Abstract
Objectives The aim of this study was to identify common risk factors for patient‐reported medical errors across countries. In country‐level analyses, differences in risks associated with error between health care systems were investigated. The joint effects of risks on error‐reporting probability were modelled for hypothetical patients with different health care utilization patterns.
Design Data from the Commonwealth Fund’s 2010 lnternational Survey of the General Public’s Views of their Health Care System’s Performance in 11 Countries.
Setting Representative population samples of 11 countries were surveyed (total sample = 19 738 adults). Utilization of health care, coordination of care problems and reported errors were assessed. Regression analyses were conducted to identify risk factors for patients’ reports of medical, medication and laboratory errors across countries and in country‐specific models.
Results Error was reported by 11.2% of patients but with marked differences between countries (range: 5.4–17.0%). Poor coordination of care was reported by 27.3%. The risk of patient‐reported error was determined mainly by health care utilization: Emergency care (OR = 1.7, P < 0.001), hospitalization (OR = 1.6, P < 0.001) and the number of providers involved (OR three doctors = 2.0, P < 0.001) are important predictors. Poor care coordination is the single most important risk factor for reporting error (OR = 3.9, P < 0.001). Country‐specific models yielded common and country‐specific predictors for self‐reported error. For high utilizers of care, the probability that errors are reported rises up to P = 0.68.
Conclusions Safety remains a global challenge affecting many patients throughout the world. Large variability exists in the frequency of patient‐reported error across countries. To learn from others’ errors is not only essential within countries but may also prove a promising strategy internationally.
Keywords: medical errors, patient‐reported outcomes, safety, survey
Introduction
Patient safety remains a major challenge for health care systems worldwide. 1 A recent chart review study conducted in the Netherlands reports the incidence of one or more adverse events as 5.7% of all hospital admissions of which 40% were deemed preventable. 2 In Sweden, the incidence of adverse events was 12.3% of hospital admissions with 70% being judged as preventable. 3 Similar data have been reported for several countries recently, including the United States, New Zealand, Canada and others. 4 , 5 , 6 , 7 On the basis of these studies, it can be concluded that approximately one of thousand hospital patients dies as a result from preventable adverse events. Many patients suffer from adverse events after discharge and are therefore not identified in record‐based studies. 8
Less research has been conducted in the outpatient care setting but the available studies suggest that patients are at considerable risk as well. In particular, preventable adverse drug events are frequent among patients in outpatient care. 9 , 10 Gurwitz et al. 11 report an overall rate of adverse drug events among older patients in the ambulatory setting of 50.1/1000 person‐years, of which 28% were considered preventable. Studies based on staff members’ incident reports in the United Kingdom yielded an error report rate of 75/1000 patient contacts in outpatient care. 12 In a similar study in the United States, errors and preventable adverse events were reported after 24% of outpatient visits. 13 In Australia, the incidence of error reported to an anonymous reporting system by general practitioners was 0.24% per patient seen per year. 14
These setting‐specific studies are valuable and important to identify and understand specific threats, e.g. hospital care or outpatient drug therapy. However, the frequency and harm of error is then investigated in isolation for specific health care sectors, settings or even therapies or treatments (e.g. medical errors in in‐patient cancer treatment). But many patients utilize several types of health care in different settings, and the associated risks accumulate or even exponentiate because of coordination and communication failures among different providers. To assess patients’ total risk, longitudinal observation of patient cohorts would be possible in theory but is methodologically challenging and has not yet been undertaken to the author’s knowledge. Another methodological approach to the accumulated likelihood of error is the survey of citizens or patients. As patients are the only individuals physically present during every treatment and consultation, they carry with them important contextualized information in particular with relation to transition between different settings. 15 , 16 Surveying patients about their experience of medical error across specific types of health care consumed, e.g. hospital care, can help to identify risk factors for error along the care continuum and relative to specific patient‐level factors and the amount and type of health care utilized.
