Abstract
Aims (i) To describe patient and public involvement (PPI) in a network promoting research in dementia and neurodegenerative diseases, in terms of activity at the different stages of the research cycle and within the different levels of the research network. (ii) To use case studies to try and answer the question: what benefits (if any) does PPI in research bring to the research process?
Background PPI in health research is a central part of government policy, but the evidence base underpinning it needs strengthening. PPI allows exploration of feasibility, acceptability and relevance of hypotheses, assists in the precise definition of research questions and increases accrual to studies. However, the measurement of outcomes is methodologically difficult, because the impact of lay researchers may occur through team interactions and be difficult to untangle from the efforts of professional researchers. Opportunities for PPI in rapidly progressive diseases may be limited, and involvement of people with marked cognitive impairment is particularly challenging.
Design (i) Description of PPI within the DeNDRoN network. (ii) Case studies of three research projects which asked for extra help from centrally organized PPI.
Results PPI in research projects on the DeNDRoN portfolio may function at different levels, occurring at project, local research network and national level. Case studies of three research projects show different roles for PPI in research and different functions for centrally organized PPI, including contribution to remedial action in studies that are not recruiting to target, solving problems because of the complexity and sensitivity of the research topic, and linking researchers to PPI resources.
Discussion The case studies suggest that centrally organized PPI can have ‘diagnostic’ and remedial functions in studies that are struggling to recruit and serve as reinforcement for study‐level PPI in the complex and sensitive research topics that are typical in neurodegenerative diseases research. PPI may be actively sought by researchers, but the infrastructure of PPI is not yet so widespread in the research community that lay researchers are easy to find; a centrally organized PPI resource can assist in this situation.
Keywords: neurodegenerative diseases, patient and public involvement, research networks
Background
Patient and public involvement (PPI) in health research is a central part of government policy 1 and may be viewed in many situations as an ethical requirement, but there is only a scanty research literature on the best methods to involve patients and the public in health research 2 and the evidence base underpinning PPI needs strengthening. 3
Research in cancer treatments appears to have the longest tradition of formally managed PPI. Thornton 4 argues from experience of working in a PPI group in cancer studies since 1995 that the benefits of PPI in the research process outweigh the disadvantages and discusses the evidence from other studies that supports this conclusion. 5 , 6 PPI allows exploration of feasibility, acceptability and relevance of hypotheses, assists in the more precise definition of research questions and increases accrual to studies, in cancer research. 7 The Alzheimer’s Society’s Research Network panel recruits people with dementia, and carers who set research priorities, prioritise and comment on grant applications sit on grant selection panels, monitor on‐going projects funded by Alzheimer’s Society and tell others about the results of research. 8
Those involved in PPI need to address uncertainty about the effectiveness of interventions and so need to develop a ‘professional’ detachment despite their personal experience of disease. According to Thornton, 4 the success of PPI in cancer research is because of its constitution as a working component of research with a collaborative ethos, not an advocacy group.
In a narrative review of seven case studies of PPI in the design stage of research, Boote et al. 9 summarized the contributions of PPI as: review of consent procedures and patient information sheets; suggestions about important and appropriate outcomes; review of the acceptability of data collection procedures; and recommendations about the timing of entry to the study and of follow‐up. The authors of this review noted that funding was needed at the design stage to allow PPI to have an impact and that this was problematic because obtaining research funding depended on the quality of the design. The only study that they could identify which tested the effect of PPI had negative findings, but the input of PPI was modest and restricted to modification of an information document. 10
In an account of patient participation in research about spinal cord injuries, Abma 11 describes the conditions necessary for effective PPI and the barriers that PPI lay researchers must overcome. The effectiveness of PPI depends on the development of a dialogue between research stakeholders, among whom there will be agnostics and adversaries as well as advocates. 12 This in turn requires time planning favourable to patients and public representatives and attention to diversity among them. For their part, professional researchers need to approach PPI in a spirit of openness, respect, inclusion and engagement. Barriers to effective dialogue include conflicting timeframes and expectations, different languages (personal experience vs. scientific objectivity) and subtle processes of exclusion of PPI researchers. Abma notes tendencies for researchers to challenge the competency of patients involved in research and to prescribe their behaviour (particularly in requiring scientific detachment), and perceived the development at times of a sense among PPI lay researchers that they are ‘betraying’ their constituency. Because PPI in research is always situated in specific practices, assumptions, attitudes, norms and beliefs, research should focus on concrete examples of cases in specific settings.
