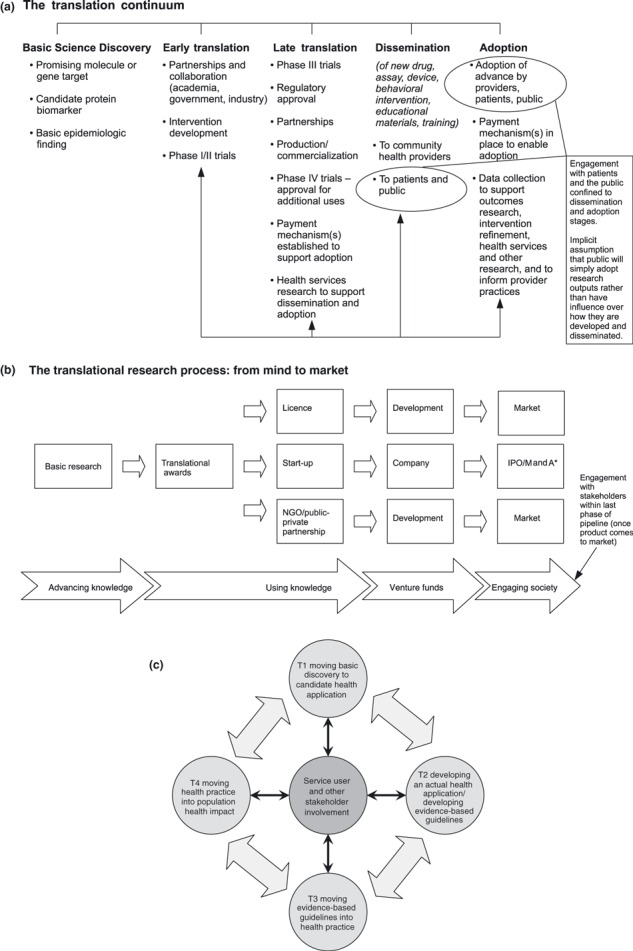
Models of translational research: The orthodox pipeline. (a) Translating research to reduce the burden of cancer: The translation continuum. Source: Suzanne H Reuben, for President’s Cancer Panel (2005) Translating Research into Cancer Care: Delivering on the Promise. 2004–2005 Annual Report: National Cancer Institute, US Department of Health and Human Services, National Institutes of Health. http://deainfo.nci.nih.gov/advisory/pcp/pcp04‐05rpt/ReportTrans.pdf (accessed 28 August 2009) [Permission for reproduction received from US Cancer Panel]. (b) The Translational Research Process: From Mind to Market. Source: The Wellcome Trust, What is Translational Research? [Permission for reproduction received from Wellcome Trust]. http://www.wellcome.ac.uk/Funding/Technology‐transfer/WTD027704.htm (accessed 30 August 2009). (c) Reconceptualized model of translational research that embeds service user and stakeholder involvement in all phases.
We argue that this orthodox conceptualization of the translational pipeline and of expertise is flawed. It confines service user and other stakeholder participation to one small channel and thereby ignores the potential for and benefits of collaborative, participatory research in all phases of the pipeline. (Our preferred term is service user rather than patient, though we retain others’ terminological choices [e.g. the commonly used ‘patient benefit’, ‘patient organizations’, etc.]. Our phrase ‘service users and other stakeholders’ equates to the term ‘public’ as it is used and defined by INVOLVE. 14 ) The orthodox conceptualization also implicitly sees service users and stakeholders as recipients of – rather than also potential generators of – knowledge, thereby rendering invisible the growing body of research conducted by, or in collaboration with, service users and stakeholders. (See for example the INVOLVE database comprising research projects in the field of health (including public health) and social care that have involved or plan actively to involve members of the public as partners in the research process. 15 )
In the remainder of this paper, we develop our argument for why the orthodox model of translational research is flawed; provide scientific, ethical and pragmatic reasons for why stakeholder involvement in required in all phases of translational research and end by calling for a reconceptualization of translational research in which service users and other stakeholders are contributors to each phase of translational research. We also briefly clarify what is required if the practice – as well as the conceptualization – of translational research is to include service users and other stakeholders in all phases.