In addition, such patient surveys of error experience conducted in a multinational design can inform health policy about common risk factors across countries and those specific to different health care systems. For example, some countries may perform better in ensuring safe transition and coordination of inpatient and outpatient care than others. The main aim of this analysis was the identification of risk factors for patient‐reported medical errors across several countries. Country‐level analyses were conducted to investigate differences in risks associated with error between different health care systems. To evaluate the joint effects of the identified risk factors, the probability that hypothetical patients with different personal and health‐related profiles and health care utilization patterns would report error in their care was modelled.
Methods
Design
This analysis is based on data from ‘The Commonwealth Fund’s 2010 lnternational Survey of the General Public’s Views of their Health Care System’s Performance in 11 Countries’, which was conducted in Australia, Canada, France, Germany, the Netherlands, New Zealand, Norway, Sweden, Switzerland, the United Kingdom and the United States in 2010 [details are available at http://www.commonwealthfund.org/Content/Surveys/2010/Nov/2010‐International‐Survey.aspx]. Computer‐assisted telephone interviews were conducted with nationally representative samples of adults aged 18 and above in each of these countries. Samples were drawn from residential phone number lists, random number lists or random digit dialing. National samples differ in the extent to which cell lines were included. The interviewee in each household was selected at random based on the most recent birthday in most countries. All sample records were called eight times or more before being abandoned as unusable. The interviews were conducted by professional interviewing staff and took on average 18–21 min across countries. Response rates varied from 13% in Norway to 54% in Switzerland.
Survey
The Commonwealth Fund’s 2010 lnternational Survey assessed public confidence in the health care system including access to care, cost and quality of care. Methods and results of earlier versions of the survey have been published previously. 17 , 18 , 19
For the purpose of this analysis, the following items relating to medical error experience are of particular relevance: whether respondents were ever been given the wrong medication or wrong dose by a doctor, nurse, hospital or pharmacist in the past 2 years (referred to as ‘medication error’ hereinafter); whether there was a time in the past 2 years the responder thought a medical mistake was made in her treatment or care (referred to as ‘medical error’ hereinafter); whether the responder has been given incorrect results for a diagnostic or laboratory test in the past 2 years (referred to as ‘lab error’ hereinafter). The response categories were yes, no, not sure and decline to answer. Participants that reported any of the above errors were also asked whether the error occurred while they were hospitalized (yes, in the hospital, no, not sure, decline to answer). Participants were also asked several questions related to demographics, their health and utilization of health care services. Responses to three items that asked for experience of poor coordination of care in the past 2 years were also included in the analysis: whether subjects reported (i) test results or medical records were unavailable at the time of a scheduled appointment; (ii) receiving conflicting information from different providers; (iii) doctors ordered medical tests that had already been performed.
Data analysis
Raw survey data were weighted for age, sex, education and region according to the most recent national census to reflect demographic distributions. To dichotomize data for analysis, ‘not sure’ and ‘decline to answer’ responses were recoded to missing.