One such case study of a PPI project over a 5‐year period concluded that long‐term, sustainable public involvement in research is possible, 13 but it reported the processes of engagement rather than the outcomes for research studies. The measurement of outcomes is methodologically difficult, because lay researchers function within a team and their impact may occur through team interactions and be difficult to untangle from the efforts of professional researchers or research managers. In some studies, there may be different levels of PPI, some recruited by the study teams as contributors to the initial proposal for funding and some operating through the research network that supports the study. Attributing effectiveness to each level may not be easy.
This paper describes the extent of PPI in research within the Dementias and Neurodegenerative Diseases Research Network (DeNDRoN), in terms of PPI activity at the different stages of the research cycle and within the different levels of the research network. It focuses particularly on the roles that can be played by central organization of PPI, as distinct from project‐ or locality‐level PPI. The paper includes three case studies in which centrally managed PPI was called on to assist three research studies conducted within the network. In our opinion, these case studies do show how PPI can alter both the processes and outcomes of studies.
DeNDRoN is a UK‐wide initiative that aims to improve the speed, quality and integration of well‐designed clinical research in dementias, Parkinson’s disease and other neurodegenerative diseases. It has been funded by the Department of Health as part of the National Health Service (NHS) National Institute for Health Research. It is committed to promoting PPI in research. DeNDRoN is organized as a managed network of seven local research networks (LRNs) directly covering approximately 65% of the population of England. It has a coordinating centre which includes a PPI organizer and clinical study groups (CSGs) of experts in the neurodegenerative diseases within its research remit. Research studies (commercial and non‐commercial) are ‘adopted’ by DeNDRoN Coordinating Centre after an assessment of feasibility and offered to LRNs as appropriate. The LRNs decide whether the study can be carried out in their locality, given their research capacity (number of researchers, current research workload), and are encouraged to have a balanced portfolio of studies that reflect the disease range of DeNDRoN, different methodological approaches (trials, observational studies) and the proportion of commercial to non‐commercial funders.
Processes and methods
Patient and public involvement
The infrastructure of PPI
PPI in DeNDRoN is organized both centrally and locally. The national coordinating centre hosts a PPI working group and a PPI forum. The seven LRNs also organize PPI panels to support research projects undertaken locally. The main aim is to ensure that the views and perspectives of people affected by the disorders inform DeNDRoN’s work in all stages of the clinical research process, both locally and nationally.
The PPI working group is comprised of representatives of relevant medical research charities (including the Alzheimer’s Society, Parkinson’s UK, the Motor Neurone Disease Association and the Huntington’s Disease Association), lay people affected by the disorders, experts in involving lay people in research, and DeNDRoN LRN managers. It oversees the PPI programme, provides the strategic lead both locally and nationally, meets three times per year and has a membership of 19.
PPI panels are convened in each of DeNDRoN’s Local Research Networks (LRNs) for patients, carers and other lay people in each region to have an input into the management and delivery of the research portfolio, in addition to lay representation in LRN steering committees.
The PPI forum is the national gathering of all the lay representation on LRN PPI panels and on national committees such as CSGs, and all other DeNDRoN lay colleagues. Its function is to share experience and knowledge, and to inform DeNDRoN’s mission to improve clinical research delivery for the benefit of patients, carers and the public. PPI members are recruited mainly through national and local medical charities and patient representative organizations, through research teams with past experience of PPI and through research‐active clinicians. They are supported by the national coordinator and by local LRN staff with training in research methods and with individual mentoring, and receive travel expenses and care costs at the rates recommended by INVOLVE. There are approximately 150 people registered as members of the PPI Forum, most of whom have, or have cared for a person with, a neurodegenerative disease.
Patient and public engagement with the DeNDRoN research portfolio
PPI in DeNDRoN is designed to have an effect on the research process at all of its stages, as shown in Fig. 1.
Figure 1.
