Abstract
Objective Allergic rhinitis is increasing globally despite treatment focussed on pharmacotherapy. This study aimed to (i) examine the range and proportion of symptoms and triggers experienced by patients with intermittent allergic rhinitis (IAR); (ii) conduct a qualitative analysis of strategies devised to control symptoms and triggers; and (iii) measure medication adherence.
Methods A qualitative and observational study of data drawn from a randomized controlled trial on patients with IAR. Strategies collaboratively devised by participants and pharmacist staff to minimize symptoms and triggers were analysed thematically. In the 10‐day observational study, the participants recorded all symptoms and triggers of IAR along with use of medications and these were analysed descriptively.
Results Number of 124 participants recorded 620 symptoms and identified 357 triggers of IAR. To minimize these, 579 strategies were devised in consultation with pharmacy staff. The frequency and type of strategy varied according to whether the goals were aimed at controlling symptoms or triggers. Adherence to a course of antihistamines over the 10‐day trial was self‐reported by participants with 36% indicating full adherence.
Conclusion A large number and range of symptoms and triggers were identified, and individualized strategies were devised to minimize symptoms and triggers. Medication adherence was poor.
Practice implications Patients with IAR can be assisted to identify their symptoms and triggers and develop relevant strategies to manage these. This approach has the potential to facilitate patient self‐management of a chronic and incapacitating condition.
Keywords: goal setting, intermittent allergic rhinitis, self‐management, strategies
Introduction
Allergic conditions and allergic rhinitis (AR)
Allergic conditions are common, and their prevalence is increasing worldwide. 1 , 2 The concomitant relationship of allergic conditions with asthma, rhinitis, sinusitis and urticaria is widely accepted. 3 The severity of these and their subsequent impact on the individual’s quality of life, as well as health and economic systems, have been under investigation as a matter of urgency. 4
As an allergic condition of the upper airways, AR has been described as the most ‘modern’ of allergic conditions, escalating over the past 50 years. 4 After exposure to an allergen, allergic rhinitis develops in susceptible individuals as a result of an IgE‐mediated inflammation 5 generally presenting as nasal discomfort with sneezing, discharge and/or congestion. However, other physical symptoms involving itching of the eyes, ears and throat may be experienced along with headaches, fatigue and sleeping difficulties.
AR is not specific to age, gender, race, ethnicity or culture, although genetic pre‐disposition, environmental and lifestyle interactions may contribute to an individual’s risk of developing the condition. 4 The prevalence of AR in Australia closely parallels the statistics observed for Europe. Approximately 16% of Australians suffer from AR including 25% of younger adults aged 25–44 years. 6
To more fully understand the impact of this condition and deliver appropriate treatment, AR is categorized according to the duration and frequency of symptoms. 7 If a patient experiences symptoms for <4 days a week and for <4 weeks continuously in a year, then the episode is categorized as ‘intermittent’ AR (IAR). If a patient experiences symptoms occurring for more than 4 days a week and for more than 4 weeks continuously in a year, then the episode is categorized as ‘persistent’ AR. 7 , 8
Treatment and management
Conservative treatment plans, with the emphasis on pharmacotherapy for immediate relief of symptoms have not reduced the allergy ‘epidemic’. 2 As an alternative to this dependency on medication, a model based on preventative management plans has been implemented via a nationally coordinated approach in the Finnish Allergy Program. 2 Others suggest that optimal treatment regimes should comprise multifaceted programmes with patient education, allergen avoidance, pharmacotherapy, allergen‐specific immunology and possibly surgery. 3 , 4 , 5 , 6 , 7 , 8 , 9
Treatment plans based primarily on pharmacology may be further constrained by patient adherence rates. An Australian AR study reported that 46% of patients who categorized themselves as having ‘moderate’ symptoms, ‘sometimes’ took their medication. 10 Similarly, poor adherence to asthma medications has been explained in terms of patient concerns about possible side effects of medications taken long term. 11 Within the general population in developed countries, non‐adherence to long‐term therapies for patients with chronic diseases has been described as a ‘world wide problem of striking magnitude’. 12 The issue of non‐adherence continues in spite of the safety profile of medications such as antihistamines being studied and positive outcomes reported. 13 This situation has given rise to the claim that ‘minimal medical intervention is universally desirable’. 3 Patients’ poor adherence supports the need for a multifaceted approach for AR treatment plans.