We write as members of a translational academic health sciences centre (AHSC); more specifically, we are part of a biomedical research centre in England funded by the National Institute for Health Research whose primary focus is on biomarkers. Our argument therefore focuses on academic translational research, 16 , 17 and in particular on academic biomarkers research. We are of course aware that a huge proportion of translational research involving biomarkers research and medical product development is carried out by the private sector. How to involve service users and other stakeholders at each stage of the development and translation process for commercially developed products is not our prime focus here. Our specific field of expertise is mental health, and we therefore draw more readily on examples from this field.
Why transform the model and practice of translational research?
The scientific and the pragmatic argument
Woolf, in an influential theoretical paper on translational research, has argued for substantial investment in T2 research so as to maximize T1 investments. As he puts it, ‘Bringing a drug to market without knowing how to bring it to patients undermines its larger purpose and can only diminish its profitability for investors’. 10 But surely his argument would be better served by ensuring that potential consumers of the drug were involved before the drug was developed (in the T1 phase), rather than waiting till we have a product, but with no guarantee that it will ever find a market (T2–T4)? Service users could, for example, be involved not only in the selection of the research agenda, but on the choice of potential drug targets, which in turn might affect design issues regarding the molecule. Consider, for example, the development of new drugs for psychotic disorders within psychiatry: many service users find some of the side effects of current medication more troubling than some of the symptoms for which that medication is prescribed. 18 This opens up complex questions in terms of how best to judge the therapeutic value of existing and yet‐to‐be‐developed medications – as well as how to determine which symptoms are most pressing when deciding on priorities for drug development. Decisions over future drug design ought, we believe, to take into account service users’ lived experience regarding which symptoms of the psychiatric diagnosis are most troubling, rather than simply drawing on scientific and clinical expertise vis‐à‐vis what kind of medication is likely to produce most therapeutic value. As regards later phases within drug development, there are grounds for optimism: empirical research on medical technological innovation is increasingly indicating the key role that users can play in ensuring functionality and usability. 19 , 20 , 21 , 22
Indeed, there is a small but growing body of evidence regarding the scientific benefits of involving service users and other stakeholders throughout the translational conduit. Table 2 documents indicative research (from systematic reviews to theoretical research) on the reasons for embedding involvement, as well as some of the mechanisms through which one might do so, as regards:
Table 2.
Embedding service user and stakeholder involvement within translational research
| Category that underpins reason for involvement1 | Reason to embed involvement | Relevant phase(s) in translational pipeline | Methods and strategies for embedding involvement2 | Indicative literature |
|---|---|---|---|---|
| Scientific | Service users’ knowledge and experiences of use in agenda setting, problem definition and hypothesis construction | T1 | Creation of ‘small innovation networks’ devoted to the transition to involvement, and comprising biomedical researchers, service users and other stakeholders, experts in participation and representatives from research funding agencies and government organizations Collaboration with patients’ organizations Dialogue Model: democratic interaction between all stakeholders in the service of an integration of different knowledge sources | Abma and Broerse 41 Ayméet al. 32 Baart and Abma 36 Caron‐Flinterman et al. 35 Wright et al. 40 |
| Service users’ knowledge and experiences of use in defining treatment targets and developing outcome measures | T1–T2 | Participatory research (e.g. service user‐led focus groups to generate outcome measures) Priority setting partnerships (that join clinician organizations and patient/carer organizations) | Buckley et al. 42 Milne et al. 43 Rose et al. 44 | |
| Service users’ knowledge and experiences of use in design of medical devices/technologies | T1–T2 | Direct and active collaboration and interaction between users and producers in early stages (concept and idea generation) rather than only or mainly in late stage of product lifecycle | Dabbs et al. 19 Gagnon et al. 45 Grocott et al. 22 Shah and Robinson 20 , 21 | |
| Service users’ knowledge and experiences of use in trial design and consent processes | T1–T2 | Stakeholder representation on trial steering committees Combination of quantitative and qualitative methods (e.g. through consultation, focus groups and questionnaires with service users) | Ali et al. 46 Guarino et al. 31 Koops and Lindley 47 Langston et al. 48 | |
| Service users’ knowledge and experiences of use in development of health services research/implementation science/dissemination | T2–4 | Principles of successful service user involvement in health research outlined in Telford et al. 49 | Crawford et al. 30 Fudge et al. 50 Nilsen et al. 51 Simpson and House 52 | |
