Abstract
Background Patients nearing the end of their lives face an array of difficult decisions.
Objective This study was designed to assess the feasibility and acceptability of a decision aid (DA) designed for patients facing advanced or terminal illness.
Design We conducted a pilot randomized clinical trial of Health Dialog’s Looking Ahead: choices for medical care when you’re seriously ill DA (booklet and DVD) applied to patients on a hospital‐based palliative care (PC) service.
Setting University of Colorado Hospital – December 2009 and May 2010.
Participants All adult, English‐speaking patients or their decision makers were potentially eligible. Patients were not approached if they were in isolation, did not speak English or if any provider felt that they were not appropriate because of issues such as family conflict or actively dying.
Intervention All participants received a standard PC consultation. Participants in the intervention arm also received a copy of the DA.
Measurements Primary outcomes included decision conflict and knowledge. Participants in the intervention arm also completed an acceptability questionnaire and qualitative exit interviews.
Results Of the 239 patients or decision makers, 51(21%) enrolled in the trial. The DA had no significant effect on decision conflict or knowledge. Exit interviews indicated it was acceptable and empowering, although they wished they had access to the DA earlier.
Conclusions While the DA was acceptable, feasibility was limited by late‐life illness challenges. Future trials of this DA should be performed on patients earlier in their illness trajectory and should include additional outcome measures such as self‐efficacy and confidence.
Keywords: decision aid, end‐of‐life, palliative care, randomized trial
Introduction
Patients and families dealing with advanced or terminal illness face an array of complicated decisions. Decisions about medical therapy include forgoing potentially life‐prolonging therapy such as chemotherapy, mechanical ventilation, and artificial nutrition and hydration. Patients are often asked to forecast these decisions through advance care planning. All this occurs in the setting of significant symptom burden and diminishing decision capacity. 1 , 2
Despite the challenges, there is ample evidence that patients desire information and control, as they approach the end of their lives. 3 , 4 , 5 Unfortunately, research suggests that patients with advanced illness are not well informed about their prognoses or their available care options. 6 , 7 , 8 For unclear reasons, patients with advanced illness tend to become more passive in their decision making, as they get older and sicker. 9 , 10
Traditional decision aids (DAs) typically focus on single‐event decisions 11 and are not as applicable to patients and families dealing with the challenges of advanced and terminal illnesses. This study utilizes a unique DA designed to inform and empower patients and family members facing advanced illness. How such a DA would best be integrated into clinical care is unknown. This pilot randomized trial was designed to assess the acceptability and feasibility of this DA.
Methods
Overview
This pilot randomized clinical trial (RCT) was designed to assess the acceptability and feasibility of a DA designed for people with advanced and/or terminal illness. The DA, entitled Looking Ahead: Choices for medical care when you’re seriously ill, was evaluated among patients on the inpatient palliative care (PC) service at University of Colorado Hospital (UCH) between December 2009 and May 2010. This trial was approved by the Colorado Multiple Institutional Review Board.
Decision aid development
The DA was developed by The Foundation for Informed Medical Decision Making and Health Dialog to encourage conversations, advance care planning and patient‐centred decision making related to advanced illness. Development entailed an extensive review of the literature; hospice and PC provider and informal caregiver focus groups; and expert review. Given the breadth of potential diagnoses and decisions that could have been included in the DA, a purposeful decision was made to focus the DA on more global issues, particularly introducing the idea of the availability of the option of PC and the importance of advance care planning and of clarifying patients’ goals and values. Specific decisions such as use of artificial nutrition and hydration, mechanical ventilation and cardiopulmonary resuscitation were not addressed in detail, given limitations on the length of the DA and the recognition that each of these specific decisions would require a full DA to address.
Participants
Adult, English‐speaking patients were recruited for this study from the inpatient palliative care consult service at UCH. In cases where the patient was unable to participate (e.g. because of mental status or extreme fatigue), the patient’s designated decision maker was eligible to participate. At UCH, patients are referred to the PC consult service for reasons including assistance with evaluation of the goals of care, discussion surrounding end‐of‐life care and symptom management. For this study, only patients who were referred for PC consults for the purposes of goal clarification or end‐of‐life discussions were eligible.