One such approach is the process of goal setting in assisting patients to self‐manage chronic disease. Studies incorporating goal setting into a disease state management service have been reported in case of asthma, 11 , 14 , 15 diabetes 16 and hypertension in older men 17 and with conditions such as pain 18 and weight loss. 19 , 20 An innovative approach using goal setting to help people self‐manage their IAR was piloted in community pharmacies in Sydney, Australia, in 2005. 21 The findings from this study demonstrated the value of a goal‐setting intervention in achieving improvements in IAR‐related clinical and humanistic outcomes 20 and supports the notion that a multifaceted individualized collaboration between patients and health‐care professionals (HCP) has the potential to ease the allergy burden to the patient. This approach was refined and implemented on a larger scale – the Pharmacy Allergic Rhinitis Intervention Service (PARIS). The outcomes of this study demonstrated the value of training non‐clinicians in the goal‐setting intervention for people with IAR. 21
The goal‐setting process involves identifying the particular symptoms and triggers that affect the individual patient and devising strategies that will enable them to meet the goal of minimizing or eliminating the said symptom(s) and trigger(s). What is not known at this stage, however, is neither an understanding of the types and range of strategies that are devised as part of a self‐management intervention conducted between HCP and patients with IAR nor their value in minimizing or controlling the symptoms and triggers of this chronic condition. This paper reports the findings of an exploration of these factors by analysing data gathered for the intervention arm of the PARIS project. That is, data were retrieved from the records of the intervention group participants and as such provides a secondary analysis of a section of the randomized control trial. Data regarding symptoms, triggers and their related strategies were not part of the control group study design.
The aims addressed in this paper are to (i) examine the range and proportion of symptoms and triggers experienced by a sample of patients suffering from IAR; (ii) conduct a qualitative analysis of strategies that were undertaken by these patients to control their symptoms and triggers; and (iii) measure medication adherence.
Methods
Ethical clearance for the conduct of this study (05‐2008/10737) was obtained through the University of Sydney’s Human Research Ethics Committee.
In presenting the methods for the research specific to this paper, its relation to a larger project needs to be explained. The method section is therefore presented in two sections. The first (Original project) describes the method used for the larger, original project (Pharmacy Allergic Rhinitis Intervention Service – PARIS), whilst the second (section ‘Participants’ to ‘Data analysis’) explains the methods (qualitative and observational) relevant to this paper.
Original project
The PARIS 22 study was a randomized controlled trial conducted through community pharmacies in metropolitan and outer metropolitan areas of Sydney, Australia, across two peak allergic rhinitis seasons: spring 2008 and autumn 2009. The study comprised of two visits, 10 days apart. The intervention involved a pharmacist or pharmacy assistant and their patient undertaking a goal setting and strategy formulation process, which was recorded on a card for the patient to keep and refer to.
At Visit 1, intervention group participants received a wallet‐sized ‘My Goals and Treatment Card’ onto which the study participants recorded the symptoms being experienced as well as the identifiable and possible triggers the patient felt were responsible for their current allergic episode. Strategies tailored to these specific symptoms and triggers were recorded on this card. These strategies were devised collaboratively between the participant and the pharmacist/pharmacy assistant and aimed at achieving the goals of ‘Minimizing/Eliminating Hayfever Symptoms’ and ‘Minimizing/Avoiding Hayfever Triggers’. Participants were asked to keep a daily record of perceived symptom severity and the days on which they took their medication over the 10‐day period. These were recorded on the back cover of their goals and treatment card. At Visit 2, participants returned to the pharmacy with their ‘My Goals and Treatment Card’ and their progress towards achieving the stated goals (‘Goals Attained’, ‘Working Towards the Goals’ or ‘Goals Not Attained’) was recorded.
The control group participants received ‘standard care’ for treating IAR. The findings of the impact of the intervention on clinical and humanistic outcomes have been reported previously. 22
Participants
Pharmacies
Recruitment: Pharmacies were randomly selected from a list of registered pharmacies obtained from the Pharmacy Board of New South Wales. Two pharmacies were recruited from each of four geographical regions of the Sydney basin (North East, North West, South East and South West). As IAR is commonly treated with non‐prescription products, both pharmacists and pharmacy assistants were recruited to deliver the service.