| Scientific/pragmatic | Assistance with recruitment into trials | T1–T2 | Involvement of consumers in trial governance so that they can then provide advice to trial participants, and promote trial to prospective participants | Langston et al. 48 |
| Ethical/socially just | Translational research that uses biomarkers for potential diagnostics, therapeutics and genetic testing raises ethical issues related to risk, susceptibility and stigma/discrimination | T1–T4 | Interdisciplinary collaborations between scientists, clinicians, social scientists, ethicists, legal scholars, policy makers, service users and those involved with industry/commercialization of biomarkers | Barr and Rose 53 Singh and Rose 27 |
| Public/civic engagement with biomedicine | T1–T4 | Citizens’ juries Town hall meetings | Nowotny et al. 54 | |
| Involvement can empower patients/service users (which can in turn improve health) | T1–T4 | Shared decision making (reduction of power differentials) | Angelmar and Berman 55 Boote et al. 56 |
1These categories are overlapping rather than discrete.
2These include methods and strategies that are evidence based, as well as those that relevant literature suggests on theoretical grounds.
-
1
Identification of biomedical research questions.
-
2
Choice of treatment targets and choice/development of outcome measures.
-
3
Design and assessment of medical devices/technologies.
-
4
Design of clinical trials (including consent processes).
-
5
Organization of health services.
-
6
Implementation science.
The potential benefits of embedding such involvement range from improving recruitment rates within clinical trials, to identifying new and important research questions and/or potential hypotheses, to developing interventions that are more likely to be taken up in the clinic and by communities. If, as Table 2 implies, there are both grounds for, and instances of, involving service users in T1 and T2 research (and not just in the phases in which attempts are being made to disseminate such research and improve adoption rates), then the orthodox model of the translational pipeline needs to be transformed. The models provided by the Wellcome Trust and the US President’s Cancer Panel are not sufficient.
The ethical argument
The search for biomarkers lies, as we have already noted, at the heart of translational research. Biomarkers promise to transform nosology and therapeutics, for example by giving clinical status to currently sub‐syndromal symptoms, and developing new modes of early intervention. 23 Within the field of Alzheimer’s research, scientists are close to identifying a blood biomarker that might be able to indicate the development of Alzheimer’s disease many years before the development of actual symptoms. 24 Research such as this promises, in time, to transform how diagnoses are made by shifting the balance away from the current reliance on clinical judgement and moving it towards the use of biomarker tests (e.g. in the form of blood tests or brain scans). It also suggests that, in time, there will be transformations in how diseases and disorders are separated out from one another. Such potential transformations raise many complex ethical and normative questions. For example, what would be the consequences of earlier biomarker‐based diagnoses in relation to a disease such as Alzheimer’s for which there is currently no cure? Biomarkers also carry great commercial value, and hence raise a number of difficult questions vis‐à‐vis the relation between academia and industry. 25 They therefore raise many ethical and legal questions. This can be discerned particularly clearly in the field in which we work – mental health. We use the example of mental health in this section to clarify our arguments, as this field is characterized by vigorous debate over aetiology; over the threshold for ‘caseness’ (how widely or narrowly the criteria for any particular diagnosis ought to be drawn); 26 the acceptability of pharmacological interventions; and which criteria to use when assessing the success of interventions. Biomarkers research is likely to have implications for all of these debates, which means that exploring the ethical and normative implications of such research is particularly pressing. But it is important to realize that psychiatric biomarkers operate through complex algorithms based around statistical probabilities rather than certainties. They are therefore unlikely definitively to resolve questions of aetiology or diagnosis, or which pharmacological intervention is most appropriate for which person. Biomarkers are, nonetheless, likely to move rapidly through translational channels and be used in a variety of therapeutic and other interventions. Singh and Rose 27 have argued in a recent article in Nature that, in the process, ‘risk profiling’ is likely to become ever more prevalent – e.g. the use of biomarkers to predict which children are likely to engage in antisocial behaviour, to justify early and preventative pharmacological intervention, or to underpin decisions over children’s education. Such risk profiles have the potential to affect personal identity, exacerbate stigma and consolidate societal assumptions about genetics, ethnic differences and behaviour. We argue, alongside Singh and Rose, that detailed research involving all those likely to be affected by biomarker research must take place before translation into and beyond the clinic. A wide range of stakeholders – including service users – must, in other words, be involved in the early translational phases, given the wide range of ethical and normative issues raised by such research.