Design
After receiving permission from the primary attending physician as well as the PC team for the patient to participate, the research assistant approached eligible participants, obtained informed consent from those willing to participate, conducted baseline assessments and randomized enrolled individuals to the control or intervention groups using a pre‐defined envelope randomization system.
Control arm participants received usual care from the PC service. The PC service at UCH is a purely consultative service consisting of two nurse practitioners, a chaplain resident and six attending physicians who rotate weekly. Patients are referred for PC consultation by primary treating physicians for a variety of reasons including assistance with symptom management, clarification of the goals of care and assistance with decision making. A typical consult includes a discussion regarding the patient’s and/or caregiver’s knowledge of their medical condition as well as the overall goals of care. Additional topics often discussed include symptoms, surrogate or proxy decision makers and advance directives. Intervention arm participants were provided the DA and asked to review it in addition to receiving the usual PC consult. We did not specify when patients should review the DA in relation to the PC consult, but rather stated that it could be performed at the participants’ convenience before, during or after the PC team consultation. By not specifying when, this allowed us to gather further insight into the real‐world feasibility of DA implementation.
Baseline assessments included a brief survey administered by the research assistant asking participants to identify the most difficult decisions that they were currently facing as well as demographic information, control preferences, 12 decision conflict 13 and knowledge assessment. A follow‐up interview was conducted several days after the baseline assessment at a time and location convenient to the patients. This interview could be conducted after the PC consult, later in their hospitalisation or after discharge. In addition to the instruments administered during the baseline assessment, follow‐up interviews in the intervention arm also included an assessment of the acceptability of the DA as well as an open‐ended exit interview regarding the participants’ reactions to the DA.
Outcomes
Decision aid implementation was evaluated using the RE‐AIM framework. 14 This framework proposes evaluating interventions in terms of reach, effectiveness, adoption, implementation and maintenance. In this analysis, reach was evaluated by determining the number of people who were eligible and approached, who ultimately enrolled in the trial. The primary effectiveness outcomes included quantitative measures of decision conflict and knowledge. Potential barriers and facilitators to adoption, implementation and maintenance were explored with qualitative interviews with the patients (reported here) and physician focus groups (reported in a forthcoming separate analysis).
Feasibility and acceptability
Feasibility was evaluated both by determining the reach of the intervention (number assessed/number enrolled and number eligible/number enrolled) as well as from the qualitative exit interviews. Acceptability was assessed via the exit interviews as well as through a modified version of DA acceptability questions developed by Barry et al. 15
Decision conflict
The Decision Conflict scale is a validated scale designed to assess patients’ perceptions of uncertainty, their feelings about modifiable factors of uncertainty and satisfaction with their ultimate choice. The original scale is 15 items, but the developers have made a 10‐item ‘low‐literacy’ version. 13 Given the severity of the illness of the patients enrolled in this trial, we used the 10‐item version to reduce participant burden.
Knowledge
Drs Matlock and Kutner developed six true/false questions designed to assess knowledge surrounding important aspects of decision making during advanced illness. These questions were reviewed by the study team as well as by the members of the University of Colorado programme in PC research to assure that they were clear and had content validity. The knowledge questions were as follows: (1) You can receive PC while receiving life‐prolonging therapy (such as chemotherapy) – True; (2) An advance directive is a signed document that tells others what is important to you regarding your medical care – True; (3) Having an advance directive means you no longer wish to receive any life‐prolonging therapies – False; (4) A ‘Do Not Resuscitate’ order means that you no longer wish to receive any life‐prolonging therapy – False; (5) A ‘proxy decision maker’ is someone who can speak for you if you become unable to speak for yourself – True; (6) A ‘Medical Power of Attorney’ controls your finances when you are unable to do so – False.
Qualitative outcomes
At the second assessment, intervention participants participated in a brief semi‐structured interview to gain further understanding of how this DA fits into the decision‐making process as well as any potential impacts (positive or negative) of the DA during this process to help provide insight into potential barriers to adoption, implementation and maintenance. Interview questions included knowledge, values and acceptability of the DA as well as self‐efficacy related to future discussions with provider.