Patients
Recruitment: Individuals were invited to join the study if they were presenting at their pharmacy with symptoms of IAR (that is experiencing symptoms for <4 days in a week or for <4 weeks consecutively) 7 and requesting an antihistamine. Additional inclusion criteria were as follows: aged above 18, able to attend a second visit at their pharmacy 10 days hence and fluency in spoken and written English. Participants were excluded if they had previously been involved in a self‐management programme at the University of Sydney, were pregnant, terminally ill or experiencing non‐AR symptoms such as sinus pain or loss of smell. This paper is reporting the data collected from participants randomized into the intervention group.
Data collection
The data records for the intervention group participants recruited into the original PARIS study were retrieved for the current study. Background data for the participants included socio‐demographic variables and IAR‐related medical history. These had been recorded by participants at Visit 1 on data collection forms.
Observational study
Data extracted which related to the observational study included the following: (i) the number and type of IAR symptoms and triggers being experienced by the participant at Visit 1, (recorded on the inside of the ‘My Goals and Treatment Card’) and (ii) a self‐reported, ‘Yes’ or ‘No’ as to whether medication had been taken for each of 10 days of the trial (recorded on the back of the ‘My Goals and Treatment Card’.
Qualitative study
Data retrieved which related to the qualitative study included the following: (i) the number and types of strategies devised by the participant and pharmacy staff to address the goal of minimizing the effects of symptoms and triggers (recorded on the inside of the ‘My Goals and Treatment Card’ and (ii) data pertaining to self‐reported goal attainment levels (recorded on a data collection form at Visit 2).
Data analysis
The background data pertaining to participants’ demographics were entered into an SPSS database and were analysed using descriptive statistics.
Observational study
The symptoms and triggers data recorded by each participant on their ‘My Goals and Treatment Card’ were entered into the SPSS database. Variable names were created for each new symptom and trigger listed by the participant on the card. The same responses made by subsequent participants were tallied under these variables, and as new symptoms or triggers data appeared on the ‘goals cards’, these were entered as new variables. This process continued until the data for symptoms and triggers from all cards had been entered. Descriptive statistics were computed for these data.
Qualitative study
The strategies recorded on the ‘My Goals and Treatment Cards’ and whether they related to a symptom or trigger were analysed for emerging themes. Initially, one researcher completed the interpretation and allocation of themes followed by discussion and cross‐checking by two others.
Results
Recruitment
Sixteen pharmacists and eight pharmacy assistants from nine community pharmacies participated in two data collection phases (spring 2008 and autumn 2009). At the close of two data collection periods, a total of 124 participants had been enrolled in the intervention arm of the original PARIS project. Four participants did not return for Visit 2; however, full data at Visit 1 were collected for two of these participants. Data pertaining to the strategies devised were incomplete for other two participants.
Demographics of the participant population
Table 1 details the socio‐demographic characteristics of the participants. There were significantly more women than men (P < 0.05), and most participants were engaged in paid employment, home duties or were students (94%). Unemployed and retirees comprised of 6% of the participant cohort. Significantly more participants reported the onset of their IAR from 12 years of age or older (P < 0.05), and half of the participants identified that they have a comorbidity, the majority reporting asthma or bronchitis (30%).
Table 1.
Participant socio‐demographics and medical history
| n (%) | |
|---|---|
| Demographic | |
| Age – years | N = 124 |
| Mean (range) | 38 (18–79) |
| Gender | N = 124 |
| Male | 45 (36) |
| Female | 79 (64) |
| Work Status | N = 118 |
| Employed | 80 (68) |
| Unemployed | 2 (2) |
| Student | 19 (16) |
| Home duties | 12 (10) |
| Retired | 5 (4) |
| Medical history | |
| Related Illnesses | N = 120 |
| Noneaact | 60 (50) |
| Asthma/Bronchitis | 36 (30) |
| Eczema | 14 (12) |
| Sinusitis | 7 (5) |
| Other | 3 (3) |
| Onset of AR | N = 119 |
| Infancy 0–2 | 8 (7) |
| Childhood 2–12 | 31 (26) |
| More than 12 | 80 (67) |
IAR symptoms and triggers
Data collected during both spring and autumn included the symptoms and triggers identified and listed by participants on their ‘My Goals and Treatment Card’. These were tabulated, and the frequencies were recorded (Table 2). Participants at Visit 1 experienced a mean number of five symptoms ranging from 1 to 11, whereas the mean number of triggers was three ranging from 1 to 7. Overall, a total of 620 symptoms and 357 triggers were recorded for 124 participants.