Ethical concerns, we emphasize, are not restricted to mental health. Any approach to health improvement carries assumptions about what health, disease and disorder are, and how best to intervene. Mol, 28 in an ethnographic study of interventions for atherosclerosis, has shown that different modes of diagnosis entail different modes of intervention (e.g. if atherosclerosis is diagnosed clinically as legs that hurt on walking, then treatment might be walking therapy; cf. if atherosclerosis is diagnosed through imaging as obstruction of the vessel lumen, then treatment might entail surgery). These different approaches entail different ways of effecting ‘patient benefit’– and different criteria to judge success. Translational biomarker research is frequently allied with particular modes of diagnosis and particular ways of judging patient benefit. It is, as already noted, closely allied with attempts more precisely to target pathophysiological pathways, and much of its impetus is directed towards pharmacological innovation rather than other modes of health intervention. Neither diagnostics nor therapeutics can ever be a simply scientific procedure: each is intimately entangled with questions of ethics and politics. Service users, alongside many other stakeholders, must be party to deliberations in the T1 phase when the priorities and goals of health research are being consolidated and decisions over the allocation of research funds made.
What is required to reconceptualize the pipeline?
Models and visions that are employed within health research and health research policy can and do have profound consequences for which research is funded and how research is organized. The NIH’s Roadmap, which disseminated the concept of ‘translational research’ to a wide audience, has already had a profound effect on how health research is being carried out both within and beyond the USA. If models of translational research restrict service user and other stakeholder involvement solely to the end of the translational pipeline, then it is likely that many of those involved in planning and carrying out translational research will have little reason to question such positioning. For this reason, we believe that a re‐conceptualization of translational research is important in bringing about potential structural and intellectual change in the actual practice of translational research. But calling for a conceptual and theoretical shift is unlikely to be sufficient. We recognize that one of the most powerful ways in which to challenge orthodox models is of course actually to start changing practice. To give such a change in practice the potential to have a greater effect, such a change should be accompanied by evaluative research – to demonstrate both that practice is changing and to ascertain what effects those changes might be having. In this section, then, we briefly indicate how existing research on service user and stakeholder involvement can be harnessed in the services of challenging both the orthodox model and the orthodox practice of translational research.
More high‐quality research on involvement in all phases of translational research
The evidence base on the impact and benefits of service user and stakeholder involvement in research is still small and uneven. 29 The systematic review by Crawford et al. 30 on involving patients in the planning and development of health care demonstrated that the effects of this involvement on the quality and effectiveness of services are as yet unknown. A recent multicentre cluster randomized trial by Guarino et al. 31 showed that a consumer modification of a clinical consent document led to neither benefit nor harm in understanding, satisfaction or study refusal and adherence rates. But the constraints of the study limited the conclusions that might be drawn from it, and the authors argued that more research is needed to assess the effects of consumer involvement in trial consent processes.