An aggregate measure was computed that captures experience of any of the specific error items. We report descriptive analysis for all individual error items and the aggregate measure per country. To identify potential predictors, several demographic, health‐related and heath care utilization variables were tested for their individual association with error experience in bivariate analyses: age, gender, education, income (relative to national averages), general health status, presence of chronic conditions (out of a specified list of conditions), having a regular doctor, number of doctors seen in the past 12 months, specialist care in the past 2 years, elective surgery in the past 2 years, hospital stay in the past 2 years, emergency care use in the past 2 years, medical tests (laboratory, X‐ray, etc.) in the past 2 years and current regular use of prescription drugs. Responses to three coordination of care items were used to compute an indicator variable indicating experience of none vs. any of these three events. All individual variables that were significantly associated with error experience in bivariate analyses at the 0.1 level were entered into the logistic regression model. Logistic regression was conducted for the aggregate measure, i.e. report of ‘any error’, and for each of the individual error items as dependent variables. Multicollinearity of the predictor variables was assessed using the variance inflation factor (VIF). VIFs > 10 were inspected, and multicollinear variables were omitted from the models. Model fit was assessed using the Archer–Lemeshow goodness‐of‐fit statistic, a F‐adjusted mean residual goodness‐of‐fit test under complex sampling. 20 To evaluate the joint effects of the identified risk factors across all countries, we predicted the probability that hypothetical subjects (patients A–F) with different personal and health‐related profiles and health care utilization patterns would report any error in their care. We also conducted country‐specific analyses for three countries (United States, United Kingdom and Germany) that represent prototypes of health care system organization, i.e. market‐driven, public and social insurance‐based health care systems. Country‐specific analyses were conducted using logistic hierarchical backward selection with the aggregate measure as outcome variable. This approach was selected because of the limited size of the country‐specific samples. Hierarchical stepwise regression differs to common stepwise regression in that potential predictors are grouped and ordered based on theory. The sequence in which groups are tested is not arbitrary. Guided by theoretical considerations, predictors were tested in the following blocks and sequences for each of the three country‐specific models: (gender) (age) (income, education) (poor health, number of chronic conditions) (specialist care, number of doctors seen) (number of prescriptions drugs) (emergency care) (surgery, hospital) (coordination of care). Beginning with the first grouping (i.e. gender), the effect of each block was tested backwards and the entire block discarded if non‐significant. Significant blocks were included as a whole. Data were analysed using the software package stata v11.2. 21
Results
Interviews were completed with 19 738 adults aged 18 and above. Sample characteristics are provided in Table 1. Self‐reported error in health care was common in all countries but with marked differences even within European countries (Table 2). For example, only 2.2% of responders in the United Kingdom but 8.6% of French participants reported a medication error in the past 2 years. Overall, one of ten citizens self‐reported a medical or medication error during the last 2 years. 18.8% of responders across countries reported that the last error in their care occurred in hospital, but this fraction varied considerably between countries and ranged from 12.3% in Sweden to 31.3% in Switzerland (P < 0.001). Across countries, the fraction of respondents that reported experience of two different types of error was 2.5%, and 0.5% reported all three types of errors. Poor coordination of care was also common in all countries: 10.9% reported that test results or medical records were not available, 19.6% perceived to have received conflicting information by care providers and 10.5% reported that tests were ordered although they had been performed before. A quarter of citizens (27.3%) reported any of these coordination problems in the past 2 years.
Table 1.
Sample characteristics, weighted data (n = 19 738)
| Characteristic | n (%) of participants |
|---|---|
| Country | |
| Australia | 3552 (18.0) |
| Canada | 3302 (16.7) |
| France | 1402 (7.1) |
| Germany | 1005 (5.1) |
| Netherlands | 1001 (5.1) |
| Norway | 1058 (5.4) |
| New Zealand | 1000 (5.1) |
| Sweden | 2100 (10.6) |
| Switzerland | 1306 (6.6) |
| United Kingdom | 1511 (7.7) |
| United States | 2501 (12.7) |
| Female gender | 11 537 (51.5) |
| Age, mean | 48.4 years |
| 18–29 years | 2212 (17.6) |
| 30–49 years | 6467 (36.9) |
| 50–64 years | 5632 (24.6) |
| 65 years and above | 5427 (20.9) |
| Education (recoded from nation‐specific response codes) | |
| High school or less | 9984 (58.4) |
| Some college | 4266 (21.4) |
| College graduate or higher | 5150 (20.3) |
| Income (relative to national averages) | |
| Much below average | 3275 (17.1) |
| Somewhat below average | 3412 (18.9) |
| Average | 4854 (26.9) |
| Somewhat above average | 4441 (24.6) |
| Much above average | 2365 (12.5) |
| Self‐rated health | |
| Excellent/very good | 10 522 (53.9) |
| Good | 6262 (31.5) |
| Fair/poor | 2876 (14.6) |
| Chronic conditions | |
| None | 7429 (42.0) |
| 1 condition | 5137 (26.0) |
| 2 or more conditions | 7119 (32.0) |
Table 2.