Table 2.
Symptoms of IAR – total participants (N = 124)
| Symptom | Participants n (%) |
|---|---|
| Nose | |
| Sneezing | 100 (80) |
| Runny | 102 (80) |
| Blocked | 52 (42) |
| Itchy | 35 (28) |
| Post‐nasal drip | 13 (10) |
| Eyes | |
| Itchy | 80 (64) |
| Watery | 54 (43) |
| Red | 14 (11) |
| Sore | 11 (8) |
| Swollen dry | 7 (6) |
| Physical | |
| Headache | 29 (23) |
| Fatigue | 19 (15) |
| Congestion | 9 (7) |
| Cough | 6 (5) |
| Thirst | 3 (2) |
| Throat | |
| Itchy | 32 (26) |
| Sore | 13 (10) |
| Other | |
| Skin, Emotional, ears | 41 (33) |
Examples of the most troublesome symptoms were as follows: sneezing and runny nose, which affected 80% of all participants. Blocked nasal passages affected 42% of the participant cohort. Eye symptoms were also prevalent with 64% of participants suffering from itchy eyes with 43% troubled by watery eyes (Table 2).
The most prevalent triggers listed by 59% of participants were pollen/flowers and dust/dust mites. Specific changes in the daily weather conditions affected 29% of study participants, whilst 24% recorded a seasonal change or grass as a trigger. Wind (16%), cats (14%) and perfumes (11%) were identified as one of the triggers for participants’ current IAR episodes (Table 3).
Table 3.
Triggers of IAR – total participants (N = 124)
| Trigger | Participants n (%) |
|---|---|
| Plants | |
| Pollen/flowers | 73 (59) |
| Grass | 30 (24) |
| Animals | |
| Cats | 17 (14) |
| Other animals | 11 (8) |
| Dogs | 6 (5) |
| Dust/Mites | 72 (59) |
| Mould | 10 (8) |
| Weather | |
| Seasons | 30 (24) |
| Weather changes | 36 (29) |
| Wind | 20 (16) |
| Chemicals | |
| Perfume | 14 (11) |
| Smoke/pollution | 10 (8) |
| Cleaning products | 7 (6) |
| Other | |
| Food | 5 (4) |
| Immune system | 6 (5) |
| Fabrics | 5 (4) |
| Environmental | 5 (4) |
Overall, within the total number of symptoms (N = 620) and triggers (N = 357), nose and eye symptoms were the most prevalent symptoms, whilst plants, weather and dust/dust mites were the most common triggers.
Thematic analysis of strategies
A total of 579 strategies were devised collaboratively between the study participants and pharmacy staff to minimize symptoms and triggers. Five themes emerged from the analysis of strategies set and recorded. These were as follows: ‘Adherence (non‐specific)’, ‘Adherence (specific)’, ‘Avoidance’, ‘Practical Actions’ and ‘Other’. An explanation and examples of the strategies for each category are listed below.
‘Adherence (non‐specific)’
Strategies in this theme reflected a non‐specific, general instruction to take or use medication.
Examples:
Take medication/antihistamine/preventative medication/right medication;
Eye drops for allergy;
Ensure I maintain pharmacist’s instructions;
Saline spray to clear nasal passages.
‘Adherence (specific)’
Strategies in this theme reflected specific and targeted instructions, including dose amounts and daily instructions. These strategies provided a greater level of guidance regarding achieving adherence.
Examples:
Nasal spray/1 puff in each nostril twice a day/one spray morning and night;
Take one tablet a day until a few days after feeling better/before going out;
Antihistamine 60 mg twice a day/10 mg daily with breakfast;
Start medication 1 week before seasonal occurrence.