Much of the existing research – and therefore, we assume, existing practices of service user and stakeholder involvement – cluster towards the latter phases of the translational pipeline (particularly health services research). More research is needed on service user involvement in T1–T2 as well as on T2–T4 – as regards scientific and other impacts, and which methods and mechanisms are likely to effect successful involvement. Service users and stakeholders are not uninterested in, nor absent from T1 research: members of patients’ organizations have designed and led biomedical research into rare diseases and have contributed to setting up biobanks. 32 There is also a growing interest in using service users’ experiential knowledge of diseases and disorders to shape biomedical hypotheses and research questions. 33
Structural and organizational changes to existing research frameworks
One of the few existing empirical investigations of service user involvement in T1 demonstrated that deliberate use of patients’ knowledge in biomedical research will require ‘a more structural and interactive approach to patient participation’ if it is to move beyond simply ad hoc use. 33 This is likely to challenge existing research cultures, and demand innovative ways of developing collaborative partnerships. In the Academic Health Sciences Centre in which we work, for example, we are attempting to develop new collaborative partnerships that will encourage the use of patients’/service users’ knowledge and expertise in biomedical research. 34 Changing research cultures and developing new collaborative partnerships is undoubtedly a difficult and slow process. But it is worth bearing in mind that the development of new research collaborations and new cultures of sharing expertise across hitherto separate domains is precisely what the translational endeavour is designed to bring about.
We are sanguine about the resistance that will undoubtedly face attempts to establish service user involvement in T1 and T2 research. Existing literature that addresses such barriers – as well as our own attempts within the translational Academic Health Sciences Centre in which we work – point to a number of intractable difficulties. These include significant asymmetries in power between scientists and service users, the prevalence amongst scientists of a ‘knowledge deficit model’ whereby they perceive their role as one of simply educating service users about the complexities of basic and translational research; many scientists’ lack of conviction that service user involvement has the potential to contribute scientifically to such research; the dominance of positivist scientific paradigms that preclude engagement with experiential knowledge and anxiety that service users lack the requisite objectivity and familiarity with high‐level abstraction adequately to participate. 35 , 36 These difficulties notwithstanding, we would remind readers of the trajectory that service user involvement has taken in applied health research over the last two decades. While resistance to involvement undoubtedly remains, many health services and public health researchers have, over the years, been persuaded of the scientific benefits of such involvement – whether through the experience of working with service users and patients, through the growing scientific stature of research that has involved service users/patients, or through the impact that such involvement has had on the design, practice and dissemination of health research. 14 We are hopeful that the domain of translational research might experience a similar journey.
Conclusion
Zerhouni, 37 the NIH director who spearheaded translational research, explicitly included ‘participation’ as one of his 4Ps of current medicine; the others are pre‐emption, prevention and personalization. Personalization of medical interventions is likely to be most effective, we argue, when the design of those interventions has not taken place at a great distance from those individuals who, it is hoped, will ultimately benefit from them. But much translational research restricts the question of participation to (i) improved recruitment and retention of subjects in clinical trials and (ii) dissemination and adoption of health applications in the latter translational phases. We have argued that restricting participation to these two arenas is likely to have deleterious effects on the translational endeavour:
-
1
through the failure to capitalize on the many scientific contributions that service users might bring to translational research in its earlier phases;
-
2
through the likelihood that many translational outputs will not be appropriately designed for their end users; and
-
3
through lack of interrogation of ethical questions raised by biomarker research and funding.
There is, then, a compelling need to challenge the dominant model and practice of ‘participation’ if the potential of translational research is fully to be realized.
Translational research is being held out as ‘an almost compulsive win–win situation’ in terms of benefits to patients and financial benefits. 38 For patients to benefit from research, they arguably need (i) the outputs of research (the intervention) to be easily available and usable, (ii) the intervention effectively to address a problem that they consider pressing and/or distressing, (iii) the treatment regimen to be tailored to the realities of their daily life 39 and (iv) the form the intervention takes to ‘fit’– more or less – with their values as regards the horizon of health, as well as acceptable levels of intrusivity and/or side‐effects. For the ‘win–win situation’ of financial as well as patient benefit to move from rhetoric to reality, translational research must therefore ensure that service users are embedded within every component of translational research.