Analysis
Quantitative analyses were performed using sas ® software v 9.2; SAS Institute Inc., Cary, NC, USA. The primary quantitative outcomes were decision conflict and knowledge of advance directives. For decision conflict, total scores for each participant were scaled from 0 to 100, while for knowledge of advance directives, the proportion correct for each study participant was utilized. Summary description of both outcomes included mean total scores, standard deviations and 95% mean confidence intervals for each study arm and pre‐ and post‐testing. A t‐test was used to determine the difference in time to follow‐up between the groups.
Decision conflict total scores were analyzed by proc mixed in SAS, which was chosen for its ability to handle the correlated nature of the data. Knowledge of advance directives scores was analyzed by proc glimmix in SAS, which fits statistical models to data with correlated responses and where the responses are not necessarily normally distributed. In our case, knowledge of advance directive responses was assumed to be binomial distributed. For both analysis methods, the responses were assumed to be correlated with an unstructured (UN) covariance matrix and fitted without an intercept. Furthermore, for comparison, both outcome responses were also analyzed by the non‐parametric Wilcoxon test, which did not demonstrate any difference in statistical conclusion obtained. All reported P values were two sided, with P ≤ 0.05 considered statistically significant.
Qualitative interviews were audio‐taped and transcribed by a professional transcriptionist, and then entered into atlas.ti qualitative software (ATLAS.ti Scientific Software Development, Berlin, Germany) for coding and analysis. 16 We utilized a general inductive approach as our primary method of analysis. 17 , 18 A codebook was built through iterative coding by three members of the team (CN, DM and TK), who met regularly to discuss codes, resolve discrepancies and reach congruence. We then presented our codebook and emerging themes to the larger team for a multidisciplinary review, considering confirming and disconfirming cases and competing explanations, next returning to the data for further contextualization and confirmation of main themes.
Results
Of the 239 patients referred to the PC service, 120 (50%) were approached and 51 (21%) of these enrolled in the study (Fig. 1) demonstrating a low reach of this intervention into this population. Of those who were unable or declined participation were 56% women and had a median age of 64 (range 21–95). Participants consisted of 31 decision makers and 20 patients who were mostly non‐Hispanic white, and all but three had at least a high school or equivalent degree or greater. The remainder of the socio‐demographic characteristics of the participants appears in Table 1. Ten patients, eight in the intervention group and two in the control group did not complete the trial. Of the ten participants who did not complete the trial, four died before the second assessment, two were decision makers who requested to no longer be included, three were decision makers who were unable to be contacted and one was a patient who lost capacity to participate for the second assessment. The time to follow‐up differed between the control and intervention groups (5 vs. 20 days, P < 0.0001), because of the allotment of additional time for the intervention group to review the DA.
Figure 1.
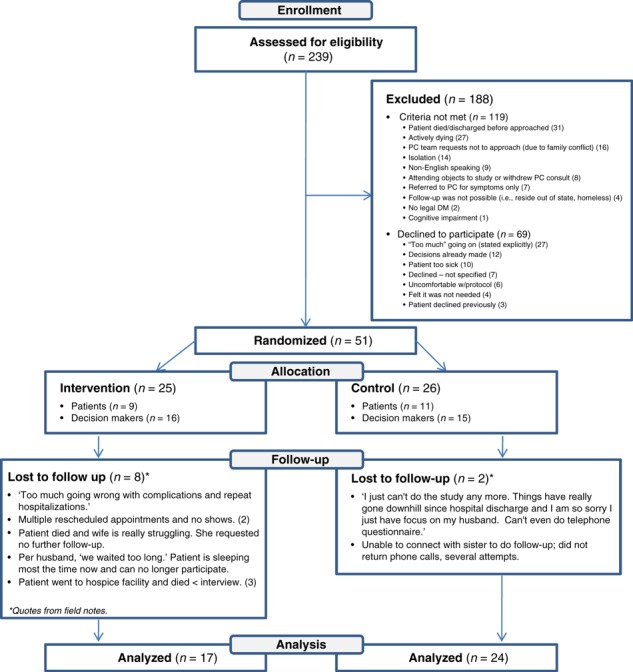
Consort diagram showing the assessment, randomization and follow‐up of study patients.
Table 1.