‘Avoidance’
Strategies in this theme were of a general nature and focused on simply avoiding triggers.
Examples:
Avoid – going outside/animals/dust/smoking areas/heavy fragrances and powders/houses with cats/bunches of flowers/spending long periods outside when windy;
Stop wearing perfume;
Be aware of triggers.
‘Practical actions’
Strategies grouped under this theme reflected specific practical actions that could be undertaken by participants, including instructions.
Examples:
Close the windows when the neighbour is mowing;
Shower and wash hair every night to remove pollens;
Wash hands after patting the cat;
Clean cat fur off furniture/floor;
Take an antihistamine before cleaning and clean regularly;
Vacuum and change bed sheets weekly;
Smear Vaseline around the nostrils to trap pollens;
Check pollen count forecast and stay indoors if possible.
‘Other’
Strategies in this theme were non‐specific and related to general health or behaviours.
Examples:
Drink water;
Take lemon juice;
Tell friends of triggers;
Rest well;
Manage asthma.
Tabulation of the coded themes revealed that more strategies were recorded for the goal of minimizing/avoiding triggers N = 315 (55%) compared with the goal of minimizing/eliminating symptoms N = 264 (45%). With respect to symptoms, strategies around the theme of adherence were most commonly recorded. These were three times more frequently recorded than practical action strategies. In contrast, when the goal was to eliminate or avoid triggers, strategies around the theme of practical actions were six times more frequently devised (Fig. 1).
Figure 1.
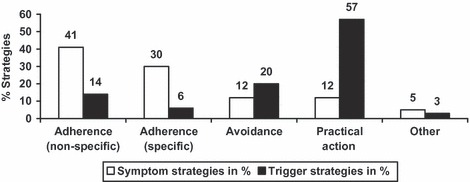
Category and frequency of strategies devised.
Goal attainment
At the conclusion of the 10‐day programme, participants self‐assessed their progress towards attaining their goals. A total of 92% of participants indicated that they had either reached their goals (57%) or were actively working towards achieving them (35%). Only 8% indicated that they had not achieved their goals.
Adherence to medication
Self‐reported data on adherence showed that only 36% of participants took their antihistamine medication every day over the 10‐day period (Fig. 2). The median number of days on which patients took their medication was 8.
Figure 2.
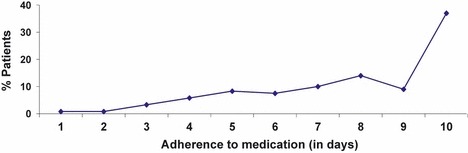
% patients adherent to medication over 10 days.
Discussion and conclusion
Discussion
The first two aims of this study were to explore the type and frequency of symptoms and triggers experienced by patients with IAR and to gain a deeper understanding of patient‐focused strategies for self‐management of IAR. The results indicated that a wide range and number of triggers and symptoms may be associated with an episode of IAR and that there appears to be wide scope for developing personalized strategies to manage symptoms and triggers of IAR. Thematic analysis of strategies showed that the type of strategy will vary according to whether it is a symptom or a trigger that is being addressed. For example, the most commonly devised strategies for symptoms related to adherence to medications, whereas triggers were most commonly dealt with through taking practical steps to manage them. This is in line with other published studies that have multifaceted recommendations for treatment. 2 , 21 The significant level of goal attainment (i.e. participants who self‐reported they were able to successfully minimize the effects of their symptoms and triggers over 10 days) suggests that these strategies were relevant and of practical utility. These outcomes suggests that individualized and practical self‐management support can be provided for patients with IAR.
The third aim of this study was to investigate the medication adherence of the participant cohort over 10 days of the study. Patient adherence to medication is a topic of debate with studies confirming patients often make the choice to alter their medication dose, discontinue it altogether or change it as they wish, for a variety of reasons. 12 Adherence to IAR medicines in this study was found to be similarly poor, further underscoring the importance of finding methods attractive to patients that can assist them in self‐managing their IAR in both shorter and longer term. Having strategies and goals collaboratively devised for the individual’s specific symptoms and triggers is a constructive approach for those patients who do not wish to rely solely upon medication during an IAR episode.