Frequency of self‐reported errors by country, weighted data
| Country | Medical error n (%) | Medication error n (%) | Either medical or medication error n (%) | Laboratory error*n (%) | Either medical, medication or laboratory error (aggregate measure) n (%) |
|---|---|---|---|---|---|
| Australia | 282 (8.3) | 155 (4.5) | 350 (10.1) | 69 (2.4) | 395 (11.4) |
| Canada | 212 (7.7) | 179 (6.0) | 322 (10.9) | 106 (4.1) | 372 (12.2) |
| France | 87 (5.9) | 110 (8.6) | 157 (11.6) | 39 (2.8) | 178 (12.5) |
| Germany | 54 (5.9) | 20 (2.2) | 64 (7.0) | 12 (1.7) | 73 (7.8) |
| Netherlands | 52 (4.8) | 45 (4.5) | 82 (7.8) | 25 (3.0) | 97 (9.3) |
| Norway | 101 (10.8) | 79 (8.1) | 147 (15.7) | 29 (3.4) | 161 (17.0) |
| New Zealand | 59 (5.6) | 39 (4.6) | 82 (8.3) | 19 (2.4) | 92 (9.0) |
| Sweden | 118 (6.1) | 92 (4.9) | 173 (8.9) | 26 (1.9) | 184 (9.5) |
| Switzerland | 81 (8.0) | 61 (5.3) | 123 (11.4) | 31 (3.2) | 136 (11.9) |
| United Kingdom | 39 (3.2) | 25 (2.2) | 55 (4.7) | 21 (2.6) | 66 (5.4) |
| United States | 204 (9.7) | 150 (6.4) | 295 (12.9) | 83 (5.0) | 331 (14.3) |
*Based on those that reported blood test, X‐rays or other tests in the past 2 years.
A number of variables were associated with patient‐reported error in bivariate analysis (Fig. 1). Across all countries, health status and health care utilization variables were associated with all three types of self‐reported errors (and the aggregate measure) with different levels of strength. Associations between demographic variables and errors were less systematic: Higher age was inversely related to all types of reported errors, except medication errors. Female gender was associated with medical error, medication error and the aggregate measure, but not the subset of laboratory errors. Low income was associated with all types of reported errors, again except laboratory errors. Education was only weakly associated with reporting medical error.
Figure 1.
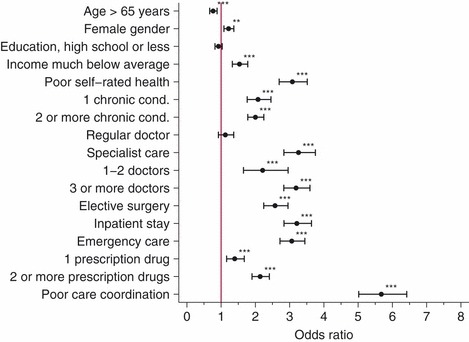
Bivariate (unadjusted) associations between demographic, health and health care utilization variables and experience of any error (aggregate measure), weighted data. Stars indicate significant associations (*P < 0.05; **P < 0.01; ***P < 0.01).
Results of the regression model for all 11 countries and three country‐specific models are presented in Table 3. All VIFs were <2.0 indicating no substantial multicollinearity. The Archer–Lemeshow goodness‐of‐fit statistic did not indicate any overall model departure from the observed data. Across countries, the risk of patient‐reported error is determined mainly by health care utilization. Emergency care, hospitalization and the number of providers involved are among the most important predictors. Having seen three or more doctors doubles the risk for reporting any error when other factors are controlled for, e.g. health status and use of prescription drugs. Experience of poor care coordination is the single most important risk factor, associated with a four‐fold increase in reporting error. Responders with chronic conditions and poor health are at considerably higher risk for reporting errors in their care, even after adjusting for a large variety of health care utilization. After controlling for health and health care utilization, patients younger than 65 years were nearly twice as likely to report any medical error.
Table 3.