Study population characteristics
| Intervention N = 25 (%) | Control N = 26 (%) | |||
|---|---|---|---|---|
| Patients | Decision makers | Patients | Decision makers | |
| n (%) | 9 (36) | 16 (64) | 11 (42) | 15 (58) |
| Gender, n (%) | ||||
| Female | 7 (78) | 7 (44) | 6 (55) | 7 (47) |
| Age | ||||
| Mean | 54 | 57 | 56 | 56 |
| Median | 54 | 61 | 55 | 57 |
| Range | 28–66 | 39–72 | 31–78 | 41–68 |
| Race/Ethnicity | ||||
| White | 7 (78) | 11 (69) | 11 (100) | 14 (93) |
| Black or African–American | 1 (11) | 0 | 0 | 0 |
| Hispanic | 0 | 4 (25) | 0 | 1 (7) |
| Other | 1 (11) | 1 (6) | 0 | 0 |
| Income | ||||
| ≤$30 000 | 5 (31) | 2 (22) | 6 (55) | 5 (33) |
| $30 001–60 000 | 3 (19) | 3 (33) | 3 (27) | 4 (27) |
| $60 001–100 000 | 4 (25) | 3 (33) | 1 (9) | 4 (27) |
| Over $100 000 | 3 (19) | 0 | 1 (9) | 1 (7) |
| Declined to answer | 1 (4) | 1 (11) | 0 | 1 (7) |
| Education | ||||
| ≤High school graduate/GED | 3 (33) | 5 (31) | 4 (36) | 5 (33) |
| Some college | 5 (56) | 5 (31) | 3 (27) | 7 (47) |
| 4‐year college graduate | 1 (11) | 2 (13) | 3 (27) | 2 (13) |
| More than college graduate | 0 | 4 (25) | 1 (9) | 1 (7) |
| Patient‐specific information relative to all participants | ||||
| Intervention | Control | |||
| Service (patients) | ||||
| ACE – Acute Care of the Elderly Service | 3 (12%) | 1 (4%) | ||
| Burn ICU | 1 (4) | 3 (12%) | ||
| Family medicine | 3 (12%) | 0 | ||
| Gynaecology/Oncology | 1 (4%) | 3 (12%) | ||
| Hepatology | 1 (4%) | 0 | ||
| Medicine/Medical ICU | 6 (24%) | 7 (27%) | ||
| Neurology | 1 (4%) | 0 | ||
| Oncology | 6 (24%) | 6 (23%) | ||
| Pulmonolgy | 1 (4%) | 1 (4%) | ||
| Rehab | 1 (4%) | 2 (8%) | ||
| Surgery | 1 (4%) | 3 (12%) | ||
| Time to second assessment | ||||
| Mean number of days (SD) | 20.1 (9.1) | 4.8 (5.3) | ||
ICU, intensive care unit; GED, general educational development; SD, standard deviation.
Each participant was asked at the baseline assessment to identify the most difficult decision they were currently facing. From the field notes, these decisions were summarized and sorted into decision categories (Table 2). The most frequently reported decision was whether or not to pursue further treatment.
Table 2.
Most difficult decisions identified
| Most difficult decisions identified: (n = 62*) | |
|---|---|
| Treatment or not (chemo, surgery, etc.) | 27 (44%) |
| Placement (home or facility, etc.) | 11 (18%) |
| Resuscitation/Remove life support | 8 (13%) |
| Hospice or not | 6 (10%) |
| Comfort (‘nerve block’; ‘pain’) | 6 (10%) |
| Diagnostic testing | 3 (5%) |
| Completing a will | 1 (2%) |
*Twelve participants identified two decisions. One participant’s answer was not categorized: ‘I’m trying to make up my mind about what I’m going to do.’
Decision conflict and knowledge both demonstrated insignificant improvements between the pre‐ and post‐assessments. Likewise, there were no significant differences between the control and the intervention groups for either outcome (Table 3). Most patients felt the DA contained the right amount of information (76%), was balanced (94%) and 88% would definitely recommend it to other people facing the same decision (Table 4).
Table 3.