The outcomes of this study address the findings from research, which showed that up to two‐third of AR sufferers will not consult their GP or (may) not have had their condition diagnosed by their GP. 23 As a result, patients may be self‐diagnosing and self‐medicating inappropriately. The role of patient‐centred health care becomes particularly important in this context as support can be provided through individualized treatment plans, which encourage the patient to review their symptoms and triggers and set relevant goals and strategies to effectively self‐manage their condition. This approach, when combined with pharmacological control of symptoms, provides patients who do not seek medical attention with professional advice regarding their condition and an expansion of their treatment options.
Limitations
The limitations of this study include the following: (i) the original research project was conducted in the Sydney metropolitan area, and therefore, any generalization of results should be undertaken with caution. It is possible that patients who were recruited into the original study may have preferred particular strategies that may not be indicative of a wider population; (ii) the study was conducted with a sample of patients with IAR; thus, results cannot be generalized to persistent AR.
Conclusion
An understanding has been gained of the type and range of strategies that may be devised to attain the goals for patient self‐management of IAR. These strategies were specific for the individual, realistic, recorded as a reminder and provide further evidence of a successful therapeutic alliance between HCPs and their patients. Future research could build on these findings by examining the extent to which particular strategies are the most effective in controlling symptoms and triggers.
Practice implications
Training pharmacy staff to enable them to discuss not only the patient’s particular IAR symptoms and triggers but also to negotiate appropriate strategies to manage these is recommended.
Financial support
This project was funded by the Australian Government Department of Health and Ageing as part of the Fourth Community Pharmacy Agreement Research & Development Program managed by the Pharmacy Guild of Australia.
Conflict of interest
There are no conflicts of interest for any of the authors. Dr Seeto is an employee of Merck, Sharp and Dohme (MSD) Pty Ltd that is one of the pharmaceutical companies that market treatments for allergic rhinitis. Materials from this research may potentially benefit all pharmaceutical companies that market such products. It should be noted that Dr Seeto’s involvement in this research is NOT in her capacity as an employee of MSD but as an expert on Immunology and also in her previous involvement in the pilot allergic rhinitis project.
Acknowledgements
The authors would like to acknowledge the support and participation of pharmacists, pharmacy assistants and patients in this project.
References
- 1. Bager P , Arnved J , Ronbaorg S . Trichuris suis ova therapy for allergic rhinitis: a randomized, double‐blind, placebo‐controlled clinical trial . Journal of Allergy and Clinical Immunology , 2010. ; 124 : 123 – 130 . [DOI] [PubMed] [Google Scholar]
- 2. Haahtela T , von Hertzen L , Makela M , Hannuksela M , the Allergy Programme Working Group . Finnish Allergy Programme 2008–2018 – time to act and change the course . Allergy , 2008. ; 63 : 634 – 645 . [DOI] [PubMed] [Google Scholar]
- 3. Hayden M , Womack C . Caring for patients with allergic rhinitis . Journal of the American Academy of Nurse Practitioners , 2007. ; 19 : 290 – 298 . [DOI] [PubMed] [Google Scholar]
- 4. Bousquet J , Cruz A , Denburg J et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA2LEN and AllerGen) . Allergy , 2008. ; 63 ( Suppl 86 ): 8 – 160 . [DOI] [PubMed] [Google Scholar]
- 5. Simoens S , Laekeman G . Pharmacotherapy of allergic rhinitis: a pharmaco‐economic approach . Allergy , 2008. ; 64 : 85 – 95 . [DOI] [PubMed] [Google Scholar]
- 6. Australian Institute of Health and Welfare . Australia’s Health 2006 . Canberra : Australian Government; , 2006. . [Google Scholar]