Results of robust logistic regression analysis, weighted data. Predictors for any self‐reported error (medical, medication or laboratory error) for 11 participating countries and country‐specific analysis for USA, United Kingdom and Germany (hierarchical regression)
| Variable | All 11 countries | USA | United Kingdom | Germany | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | CI | P | OR | CI | P | OR | CI | P | OR | CI | P | |
| Age > 65 years | 0.59 | 0.50–0.71 | <0.001 | 0.43 | 0.30–0.62 | <0.001 | ||||||
| Female gender | 1.05 | 0.91–1.20 | 0.522 | |||||||||
| College education (vs. High school or less) | 1.12 | 0.94–1.34 | 0.191 | 1.01 | 0.55–1.87 | 0.966 | ||||||
| Income much below national averages (vs. no) | 1.15 | 1.00–1.32 | 0.045 | 3.96 | 1.54–10.17 | 0.004 | ||||||
| Fair/poor self‐rated health (vs. excellent/good) | 1.48 | 1.25–1.76 | <0.001 | 1.38 | 0.96–1.98 | 0.083 | ||||||
| Chronic conditions (base: none) | ||||||||||||
| 1 chronic condition | 1.58 | 1.29–1.92 | <0.001 | 2.34 | 1.42–3.86 | 0.001 | ||||||
| 2 or more chronic conditions | 1.56 | 1.27–1.93 | <0.001 | 2.84 | 1.80–4.49 | <0.001 | ||||||
| Specialist care (vs. no) | 1.51 | 1.27–1.81 | <0.001 | 1.47 | 1.02–2.11 | 0.037 | ||||||
| Number of doctors seen (base: none) | ||||||||||||
| 1–2 doctors | 1.43 | 1.02–2.01 | 0.036 | 0.95 | 0.47–1.90 | 0.877 | ||||||
| 3 or more doctors | 2.01 | 1.41–2.87 | <0.001 | 1.58 | 0.78–3.19 | 0.206 | ||||||
| Elective surgery (vs. no) | 1.28 | 1.08–1.51 | 0.004 | 0.82 | 0.29–2.30 | 0.708 | 1.89 | 0.93–3.85 | 0.078 | |||
| Hospital stay (vs. no) | 1.60 | 1.37–1.88 | <0.001 | 3.90 | 1.71–8.93 | 0.001 | 5.50 | 2.57–11.77 | <0.001 | |||
| Emergency care (vs. no) | 1.66 | 1.44–1.91 | <0.001 | 1.74 | 1.27–2.37 | 0.001 | ||||||
| Number of prescription drugs (base: none) | ||||||||||||
| 1 prescription drug | 1.00 | 0.81–1.25 | 0.968 | 1.23 | 0.46–3.26 | 0.679 | ||||||
| 2 or more prescription drugs | 1.32 | 1.09–1.60 | 0.004 | 2.69 | 1.34–5.40 | 0.005 | ||||||
| Poor coordination of care experiences (vs. no) | 3.85 | 3.35–4.41 | <0.001 | 4.23 | 3.09–5.78 | <0.001 | 4.56 | 2.38–8.73 | <0.001 | 4.28 | 2.31–7.92 | <0.001 |
| n | 17 825 | 2426 | 1483 | 952 | ||||||||
| Archer–Lemeshow test statistic | 1.259 | 0.254 | 0.762 | 0.652 | 0.360 | 0.876 | 0.091 | 0.997 | ||||
The joint influence of the risk factors on the probability that patients report error in their care is substantial (illustrated in Fig. 2). For example, the differences between hypothetical patients B and F (chronic conditions, emergency care, prescription drugs, number of doctors seen, specialist care and coordination of care problems) account for a 14‐fold increase in probability of reporting error, keeping younger age, low income, poor self‐reported health, hospital stay and surgery constant (p B = 0.049, p F = 0.679, P < 0.001).
Figure 2.