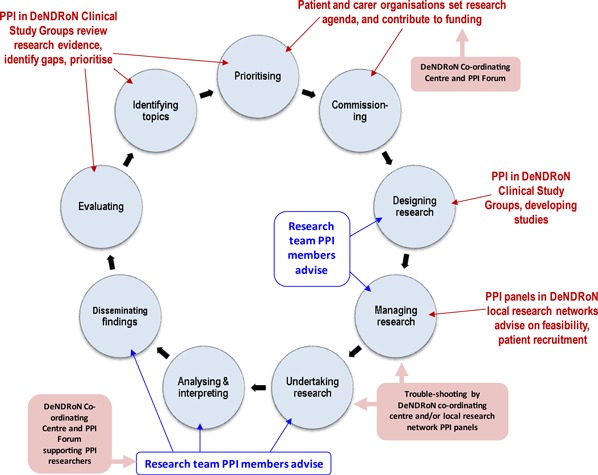
Patient and public involvement in DeNDRoN and the INVOLVE research cycle.
Text in blue shows how PPI lay researchers within studies influence study implementation. Text in red shows where PPI organized through the different structures within the DeNDRoN network has an impact on research. Text in pink boxes shows the role of the DeNDRoN Coordinating Centre in promoting PPI throughout the network. This role is the focus of the following case studies.
Case study methodology
Case study methods are appropriate when investigators desire or are forced by circumstances to define research topics broadly, to cover contextual or complex multivariate conditions and to rely on multiple sources of evidence. 14 They enable researchers to understand emerging problems and their practical solutions in the system under study, and gain insights that are potentially profitable in optimizing future development and policy advice. 15 Case studies can be exploratory, descriptive or explanatory, and findings from explanatory case studies can be amassed for cross‐case analyses. The all‐encompassing feature of a case study is its focus on a single phenomenon within its real‐life context. 16 Explanatory case studies can suggest important clues to causal relationships, but not with the certainty of true experiments. In the situation of the DeNDRoN research network, which is actively encouraged to promote PPI in all research activities, the question researchers want to answer is: what benefits (if any) does PPI in research bring to the research process?
In attempting to answer this question, we report here three examples of how the DeNDRoN Coordinating Centre has helped with PPI in individual studies, when asked to do so. One example (RESULT) is a study reviewing aspects of long‐term neurological conditions. The other two are drug studies, one around dementia in Parkinson’s disease (MUSTARDD‐PD) and the other concerning Alzheimer’s disease (DOMINO‐AD). The reasons for DeNDRoN Coordinating Centre’s PPI work for each study varied. Two of the study teams approached DeNDRoN asking for help with PPI in the development stage, but the first discussed here (DOMINO‐AD) was an established study where DeNDRoN Coordinating Centre became involved at the request of the LRNs and the study’s chief investigator when recruitment proved slower than desired in some areas.
Case studies
DOMINO‐AD – donepezil and memantine in moderate to severe Alzheimer’s disease
DeNDRoN Coordinating Centre asked our LRNs to suggest on‐going studies where PPI ideas might help with overcoming any challenges to meeting recruitment targets, and DOMINO‐AD was one such identified. This large clinical trial recruits people with Alzheimer’s disease who have been taking donepezil but have reached the point where NICE guidance would mean they would have to stop treatment. It investigates the benefits of an extra 12 months of treatment with donepezil, or memantine, or a combination, or placebo. DeNDRoN Coordinating Centre decided to organise two PPI focus groups to explore why, in some areas, recruitment rates had been below what was hoped.
Outcome
The two PPI focus groups were facilitated by staff in South West DeNDRoN and East Anglia DeNDRoN Local Research Network (LRNs) with a total of 27 patients with mild cognitive impairment, carers of patients with dementia, and two patients without direct dementia‐related experience but with motor neurone disease and Parkinson’s disease.
The discussions identified a range of key issues which were affecting the level of recruitment to DOMINO‐AD and specific ideas which could be useful in boosting recruitment. These included engaging with patients’ and carers’ perceptions of risks around stopping donepezil to join the study. The study was seen as potentially ‘inviting unnecessary disruptions’ at a particularly difficult stage of the illness. Fatigue and time pressures for carers was also identified as potential barriers to retention in the study, with ideas for making research study visits a more positive experience for carers.
Direct causal evidence of particular focus group ideas impacting on recruitment to DOMINO‐AD is not easy to identify, but Fig. 2 shows that the relative recruitment rates went up significantly within these two LRNs following the focus groups, in comparison with all other DeNDRoN LRN recruitment patterns for the DOMINO‐AD study.
Figure 2.