- 7. Bousquet J , Van Cauwenberge P , Khaltaev N , the WHO panel . Allergic rhinitis and its impact on asthma (ARIA), in collaboration with the World Health Organisation . Journal of Allergy Clinical Immunology , 2001. ; 5 ( Suppl ): S147 – S334 . [DOI] [PubMed] [Google Scholar]
- 8. Bousquet J , van Cauwenberge P , Aït Khaled N et al. Pharmacologic and anti‐IgE treatment of allergic rhinitis ARIA update (in collaboration with GA2LEN) . Allergy , 2006. ; 61 : 1086 – 1096 . [DOI] [PubMed] [Google Scholar]
- 9. Bousquet J , van Cauwenberge P , Khaltaev N , Members of the Workshops . ARIA in the pharmacy: management of allergic rhinitis symptoms in the pharmacy . Allergy , 2004. ; 59 : 373 – 387 . [DOI] [PubMed] [Google Scholar]
- 10. Sharp T , Seeto C . The psychosocial impact of self‐reported morning allergy sumptoms: findings from an Australian internet‐based survey . Journal of Allergy 2010. , Art. No.: 710926 [doi : 10.1155/2010/710926 ] . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loignon C , Bedos C , Sévigny R , Leduc N . Understanding the self‐care strategies of patients with asthma . Patient Education and Counseling , 2009. ; 75 : 256 – 262 . [DOI] [PubMed] [Google Scholar]
- 12. Sabate E . Adherence to long term therapies: evidence for action . Geneva : World Health Organisation; , 2003. . Available at : http://www.who.int/chp/knowledge/publications/adherencerep.pdf . [Google Scholar]
- 13. Bousquet J , Bachert C , Canonica G et al. Efficacy of desloratadine in intermittent allergic rhinitis: a GA2LEN study . Allergy , 2009. ; 64 : 1516 – 1523 . [DOI] [PubMed] [Google Scholar]
- 14. Smith L , Bosnic‐Anticevich S , Mitchell B , Saini B , Krass I , Armour C . Treating asthma with a self‐management model of illness behaviour in an Australian community pharmacy setting . Social Science and Medicine , 2007. ; 64 : 1501 – 1511 . [DOI] [PubMed] [Google Scholar]
- 15. Kahawati C , Smith L , Armour C . Goal setting by people with asthma – what do they want? Australian Pharmacist , 2008. ; 27 : 674 – 678 . [Google Scholar]
- 16. DeWalt D , Davis T , Wallace A et al. Goal setting in diabetes self‐management: taking the baby steps to success . Patient Education and Counseling , 2009. ; 77 : 218 – 223 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown V , Bartholomew L , Naik A . Management of chronic hypertension in older men: an exploration of patient goal‐setting . Patient Education and Counseling , 2007. ; 3 : 93 – 99 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Filoramo MA . Improving goal setting and goal attainment in patients with chronic noncancer pain . Pain Management Nursing 2007. ; 8 : 96 – 101 [DOI : 10.1016/j.pmn.2007.03.005 ] . [DOI] [PubMed] [Google Scholar]
- 19. Weber Cullen K , Baranowski T , Smith S . Using goal setting as a strategy for dietary behavior change . Journal of the American Dietetic Association , 2001. ; 101 : 562 – 566 . [DOI: 10.1016/S0002‐8223(01)00140‐7] . [DOI] [PubMed] [Google Scholar]
- 20. Bagozzi R , Edwards E . Goal setting and goal pursuit in the regulation of body weight . In : Norman P , Abraham C , Conner M. ( eds ) Understanding and Changing Health Behaviour from Health Beliefs to Self‐regulation . Newark, NJ : Hardwood Academic Publishers; , 2000. : 262 – 300 . [Google Scholar]
- 21. O’Connor J , Seeto C , Saini B et al. Healthcare professional versus patient goal setting in intermittent allergic rhinitis . Patient Education and Counseling , 2008. ; 70 : 111 – 117 . [DOI] [PubMed] [Google Scholar]
- 22. Smith L , Nguyen T , Seeto C , Saini B , Brown L . The role of non‐clinicians in a goal setting model for the management of allergic rhinitis in community pharmacy settings . Patient Education and Counseling 2010. ; 81 : e26 – e32 . [DOI] [PubMed] [Google Scholar]
- 23. Walls RS , Heddle RJ , Tang MLK , Basger BJ , Solley GO , Yeo GT . Optimising the management of allergic rhinitis: an Australian perspective . In : Kemp SA , Mullins RJ , Weiner JM. ( eds ) MJA Practice Essentials – Allergies . Pyrmont, NSW : Australasian Medical Publishing Company Pty Ltd; , 2007. : 48 – 53 . [Google Scholar]