Figure 1c presents a new model of translational research that indicates how such research might be both conceptualized and practised if this were the case. Instead of a pipeline, it is constructed as an interlocking loop with service user and stakeholder involvement feeding into each element (T1, T2, T3 and T4). There is, notably, two‐way interaction between each element – including service user and stakeholder involvement – within the model. Undoubtedly the model requires fine‐tuning and development through further empirical and theoretical research. The original NIH Roadmap charted, as any roadmap does, a direction for travel rather than a completed journey. In a similar way, our reconceptualized model of translational research presented in Fig. 1c is envisaged as a starting‐off point in conceptualizing service user involvement in translational research rather than as a final destination.
Competing interests
All authors are members of the Stakeholder Participation Theme of the NIHR Specialist Biomedical Research Centre (BRC) for Mental Health, South London and Maudsley NHS Foundation Trust and Institute of Psychiatry, King’s College London.
Acknowledgements and sources of funding
The authors acknowledge financial support from the National Institute for Health Research (NIHR) Specialist Biomedical Research Centre for Mental Health award to the South London and Maudsley NHS Foundation Trust and the Institute of Psychiatry, King’s College London. This funding source had involvement neither in the writing of this Review article nor in the decision to submit it for publication.
References
- 1. Zerhouni E. The NIH Roadmap. Science, 2003; 302: 63–72. [DOI] [PubMed] [Google Scholar]
- 2. National Institute for Health Research (NIHR) . Biomedical Research Centres. n.d. Available at: http://www.nihr.ac.uk/infrastructure/Pages/infrastructure_biomedical_research_centres.aspx, accessed 7 January 2011.
- 3. European Commission CORDIS . Health Research Homepage. n.d. Available at: http://cordis.europa.eu/fp7/health/, accessed 5 January 2010; cited 7 January 2011.
- 4. Butler D. Crossing the valley of death. Nature, 2008; 453: 840–842. [DOI] [PubMed] [Google Scholar]
- 5. Contopoulos‐Ioannidis DG, Ntzani EE, Ioannidis JPA. Translation of highly promising basic science research into clinical applications. The American Journal of Medicine, 2003; 114: 477–484. [DOI] [PubMed] [Google Scholar]
- 6. Mankoff S, Brander C, Ferrone S, Marincola F. Lost in translation: obstacles to translational medicine. Journal of Translational Medicine, 2004; 2: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Biomarker Definitions Working Group . Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clinical Pharmacology and Therapeutics, 2001; 69: 89–95. [DOI] [PubMed] [Google Scholar]
- 8. Wehling M. Translational medicine: science or wishful thinking? Journal of Translational Medicine, 2008; 6: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marincola FM. Translational medicine: a two way road. Journal of Translational Medicine, 2003; 1: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Woolf SH. The meaning of translational research and why it matters. JAMA, 2008; 299: 211–213. [DOI] [PubMed] [Google Scholar]
- 11. Tansella M, Thornicroft G. Implementation science: understanding the translation of evidence into practice. The British Journal of Psychiatry, 2009; 195: 283–285. [DOI] [PubMed] [Google Scholar]
- 12. Khoury MJ, Gwinn M, Yoon PW, Dowling N, Moore CA, Bradley L. The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genetics in Medicine, 2007; 9: 665–674. [DOI] [PubMed] [Google Scholar]
- 13. van den Hoonaard DK. Moving toward a three‐way intersection in translational research: a sociological perspective. Qualitative Health Research, 2009; 19: 1783–1787. [DOI] [PubMed] [Google Scholar]
- 14. Staley K. Exploring Impact: Public Involvement in NHS, Public Health and Social Care Research. Eastleigh: INVOLVE, 2009. [Google Scholar]
- 15. INVOLVE (National Institute for Health Research) . INVOLVE database. n.d. Available at: http://www.invo.org.uk/Database.asp, accessed 24 February 2010; cited 7 January 2011.