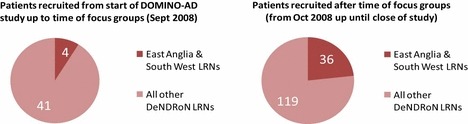
Numbers of patients recruited to DOMINO‐AD study comparing Local Research Networks which held focus groups with those which did not hold focus groups.
One suggestion made by the East Anglia focus group was to promote the study more effectively in primary care and to make GP referrals of patients into the study easier, by informing practices of the study and inviting them to engage with the research team and identify potential participants. This had a positive impact on East Anglia LRN’s efforts to increase local awareness, confirming that there would be patients who would want to participate.
Figure 3 shows that DOMINO‐AD recruitment in East Anglia LRN was struggling before the focus group. The discussions in the group encouraged researchers that committing resources to the lengthy processes of developing links through the Primary Care Research Network (PCRN) would bear fruit, and the Figure shows that rolling out the Primary Care recruitment clearly boosted levels.
Figure 3.
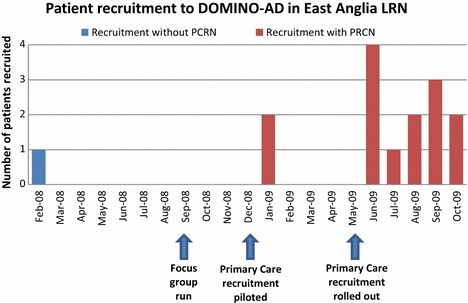
The impact on number of patients recruited to DOMINO‐AD in the East Anglia LRN when rolled out to General Practices with the support of the Primary Care Research Network (PCRN).
MUSTARDD‐PD – multicentre UK study of the acetylcholinesterase inhibitor donepezil in early dementia associated with Parkinson’s disease
This study evaluates the effectiveness of donepezil in the management of people with mild dementia associated with Parkinson’s disease. Its outcomes will inform prescribing policy for the use of these agents in this condition will also make a significant contribution to removing uncertainty regarding clinical effectiveness and reduce variability in the use of ‘anti‐dementia’ drugs in this context. The study has two lay researchers on its steering group, who identified study issues needed exploration in more depth, particularly appropriate and sensitive ways of broaching the topic of dementia with patients with Parkinson’s disease. DeNDRoN Coordinating Centre was asked for support in widening the discussion about these issues.
There is an expectation in DeNDRoN among patients and carers on LRN PPI ‘panels’ that they will become progressively more involved in study‐specific research tasks, such as advising in the developmental and implementation phases of studies. This opportunity was taken to use the development of patient information sheets as a training opportunity for PPI representatives that could also help the research team improve its documentation for the trial. Therefore, with the agreement of the MUSTARDD‐PD study team, DeNDRoN LRNs circulated the draft Patient and Carer Information Sheets to individual members of LRN PPI patient/carer panels.
Outcome
Fifteen PPI panel members in LRNs across DeNDRoN responded to the request to review the MUSTARD‐DD patient information sheet. These reviewers did not all necessarily have personal experience of Parkinson’s disease but also included others who offered perspectives of dementias from their patient or carer experience. They focused on whether the language used to introduce the subject of dementia to patients with early Parkinson’s disease was appropriate. There was consensus that the word – and topic of –‘dementia’ raises anxieties. PPI reviewers all felt that it was important that someone would take time to talk it through these anxieties fully with PD patients who were considering entering the trial. The dominant view was that ‘dementia’ has a different meaning to the medical profession, with less serious symptoms, from the frequent public perception of more serious and advanced symptoms. So the recommendation was that the word ‘dementia’ should be used less and be replaced by terminology which pertained to the range of potential symptoms and signs, was more sympathetically worded, and encompassed differing aspects of the problem. One PPI reviewer put it in these terms:
The medical profession uses a catch‐all phrase of Dementia to cover a range of, often slowly progressing, mental conditions. There are several neurodegenerative conditions where, over a period of time, whatever physical symptoms are present, there may also be some mental symptoms. These can include one, or any number of some degree of memory loss, muddled thinking, impaired judgment, impaired ability to think abstract thoughts, personality changes, slowness of thought process, etc.
Although to the lay person the very word dementia can be emotive and have negative connotations, often these symptoms are very mild, and remain so for many years. It is these very mild symptoms that the MUSTARRD‐PD study is seeking to investigate, as applicable to Parkinson’s Disease.