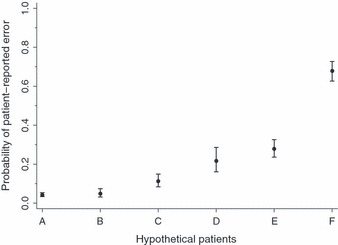
Predicted probability for patient‐reported error (aggregate measure) across 11 countries for six hypothetical patients (A–F), weighted data. Six hypothetical patients (A–F) were modelled with the following characteristics: Patient A: Aged >65 years, average income, good self‐reported health, two or more chronic conditions, emergency care, no hospital or surgery, two or more prescription drugs, one or two doctors, no specialist, no coordination of care problems. Patient B: Aged <65 years, much below average income, poor self‐reported health, no chronic conditions, no emergency care, hospital stay and surgery, no prescription drugs, no doctors seen, no specialist, no coordination of care problems. Patient C: Aged <65 years, average income, poor self‐reported health, one chronic condition, no emergency care, hospital stay, no surgery, one prescription drug, one or two doctors, specialist, no coordination of care problems. Patient D: Aged >65 years, much below average income, poor self‐reported health, no chronic conditions, emergency care, hospital stay and surgery, no prescription drugs, one or two doctors, no specialist, poor coordination of care. Patient E: Aged <65 years, average income, good self‐reported health, one chronic condition, no emergency care, no hospital stay or surgery, two or more prescription drugs, three or more doctors, specialist, poor coordination of care. Patient F: Aged <65 years, much below average income, poor self‐reported health, two or more chronic conditions, emergency care, hospital stay and surgery, two or more prescription drugs, three or more doctors, specialist, poor coordination of care.
Three country‐specific models yield common and country‐specific predictors for self‐reported error. Poor coordination of care experiences was the strongest predictor for patient‐reported error in all three countries. Hospital care in the past 2 years was associated with reporting error in the United Kingdom and Germany, but not in the United States. On the contrary, poor health, specialist care and emergency care increase the likelihood of self‐reported error in the United States, but not in the United Kingdom and Germany. Use of prescription drugs was a significant predictor only in the United Kingdom. Having a much below average income was a strong predictor for reporting error experience in Germany.
Discussion
This study reports new data on patients’ perceptions of error in 11 countries and identified a number of important risk factors. Overall, one of ten surveyed patients reported either medical, medication or laboratory errors in their care but this risk varies markedly by a factor of 3 across countries (5.4% in the United Kingdom and 17.0% in Norway). Several factors may help to explain this finding: Different health care systems may in fact perform better in preventing errors and can thus deem to be safer. However, observed differences between countries may also stem from differences in patients’ likelihood to identify and report error, rather than differences in true incidences. While evidence shows that patients’ reports of adverse events are often in well concordance with other detection methods, e.g. record review, it is unclear whether this degree of concordance is similar across countries. 22 , 23 , 24 , 25 For example, safety in health care may be an issue of high public awareness in some countries and largely unrecognized in others. As a result, patients may be more or less vigilant and educated about safety and have different abilities or motivation to detect errors. ‘Medical error’ may also be defined differently in diverse cultural contexts. In addition, patients’ reports of errors are likely to be affected by official standards and cultural norms among health care workers on how openly to communicate errors towards patients. Thus, patients’ reports of error do not only reflect incidence of error but are also ‘contaminated’ by identification and reporting biases. Reporting effects rather than differences in frequency may also help to explain why younger patients were systematically more likely to report errors compared to respondents aged 65 and above, a finding that has been reported in the previous studies. For example, in a recent survey study among Swiss hospital patients, the likelihood for reporting adverse events during hospital stay decreased significantly with higher age by a comparable magnitude. 26 Younger patients may be more aware of safety problems and less reluctant to report these.