Knowledge and decision conflict scores
| Pre | Post | P‐value | |
|---|---|---|---|
| Knowledge scores (based on 6 true false questions) | |||
| Control (n = 24), % correct, mean (SD) | 56%, 3.4 (1.5) | 62%, 3.7 (3.5) | 0.40 |
| Intervention (n = 17), % correct, mean, SD | 72%, 4.3 (1.3) | 78%, 4.7 (1.3) | 0.33 |
| Control vs. Intervention | 0.35 | ||
| Decision conflict scores (100‐point scale, higher scores mean greater conflict) | |||
| Control (n = 24), mean, SD | 17.5, (20.3) | 15.8, (7.5) | 0.45 |
| Intervention (n = 17), mean, SD | 11.0, (11.8) | 5.0, (0) | 0.09 |
| Control vs. Intervention | 0.41 | ||
Table 4.
Acceptability ranking (intervention participants only)
| Questions | Responses | N (%) |
|---|---|---|
| 1. How would you rate the amount of information in the decision aid? | Much less than I needed | 0 |
| A little less than I needed | 1 (5.88) | |
| About the right amount of information | 13 (76.47) | |
| A little more information than I needed | 2 (11.76) | |
| A lot more information than I needed | 1 (5.88)* | |
| 2. How balanced was the information about palliative care versus the other options? | Clearly slanted towards palliative care | 0 |
| A little slanted towards palliative care | 0 | |
| Completely balanced | 16 (94.12) | |
| A little slanted away from palliative care | 1 (5.88) | |
| Clearly slanted away from palliative care | 0 | |
| 3. Did the decision aid present one option as the best overall choice? | No the decision aid was neutral and balanced | 15 (88.24) |
| Yes, the decision aid favoured palliative care | 1 (5.88) | |
| Yes, the decision aid favoured hospice | 0 | |
| Yes, the decision aid favoured life‐prolonging care | 0 | |
| No answer | 1 (5.88) | |
| 4. How clear was the information in the decision aid? | Everything was clear | 8 (47.06) |
| Most things were clear | 9 (52.94) | |
| Some things were clear | 0 | |
| Many things were unclear | 0 | |
| 5. How helpful is the decision aid in helping you make a decision about treatment options? | Very helpful | 10 (58.82) |
| Somewhat helpful | 6 (35.29)† | |
| A little helpful | 0 | |
| Not helpful | 1 (5.88)‡ | |
| 6. Would you recommend this decision aid to other people who are facing the same decision? | I would definitely recommend | 15 (88.24) |
| I would probably recommend it | 2 (11.76) | |
| I would probably not recommend it | 0 | |
| I would definitely not recommend it | 0 |
*One participant noted: ‘most of the information was not pertinent because I got it too late’.
†One participant noted: ‘my mind was already made up’.
‡One participant noted: ‘decisions were already made’.
Qualitative exit interviews
A total of 14 participants in the intervention arm completed exit interviews ranging between 18 and 65 min, with a mean 40 min. Three intervention participants only completed the follow‐up questionnaire and declined the in‐depth interview. Key themes identified included: (1) an increased sense of empowerment and control, including (1a) validation of recent medical decisions, (1b) anticipated impact on future discussions with physicians and (1c) heightened motivation for completion of one’s own advance directives; and (2) receiving the DA at an earlier time‐point would have been more useful to most of the participants. Illustrative quotes for these themes are included below.
(1a). Increased empowerment and control: validation of decisions
Some participants indicated that the DA affirmed their choices, resulting in peace of mind about their decisions. Several spoke of the importance of including the patient’s perspective in decisions if at all possible.
The one thing that the booklet and the DVD did for me is to validate the course of action we took and why we are here now… (Husband, age 70)
I would feel reinforced that I was going along the right path and doing the right thing for my husband. And for my family I’m sure… had I made the same decisions before I… read this book or watched the DVD I would have gone ok, good. I did the best (Wife, age 64)
I think just to follow along with the last statement I made, is we do everything possible from the patient’s point of view as opposed to the institution point of view. (Husband, age 71)
(1b). Increased empowerment and control: anticipated impact on future discussions with physicians
Many participants felt they would handle future discussions with physicians quite differently, as a result of viewing the DA. Asking questions, taking a proactive role and becoming more assertive in their loved ones’ health care were mentioned as future changes.