In addition, the lay PPI reviewers identified problems that were unrelated to the study’s disease focus, including the use of language, characterization of risks and the potential burden of assessments. One reviewer said:
The consent forms are incomprehensible…the sentence length is breathtaking’.
Questions of risk classification included the risks of discovering previously unknown problems during CT scanning that would affect participants’ ability to obtain insurance, and the real meaning of ‘small risk’ when used to describe blood sampling. The effect of lengthy visits to clinics (six, of about 2 h’s duration) on impaired participants was questioned, and the implications for retention in the study were noted.
Organizing face‐to‐face meetings was not attempted because of time considerations, patient mobility restrictions and the wide geographical spread of the LRNs; therefore, we asked lay members to comment individually. As a result, suggestions sometimes appeared contradictory and differences in recommendations had to be resolved by the research team (including its own PPI representatives). Although some of the key terminology in the patient information sheets is constrained by the formats encouraged by ethics committees, the study team reported back to DeNDRoN Coordinating Centre that many suggestions made were valid and constructive and that they would be making changes to their documents as a result of the consultation process.
RESULT – review of epidemiology and service use in rare long‐term neurological conditions
The RESULT study is studying service provision for people with rare long‐term neurological conditions, to inform the implementation of the National Service Framework for Long‐Term Neurological Conditions. Specifically, the study focuses on people with motor neurone disease, Huntington’s disease, multiple system atrophy, dominantly inherited ataxias, progressive supranuclear palsy, post‐polio syndrome and Charcot–Marie tooth disease. This study aims to investigate the current provisions of care and treatment and how they could beneficially change. Among many other aims is to provide detail of care management such as the timing of referral, drug history, access to rehabilitation and palliative services. This information will be available by population group, enabling breakdown by age, sex, ethnicity and locality. Full details at: http://www.ltnc.org.uk/research_files/RESULT_study.html, accessed 26 August 2011.
The study team had already budgeted in the research proposal for PPI to be ‘an integral part of all stages of the project’. They approached DeNDRoN asking for support in forming a reference panel of patients, carers and others to oversee the study.
Outcome
DeNDRoN Coordinating Centre succeeded in identifying and inviting lay people (and organizations where relevant) to take part in the reference panel. Members of the DeNDRoN PPI forum suggested ideas for the panel’s remit, including the potential for the panel to exist as a ‘virtual’ group in which not all members would necessarily meet face‐to‐face but instead use the DeNDRoN section of the National Institute for Health Research Portal as a forum for communication. In the first stage of the RESULT study user‐led consultations were organized by email and electronic discussion, but also through interviews with people whose disease (for example, motor neurone disease) limited their participation. These consultations were designed to prioritise those aspects of the course and consequences of neurological disease that should be captured in the study. Members of the PPI reference panel of RESULT also contributed to designing a systematic review of literature and available data, reviews of GP records and hospital databases, and economic modelling of disease progression and cost at different stages.
PPI representatives had a significant role in the final stage of the study. A questionnaire was developed with help of the patients and carers on the reference panel, and approved by an ethics committee, which allowed lay people to review the intended outcomes of studies, dissemination plans and implementation strategies. Posters, including one entitled ‘The Delphi technique to identify service user priorities in rare neurological conditions’, were disseminated, and a future programme of studies was designed.
The RESULT project has demonstrated that the model of PPI championed by DeNDRoN can be applied to a whole research process, engaging with people whose neurological disease in many circumstances impairs involvement. Involvement in this study has also been very helpful to DeNDRoN, because it has highlighted the need for clarity about where the responsibility lies for PPI activities in specific studies. For example, what help and guidance on PPI can study teams expect from research networks before and after adoption? Which PPI tasks should rest with the study team itself, which with the national coordinating centre of the Research Network, and which with the Local Research Networks where the study delivery will take place? For example, what training and on‐going support should research networks to provide for PPI volunteers identified for a specific study?