Across 11 countries, our data clearly show that risk of self‐reported error increases steadily with the amount and categories of health care consumed. However, across countries, patients with poor health and low income are at increased risk even after adjusting for various health care utilization‐related variables. It is not surprising that poor care coordination experience is the most important single risk factor for reporting errors across countries and in our country‐specific analyses. Unavailable records, conflicting information and repetition of tests can signal, cause or coincide with safety events and can themselves be regarded as ‘error’, even if they may not cause harm. Thus, it seems likely that an unknown fraction of responders had the same event in mind when reporting coordination of care problems and error. This would lead to an overestimation of the association of coordination of care problems with error. Indeed, Rathert et al. 27 recently reported from a qualitative study that patients seem to share a broader interpretation of safety compared with health professionals and often include communication and coordination failures. Our country‐level analyses reveal that the risk associated with different health care services varies considerably between countries. This strengthens the assumption that systems differ in their abilities to manage specific threats for patient safety. This view is also supported by the large variance observed in reported occurrence of error. Hospital‐associated error was much more frequent in some countries (e.g. Switzerland) compared to the cross‐national average. These results may reflect differences between countries in how care is organized. For example, access to specialist outpatient care is far more restrictive in some countries compared to others.
While our results clearly indicate that various types of health care consumed increase the risk of error, the relative magnitude of predictor variables should be compared with care. As with all surveys, health care utilization had to be operationalized for measurement and this operationalization may interact with specific forms of care organizations and is thus important for interpretation: For example, a single hospital stay is longer and patients are exposed to risk (and error identification) simply for a longer duration as compared to a short outpatient consultation. The number of doctors seen does reflect the increasing need for coordination, but not necessarily treatment intensity. Thus, for countries that restrict access to the number of providers involved, treatment intensity per provider may be more important.
This study has some limitations: First, the samples for each of 11 countries have been drawn and weighted to be representative for each individual country.
The sample sizes did not allow more extensive analyses of country‐level data, e.g. selection of predictors based on bivariate analyses or including the same predictors in all country‐specific models irrespective of their significance. In addition, the reasons for and potential effects of the very different survey response rates remain unclear. For example, Norway had the lowest response rate (13%) and the highest fraction of patients that reported any error in their care (17%). It seems likely that individuals that experienced error were more likely to participate than others. Second, we used an aggregate measure of error as outcome variable in regression analyses. Distinct associations with specific types of errors, i.e. medication or laboratory errors, may thus have gone undetected. Finally, owing to the nature of the data, we cannot demonstrate causal or temporal relationship between health care utilization and error. While responders were asked to consider the past 2 years in most of the questions, we do not know whether health care was utilized before or after the reported events occurred and how they are connected.
Despite these limitations, the results of this study are alarming. Our modelling of hypothetical patients shows that for high utilizers of health care that unify multiple risk factors it is nearly rule rather than exception that errors occur. Patients who utilize various types of health care in different settings accumulate a high risk of errors, which is largely underestimated in isolated setting‐specific adverse event studies. Despite the associated health‐related harm, the common experience of error in these populations may also cause considerable loss of trust in the health care system as a whole. Medical error is communized with poor coordination of care experiences, and obviously, health care systems fail to overcome risks associated with the segmentation of health care. This is also indicated by the fact that having a regular doctor had no substantial protecting effects on patient safety. These results emphasize that patient safety remains a global challenge affecting many patients throughout the world. However, large variability exists in the frequency of patient‐reported error across countries. Taking the opportunity to learn from others’ errors is not only essential within individual institutions or systems but may also prove a promising strategy internationally.
Ethics approval
Ethics approval was not necessary for this study.
Competing interests
None.
Funding
The author obtained no funding for this particular research. Core funding for the ‘Commonwealth Fund’s 2010 lnternational Survey of the General Public’s Views of their Health Care System’s Performance in Eleven Countries’ was by the Commonwealth Fund with co‐funding from the following organizations: the Australian Commission on Safety and Quality in Health Care; the Ontario Health Quality Council; the Health Council of Canada; the Quebec Health Commission; La Haute Autorité de Santé; the Caisse Nationale d’Assurance Maladie Ces Travailleurs Salaries; the German lnstitute for Quality and Efficiency in Health Care; the Dutch Ministry of Health, Welfare and Sport; the Scientific lnstitute for Quality of Healthcare, Radboud University Nijmegen; the Norwegian Knowledge Centre for the Health Services; the Health Foundation; the Swedish Ministry of Health and Social Affairs; the Swiss Federal Office of Public Health.