- 16. Dzau VJ, Ackerly DC, Sutton‐Wallace P et al. The role of academic health science systems in the transformation of medicine. Lancet, 2010; 375: 949–953. [DOI] [PubMed] [Google Scholar]
- 17. Lord GM, Trembath RC. A strategy for translation. Lancet, 2007; 369: 1771–1773. [DOI] [PubMed] [Google Scholar]
- 18. Read J. Psychiatric Drugs: Key Issues and Service User Perspectives. Basingstoke: Palgrave Macmillan, 2009. [Google Scholar]
- 19. Dabbs AV, Myers BA, McCurry KR et al. User‐centered design and interactive health technologies for patients. Computers, Informatics, Nursing, 2009; 27: 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shah SGS, Robinson I. User involvement in healthcare technology development and assessment: structured literature review. International Journal of Health Care Quality Assurance Incorporating Leadership in Health Services, 2006; 19: 500–515. [DOI] [PubMed] [Google Scholar]
- 21. Shah SGS, Robinson I. Benefits of and barriers to involving users in medical device technology development and evaluation. International Journal of Technology Assessment in Health Care, 2007; 23: 131–137. [DOI] [PubMed] [Google Scholar]
- 22. Grocott P, Weir H, Bridgelal Ram M. A model of user engagement in medical device development. International Journal of Health Care Quality Assurance Incorporating Leadership in Health Services, 2007; 20: 484–493. [DOI] [PubMed] [Google Scholar]
- 23. Woodcock J. The prospects for ‘personalized medicine’ in drug development and drug therapy. Clinical Pharmacology and Therapeutics, 2007; 81: 164–169. [DOI] [PubMed] [Google Scholar]
- 24. Thambisetty M, Simmons A, Velayudhan L et al. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Archives of General Psychiatry, 2010; 67: 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kolata G. In push to detect early Alzheimer’s markers, hopes for prevention. The New York Times. August 4, 2010. Available at: http://www.nytimes.com/2010/08/05/health/05alzheimers.html, accessed 13 April 2011. [Google Scholar]
- 26. Wykes T, Callard F. Diagnosis, diagnosis, diagnosis: towards DSM‐5. Journal of Mental Health, 2010; 19: 301–304. [DOI] [PubMed] [Google Scholar]
- 27. Singh I, Rose N. Biomarkers in psychiatry. Nature, 2009; 460: 202–207. [DOI] [PubMed] [Google Scholar]
- 28. Mol A. The Body Multiple: Ontology in Medical Practice. Durham and London: Duke University Press, 2002. [Google Scholar]
- 29. Staniszewska S, Herron‐Marx S, Mockford C. Measuring the impact of patient and public involvement: the need for an evidence base. International Journal for Quality in Health Care, 2008; 20: 373–374. [DOI] [PubMed] [Google Scholar]
- 30. Crawford MJ, Rutter D, Manley C et al. Systematic review of involving patients in the planning and development of health care. BMJ, 2002; 325: 1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guarino P, Elbourne D, Carpenter J, Peduzzi P. Consumer involvement in consent document development: a multicenter cluster randomized trial to assess study participants’ understanding. Clinical Trials, 2006; 3: 19–30. [DOI] [PubMed] [Google Scholar]
- 32. Aymé S, Kole A, Groft S. Empowerment of patients: lessons from the rare diseases community. Lancet, 2008; 371: 2048–2051. [DOI] [PubMed] [Google Scholar]
- 33. Caron‐Flinterman JF, Broerse JEW, Bunders JFG. The experiential knowledge of patients: a new resource for biomedical research? Social Science & Medicine, 2005; 60: 2575–2584. [DOI] [PubMed] [Google Scholar]
- 34. Callard F, Wykes T. Mental health and perceptions of biomarker research – possible effects on participation. Journal of Mental Health, 2008; 17: 1–7. [Google Scholar]
- 35. Caron‐Flinterman JF, Broerse JEW, Bunders JFG. Patient partnership in decision‐making on biomedical research: changing the network. Science Technology Human Values, 2007; 32: 339–368. [Google Scholar]
- 36. Baart ILMA, Abma TA. Patient participation in fundamental psychiatric genomics research: a Dutch case study. Health Expectations, 2010; doi: 10.1111/j.1369‐7625.2010.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zerhouni EA. Translational research: moving discovery to practice. Clinical Pharmacology and Therapeutics, 2007; 81: 126–128. [DOI] [PubMed] [Google Scholar]