Discussion
Staniszewska, reviewing studies of PPI in research, describes PPI as complex, diverse, rich but also conceptually muddled and theoretically poor, with sparse impact data. She calls for consistent terminology in the reporting of PPI, with descriptions of what works for whom, and in what circumstances. 17
The three case studies presented here are examples of how centrally managed PPI can contribute positively to clinical research, in different ways. The benefits of PPI in these studies were perceived positively by both research network staff and researchers, as they solved problems identified by the research teams themselves. We argue that they allow us to tentatively answer the question: what benefits (if any) does PPI in research bring to the research process? They also offer insights that are potentially applicable to the future development of and policy about PPI in research. In arguing this we accept that case‐to‐case transfer, 18 transferability 19 and usability 20 of findings from case studies are dependent on the readers’ judgements, not ours.
In the DOMINO‐AD study, lay researchers were able to solve issues that were interfering with expected recruitment. This is an example of PPI used in a ‘diagnostic’ way which allows the causes of problems to be identified and remedial action taken. It is possible that earlier contributions by lay researchers could have averted these problems.
Because of the complexity and sensitivity of the research topic, the MUSTARDD‐PD study required a wider input from lay researchers than the PPI members of its steering committee could give and is an example of how promoting PPI at different levels may be helpful. Group discussions with lay people might have been preferable to individual feedback because they could potentially produce a greater degree of consensus and less diverse advice, but they require more organization. This reinforces the need for PPI planning at the early stage of study development, especially to meet the challenges when seeking involvement from people with mobility problems. Longer‐term evaluation will tell how and whether PPI inputs have a beneficial impact on study implementation.
The RESULT study is an example of the limited penetration of the research community by the PPI approach, because a research team wanting extensive PPI input to gain maximum involvement of patients in an epidemiological study – a group of advocates of PPI – did not have access ‘in house’ to the expertise or resources necessary.
These experiences demonstrated the complexity of developing PPI in research. We suggest that more specific written guidance is needed to optimize the contribution of PPI in clinical research at all stages from design to implementation. Study teams may well have unrealistic expectations of what help will be available from research networks, in terms of hands‐on facilitation, ‘banks’ of patients and carers, expertise and resources. This guidance should contain clear information about the level of support that is on offer for study teams, practical advice about PPI methods, and details of the resources available. Practical advice about PPI might include sending patient‐facing study information out for reviewing, running study‐specific focus groups, including lay members on study steering committees and establishing lay reference panels. Any guidance should include advice on what resources will be needed. An unresolved question, which these three examples have highlighted, is whether involvement expenses are a research cost and therefore provided from study grants, or a service support cost and therefore provided by the LRNs.
The level of PPI input required by individual studies may vary. Some projects may require significantly enhanced levels as exemplified by the RESULT study. Any group of people who have gained such an in‐depth knowledge of a study over time will be well placed to review study progress, look critically at problems that arise, interpret findings, and generate ideas for dissemination and for further related studies. The RESULT study example has been useful in highlighting the need for detailed guidance to help determine the composition of PPI panels, the remit of such panels, including managing expectations, how the groups should be administered within research networks and study teams as well as issues around training (who does what), finance and payment.
An important question which arises in this context is who should be involved in such panels. This flows from whether the PPI model aims at being highly representative of those with direct disease‐specific experience (as in RESULT), or the prioritises factors such as knowledge of clinical research generally, expertise in patient advocacy, social issues or ethical issues, even if they have no experience with any of the identified conditions (as in MUSTARD‐PD). If it is the former model, decisions are needed about the balance of patients and carers, ethnic mix and the degree to which to be representative of the relevant patient organizations as well as patients or carers themselves.
The contents of this paper are based on experiences obtained during the first 4 years activity of a UK research network on dementia and neurodegenerative diseases (DeNDRoN). The coordination of active PPI has presented a particular challenge for DeNDRoN. A number of issues have had to be addressed to enable people affected by neurodegenerative diseases to become more actively involved in the work of the network. Although many of these issues cut across all the diseases within the DeNDRoN remit, disease‐specific issues also need to be considered. Opportunities for PPI in rapidly progressive disease may be limited. Involvement of people with marked cognitive impairment is particularly challenging, and carers of people living with dementia have, in practice, been much more involved in DeNDRoN discussion groups. There are also challenges to overcome with involving people with mobility and speech problems.
Conclusions
Although it might be argued that neurodegenerative diseases raise unique disease‐specific questions about PPI, we hope that the cases described here demonstrate how centrally managed user involvement can positively influence the development and evolution of clinical research more generally. PPI may have diagnostic and potentially remedial functions in studies that are struggling, while PPI operating at different levels may help to carry out research in very sensitive domains. The research environment is not so saturated with experienced lay researchers that professional researchers aware of the need for PPI can easily obtain it.