I didn’t ask any damn questions…’cause…I thought they were too busy for me to ask all these small… questions. …I thought doctors were so busy, why should I bother them with all these little…questions. Which that wasn’t the case. Now that I see, I’m going to ask them a lot more damn questions. Honestly…because of what I saw on that video. Because no question is too small. (Fiancée, age 41)
…knowing enough to say no. Are there any other options? And listening to the people being interviewed in the various different situations, which was good, because of coming at it from different perspectives… so knowing you have the right to say No. I’m done. I don’t want to do anything. Or I’d like to try something else…we have more control…or I feel more secure in the fact that we have that control…it feels good. I mean it gives us hope you know? I mean we are certainly not ready to give up. So it’s empowering. (Wife, age 59)
(1c). Increased empowerment and control: heightened motivation for completing one’s own advance directives
The DA served to alert some decision makers to the value of expressing their own wishes to loved ones sooner rather than later to reduce the last‐minute distress concomitant with end‐of‐life decisions, where the patient’s wishes are not known.
And I think that was a very valuable lesson for me… it is time that I need to consider something for myself in the future too and that I shouldn’t be waiting until it becomes such a dire need that there is a scramble between…my children to decide what to do about my future. And now would be a good time to discuss that and I think that was …a big part of what I got from the booklet and the DVD… I could no longer make decisions concerning my husband, but I certainly need to be making decisions about myself. (Wife, age 62)
(2). DA would have been more useful at an earlier point in the disease process:
Many of those interviewed viewed the timing of exposure to the DA as too late in the disease process; some indicated regret that it had not been introduced to them months prior, either at diagnosis or even earlier, potentially available to all through a web‐based format.
I would think that this would be more beneficial at the time of diagnosis, as opposed to when it’s almost too late to do anything…and in our case, it was too late. I wish this had been available the day he was diagnosed 8 months ago. (Wife, age 63)
Had I gotten the materials when he first got in the hospital, we probably could have talked about a lot of the things in there, when he was still able...because it was like he wasn’t there. I mean he was there ‐ he knew who everybody was – and you know, he knew that there was love in the room, but like I said, he was hallucinating and talking out of his head. (Fiancée, age 41).
Discussion
This pilot trial improves our understanding of how a DA could be used by patients and families dealing with advanced or terminal illness. While study participants found the DA to be acceptable and empowering, they noted that it came too late in their illness, after many of the major decisions had already been made. Also, many participants were too sick or dealing with too many other issues to participate in the trial as evidenced by the ineligibility, low enrolment and the high rate of incompletion for the follow‐up interviews (Fig. 1). While we found no difference in the primary outcomes of decision conflict and knowledge, this pilot feasibility trial only had sufficient power to detect very large differences. Also, the heterogeneity of decisions that people are making suggests that traditional decision‐making outcomes linked to a single decision, such as decision conflict, are not appropriate for the study of this type of DA.
To our knowledge, this is the first randomized trial of a DA on an inpatient PC population. Until recently, decision making for patients late in their lives has been an underrepresented area of research in the decision sciences. Indeed, DAs surrounding general advance directives or education programmes not geared to a specific decision were specifically excluded from the Cochrane review of DAs. 11 A pre–post analysis of a DA for surrogate decision makers facing the decision to place a feeding tube into a cognitively impaired patient found that the DA reduced decision conflict and improved knowledge surrounding that decision. 19 A DA regarding cardiopulmonary resuscitation was found to be highly acceptable among patients and families. 20 Another DA about mechanical ventilation for patients with chronic obstructive pulmonary disease was helpful in improving knowledge and decreasing conflict, although over a quarter of the participants found the experience to be stressful. 21 However, these trials were limited by the pre–post design, as none were formally evaluated against a control group. Other DAs surrounding late‐life issues are available on the University of Ottawa A–Z inventory of DAs include autopsy, dialysis, ICU care and stopping other treatments, but these have not yet been evaluated in formal trials. 22 Recently, video DAs have been shown to improve decision making around various aspects of advance directives, particularly for patients with lower literacy, but these were not explicitly in a PC population. 23 , 24 , 25 Thus, there is little guidance in the literature regarding how to incorporate a DA into an inpatient PC population.