- 38. Wehling M. Translational medicine: can it really facilitate the transition of research “from bench to bedside”? European Journal of Clinical Pharmacology, 2006; 62: 91–95. [DOI] [PubMed] [Google Scholar]
- 39. May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ, 2009; 339: b2803. [DOI] [PubMed] [Google Scholar]
- 40. Wright D, Corner J, Hopkinson J, Foster C. Listening to the views of people affected by cancer about cancer research: an example of participatory research in setting the cancer research agenda. Health Expectations, 2006; 9: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Abma TA, Broerse JEW. Patient participation as dialogue: setting research agendas. Health Expectations, 2010; 13: 160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buckley BS, Grant AM, Tincello DG, Wagg AS, Firkins L. Prioritizing research: patients, carers, and clinicians working together to identify and prioritize important clinical uncertainties in urinary incontinence. Neurourology and Urodynamics, 2010; 29: 708–714. [DOI] [PubMed] [Google Scholar]
- 43. Milne JL, Magali R, Selphee T, Drummond N, Ross S. Goal achievement as a patient‐generated outcome measure for stress urinary incontinence. Health Expectations., 2009; 12: 288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rose D, Wykes T, Farrier D, Doran A‐M, Sporle T, Bogner D. What do clients think of cognitive remediation therapy? A consumer‐led investigation of satisfaction and side effects. American Journal of Psychiatric Rehabilitation, 2008; 11: 181–204. [Google Scholar]
- 45. Gagnon M‐P, Lepage‐Savary D, Gagnon J et al. Introducing patient perspective in health technology assessment at the local level. BMC Health Services Research, 2009; 9: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ali K, Roffe C, Crome P. What patients want: consumer involvement in the design of a randomized controlled trial of routine oxygen supplementation after acute stroke. Stroke, 2006; 37: 865–871. [DOI] [PubMed] [Google Scholar]
- 47. Koops L, Lindley RI. Thrombolysis for acute ischaemic stroke: consumer involvement in design of new randomised controlled trial. BMJ, 2002; 325: 415–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Langston AL, McCallum M, Campbell MK, Robertson C, Ralston SH. An integrated approach to consumer representation and involvement in a multicentre randomized controlled trial. Clinical Trials, 2005; 2: 80–87. [DOI] [PubMed] [Google Scholar]
- 49. Telford R, Boote JD, Cooper CL. What does it mean to involve consumers successfully in NHS research? A consensus study. Health Expectations, 2004; 7: 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fudge N, Wolfe CDA, McKevitt C. Assessing the promise of user involvement in health service development: ethnographic study. BMJ, 2008; 336: 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nilsen E, Myrhaug H, Johansen M, Oliver S, Oxman A. Methods of consumer involvement in developing healthcare policy and research, clinical practice guidelines and patient information material. Cochrane Database of Systematic Reviews, 2006. (3): CD004563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Simpson EL, House AO. Involving users in the delivery and evaluation of mental health services: systematic review. BMJ, 2002; 325: 1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Barr M, Rose D. The great ambivalence: factors likely to affect service user and public acceptability of the pharmacogenomics of antidepressant medication. Sociology of Health & Illness, 2008; 30: 944–958. [DOI] [PubMed] [Google Scholar]
- 54. Nowotny H, Scott P, Gibbons M. Re‐Thinking Science: Knowledge and the Public in an Age of Uncertainty. Cambridge: Polity Press, 2001. [Google Scholar]
- 55. Angelmar R, Berman PC. Patient empowerment and efficient health outcomes. Report 3 in: Financing Sustainable Healthcare in Europe: New Approaches for New Outcomes. February 2007. Available at: http://elibrary.zdrave.net/document/EU/Commission%20of%20the%20EC/eure324972771_en.pdf, accessed: 13 April 2011. [Google Scholar]
- 56. Boote J, Telford R, Cooper C. Consumer involvement in health research: a review and research agenda. Health Policy, 2002; 61: 213–236. [DOI] [PubMed] [Google Scholar]