Funding source
DeNDRoN.
Conflicts of interest
SI is an Associate Director of DeNDRoN responsible for the promotion of PPI; TMcG leads PPI development at the DeNDRoN coordinating centre; The late DM was chair of the DeNDRoN working group for PPI.
Acknowledgements
We thank all DeNDRoN staff, PPI representatives and researchers who discussed the ideas in this paper with us. The work of the Preston MND Care & Research Centre is supported by the MND Association, NIHR, George Barton Trust, ALSA and the Fisher Foundation.
References
- 1. Department of Health . Research Governance Framework for Health and Social Care, 2nd edn, London: Department of Health, 2005. [Google Scholar]
- 2. Staley K. Exploring Impact: Public Involvement in the NHS, Public Health and Social Care Research. Eastleigh: INVOLVE, 2009. [Google Scholar]
- 3. Staniszewska S, Herron‐Marx S, Mockford C. Measuring the impact of patient and public involvement: the need for an evidence base. International Journal for Quality in Health Care, 2008; 20: 373–374. [DOI] [PubMed] [Google Scholar]
- 4. Thornton H. Patients and health professionals working together to improve clinical research: where are we going? European Journal of Cancer, 2006; 42: 2454–2458. [DOI] [PubMed] [Google Scholar]
- 5. Hanley B, Truesdale A, King A, Elbourne D, Chalmers I. Involving consumers in designing, conducting and interpreting randomised controlled trials: questionnaire survey. BMJ, 2001; 322: 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Telford R, Boote J, Cooper C. What does it mean to involve consumers successfully in NHS research? A consensus study Health Expectations, 2004; 7: 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thornton H, Dixon‐Woods M. Recruitment of women into trials. Lancet, 2002; 359: 164. [DOI] [PubMed] [Google Scholar]
- 8. Alzheimers Society . The Quality research in dementia consumer network. Available at: http://www.alzheimers.org.uk/site/scripts/documents.php?categoryID=200296, accessed 26 August 2011.
- 9. Boote J, Baird W, Beecroft C. Public involvement at the design stage of primary health research: a narrative review of case examples. Health Policy, 2010; 95: 10–23. [DOI] [PubMed] [Google Scholar]
- 10. Guarino P, Elbourne D, Carpenter J, Peduzzi P. Consumer involvement in consent document development; a multicentre cluster randomised trial to assess study participants’ understanding. Clinical Trials, 2006; 3: 19–30. [DOI] [PubMed] [Google Scholar]
- 11. Abma T. Patient participation in health research: research with and for people with spinal cord injuries. Qualitative Health Research, 2005; 15: 1310. [DOI] [PubMed] [Google Scholar]
- 12. Becker S, Sempik J, Bryman A. Advocates, Agnostics and Adversaries: researchers’ perceptions of service user involvement in social policy research. Social Policy & Society, 2010; 9: 355–366. [Google Scholar]
- 13. Howe A, Delaney S, Romero J, Tinsley A, Vicary P. Public involvement in health research: a case study of one NHS project over 5 years. Primary Health Care Research & Development, 2010; 11: 17–28. [Google Scholar]
- 14. Yin RK. Applications of Case Study Research, 2nd edn London: Sage, 2003: xi–xvii. [Google Scholar]
- 15. Swanborn P. Case Study Research: What, Why and How? London: Sage, 2010: 33. [Google Scholar]
- 16. Yin RK. Enhancing the quality of case studies in health services research. Health Services Research, 1999; 34 (5 Pt 2): 1209–1224. [PMC free article] [PubMed] [Google Scholar]
- 17. Staniszewska S. Patient and Public Involvement in Research. Does it Really Make a Difference? Society of Academic Primary Care conference, July 8th 2010, Norwich. [Google Scholar]
- 18. Firestone WA. Alternative arguments for generalising from data as applied to qualitative research. Educational Researcher, 1993; 22: 16–23. [Google Scholar]
- 19. Lincoln YS, Guba EG. Naturalistic Inquiry. San Francisco, CA: Sage, 1985. [Google Scholar]
- 20. Swanborn P. A common base for quality control criteria in quantitative and qualitative research. Quality & Quantity, 1996; 30: 19–35. [Google Scholar]