The primary purpose of this study was to determine feasibility and acceptability. In regard to feasibility, the low eligibility, low enrolment and loss to follow‐up because of death raise concerns about the real‐world feasibility of incorporating a DA into an inpatient PC service. In regard to acceptability, participants who completed the follow‐up interview found the DA to be highly acceptable (Table 4); the exit interviews demonstrated that the DA empowered participants to take a more active role in their decision making, although they did note that this DA came too late in their disease process. Despite our concerns about feasibility, the empowerment theme suggests that this DA does have a place in the care of patients with serious illness. Indeed, this is in line with recent recommendations that good advance care planning is more focused on ‘preparing’ patients to make decisions rather than ensuring that they make decisions. 1
While this trial was not powered to detect small differences, the lack of effect on knowledge and decision conflict should be discussed. Certainly, smaller trials of DAs have shown statistically significant effects on both knowledge and decision conflict. 19 , 21 One potential explanation is that patients in the control arm received a PC consult. Palliative care seeks to assist patients in clarifying their knowledge and in making sure that the treatments are concordant with their values. Essentially, PC is expert decision coaching. 26 A trial design, where the control group is not receiving this expert decision coaching in the form of PC, would mimic the real‐world situation more clearly and be more likely to show the benefits of this DA.
Several limitations of this study should be noted. First, the small sample size in this pilot trial was not able to detect small differences between populations. Second, the exit interviews suggest that we may have been measuring the wrong outcomes. Outcomes measuring empowerment, such as confidence or self‐efficacy, may be more appropriate end points. Third, drop‐out, particularly in the intervention arm, limits our ability to draw conclusions regarding the effectiveness of the intervention. This is a challenge in all research of populations who are very ill. To address this, we used conservative statistical tests, which assume that the missing data were not at random. 27
Trials of DAs for an inpatient palliative care population need to be carefully designed. First, the significant drop‐out needs to be carefully considered both in the design and the analysis. Second, the wide array of decisions that people in this state are facing necessitates that these DAs should be broadly defined. Future trials should be performed earlier in the illness process, should not be performed against palliative care and should use end points measuring patient empowerment and utilisation.
Acknowledgements
None, all persons who contributed significantly to this work have been included in the author list.
Presentations. This was presented as a poster presentation at the 2010 Society for Medical Decision Making in Toronto, Canada.
Grant or Financial Support. This research was conducted while Dan Matlock was a Hartford Geriatric Health Outcome Research Scholar. The project was funded by the Foundation for Informed Medical Decision Making.
This trial is registered at clinicaltrials.gov: NCT01235611.
Reference
- 1. Sudore RL, Fried TR. Redefining theplanning in advance care planning: preparing for end‐of‐life decision making. Annals of Internal Medicine, 2010; 153: 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goldstein NE, Back AL, Morrison RS. Titrating guidance: a model to guide physicians in assisting patients and family members who are facing complex decisions. Archives of Internal Medicine, 2008; 168: 1733–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Apatira L, Boyd EA, Malvar G et al. Hope, truth, and preparing for death: perspectives of surrogate decision makers. Annals of Internal Medicine, 2008; 149: 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fried TR, O’Leary JR. Using the experiences of bereaved caregivers to inform patient‐ and caregiver‐centered advance care planning. Journal of General Internal Medicine, 2008; 23: 1602–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singer PA, Martin DK, Kelner M. Quality end‐of‐life care. The Journal of the American Medical Association, 1999; 281: 163–168. [DOI] [PubMed] [Google Scholar]
- 6. Back AL, Arnold RM. Discussing prognosis: “how much do you want to know?” talking to patients who are prepared for explicit information. Journal of Clinical Oncology, 2006; 24: 4209–4213. [DOI] [PubMed] [Google Scholar]
- 7. Mattimore TJ, Wenger NS, Desbiens NA et al. Surrogate and physician understanding of patients’ preferences for living permanently in a nursing home. Journal of the American Geriatrics Society, 1997; 45: 818–824. [DOI] [PubMed] [Google Scholar]
- 8. Pritchard RS, Fisher ES, Teno JM et al. Influence of patient preferences and local health system characteristics on the place of death. SUPPORT Investigators. Study to understand prognoses and preferences for risks and outcomes of treatment. [see comment]. Journal of the American Geriatrics Society, 1998; 46: 1242–1250. [DOI] [PubMed] [Google Scholar]
- 9. Matlock DD, Nowels CT, Bekelman DB. Patient perspectives on decision making in heart failure. Journal of Cardiac Failure, 2010; 16: 823–826. [DOI] [PubMed] [Google Scholar]
- 10. Say R, Murtagh M, Thomson R. Patients’ preference for involvement in medical decision making: a narrative review. [Review] [57 refs]. Patient Education and Counseling, 2006; 60: 102–114. [DOI] [PubMed] [Google Scholar]
- 11. O’Connor AM, Bennett CL, Stacey D, Barry M, Col NF, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews, 2009, Art. No.: CD001431. DOI: 10.1002/14651858.cd001431. pub2. [DOI] [PubMed] [Google Scholar]
- 12. Degner LF, Sloan JA, Venkatesh P. The control preferences scale. Canadian Journal of Nursing Research, 1997; 29: 21–43. [PubMed] [Google Scholar]
- 13. O’Connor AM. Validation of a decisional conflict scale. Medical Decision Making, 1995; 15: 25–30. [DOI] [PubMed] [Google Scholar]
- 14. Glasgow RE, McKay HG, Piette JD, Reynolds KD. The RE‐AIM framework for evaluating interventions: what can it tell us about approaches to chronic illness management? Patient Education and Counseling, 2001; 44: 119–127. [DOI] [PubMed] [Google Scholar]
- 15. Barry MJ, Fowler FJ Jr, Mulley AG Jr, Henderson JV Jr, Wennberg JE. Patient reactions to a program designed to facilitate patient participation in treatment decisions for benign prostatic hyperplasia. Medical Care, 1995; 33: 771–782. [DOI] [PubMed] [Google Scholar]
- 16. ATLAS‐ti [computer program]. Version 6.0. Berlin, Germany, 2010.
- 17. Thomas DR. A general inductive approach for analyzing qualitative evaluation data. American Journal of Evaluation, 2006; 27: 237–246. [Google Scholar]
- 18. Patton MQ. Qualitative Evaluation and Research Methods. Newbury Park, CA: Sage Publications, 1990. [Google Scholar]
- 19. Mitchell SL, Tetroe J, O’Connor AM. A decision aid for long‐term tube feeding in cognitively impaired older persons. Journal of the American Geriatrics Society, 2001; 49: 313–316. [DOI] [PubMed] [Google Scholar]
- 20. Christopher F, Deborah P, Jeannette S, Daren H. Development and use of a decision aid for communication with hospitalized patients about cardiopulmonary resuscitation preference. Patient Education and Counseling, 2010; 79: 130–133. Ref Type: Abstract. [DOI] [PubMed] [Google Scholar]
- 21. Wilson KG, Aaron SD, Vandemheen KL et al. Evaluation of a decision aid for making choices about intubation and mechanical ventilation in chronic obstructive pulmonary disease. Patient Education and Counseling, 2005; 57: 88–95. [DOI] [PubMed] [Google Scholar]
- 22. University of Ottawa A–Z Inventory of Decision Aids. http://decisionaid.ohri.ca/azinvent.php, accessed 1 September 2011.
- 23. El‐Jawahri A, Podgurski LM, Eichler AF et al. Use of video to facilitate end‐of‐life with discussions patients with cancer: a randomized controlled trial. Journal of Clinical Oncology, 2010; 28: 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Volandes AE, Paasche‐Orlow MK, Barry MJ et al. Video decision support tool for advance care planning in dementia: randomised controlled trial. British Medical Journal, 2009; 338: b2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Volandes AE, Ariza M, Abbo ED, Paasche‐Orlow M. Overcoming educational barriers for advance care planning in Latinos with video images. Journal of Palliative Medicine, 2008; 11: 700–706. [DOI] [PubMed] [Google Scholar]
- 26. O’Connor AM, Stacey D, Légaré F. Coaching to support patients in making decisions. BMJ, 2008; 336: 228–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fairclough DL. Patient reported outcomes as endpoints in medical research. [Review] [73 refs]. Statistical Methods in Medical Research, 2004; 13: 115–138. [DOI] [PubMed] [Google Scholar]