Acknowledgements
The author thanks the Commonwealth Fund for permission to analyse the data. The support by Markus Weber (Swiss Federal Office of Public Health, BAG) is highly appreciated. Three anonymous referees are acknowledged for their thorough comments on an earlier draft. The contents are the sole responsibility of the author and do not represent the views of the Commonwealth Fund or local agencies of the participating countries.
References
- 1. von Laue NC , Schwappach DL , Koeck CM . The epidemiology of medical errors: a review of the literature . Wiener Klinische Wochenschrift , 2003. ; 115 : 318 – 325 . [DOI] [PubMed] [Google Scholar]
- 2. Zegers M , de Bruijne MC , Wagner C et al. Adverse events and potentially preventable deaths in Dutch hospitals: results of a retrospective patient record review study . Quality and Safety in Health Care , 2009. ; 18 : 297 – 302 . [DOI] [PubMed] [Google Scholar]
- 3. Soop M , Fryksmark U , Koster M , Haglund B . The incidence of adverse events in Swedish hospitals: a retrospective medical record review study . International Journal for Quality in Health Care , 2009. ; 21 : 285 – 291 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baker GR , Norton PG , Flintoft V et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada . CMAJ , 2004. ; 170 : 1678 – 1686 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis P , Lay‐Yee R , Briant R , Ali W , Scott A , Schug S . Adverse events in New Zealand public hospitals 1: occurrence and impact . New Zealand Medical Journal , 2002. ; 115 : U271 . [PubMed] [Google Scholar]
- 6. Thomas EJ , Studdert DM , Burstin HR et al. Incidence and types of adverse events and negligent care in Utah and Colorado . Medical Care , 2000. ; 38 : 261 – 271 . [DOI] [PubMed] [Google Scholar]
- 7. Vincent C , Neale G , Woloshynowych M . Adverse events in British hospitals: preliminary retrospective record review . BMJ , 2001. ; 322 : 517 – 519 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Forster AJ , Murff HJ , Peterson JF , Gandhi TK , Bates DW . The incidence and severity of adverse events affecting patients after discharge from the hospital . Annals of Internal Medicine , 2003. ; 138 : 161 – 167 . [DOI] [PubMed] [Google Scholar]
- 9. Thomsen LA , Winterstein AG , Sondergaard B , Haugbolle LS , Melander A . Systematic review of the incidence and characteristics of preventable adverse drug events in ambulatory care . Annals of Pharmacotherapy , 2007. ; 41 : 1411 – 1426 . [DOI] [PubMed] [Google Scholar]
- 10. Miller GC , Britth HC , Valenti L . Adverse drug events in general practice patients in Australia . Medical Journal of Australia , 2006. ; 184 : 321 – 324 . [DOI] [PubMed] [Google Scholar]
- 11. Gurwitz JH , Field TS , Harrold LR et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting . JAMA , 2003. ; 289 : 1107 – 1116 . [DOI] [PubMed] [Google Scholar]
- 12. Rubin G , George A , Chinn DJ , Richardson C . Errors in general practice: development of an error classification and pilot study of a method for detecting errors . Quality and Safety in Health Care , 2003. ; 12 : 443 – 447 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elder NC , Meulen MV , Cassedy A . The identification of medical errors by family physicians during outpatient visits . Annals of Family Medicine , 2004. ; 2 : 125 – 129 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Makeham MA , Kidd MR , Saltman DC et al. The threats to australian patient safety (TAPS) study: incidence of reported errors in general practice . Medical Journal of Australia , 2006. ; 185 : 95 – 98 . [DOI] [PubMed] [Google Scholar]
- 15. Unruh KT , Pratt W . Patients as actors: the patient’s role in detecting, preventing, and recovering from medical errors . International Journal of Medical Informatics , 2007. ; 76 ( Suppl 1 ): S236 – S244 . [DOI] [PubMed] [Google Scholar]
- 16. Schwappach DL . Engaging patients as vigilant partners in safety: a systematic review . Medical Care Research and Review , 2010. ; 67 : 119 – 148 . [DOI] [PubMed] [Google Scholar]
- 17. Schoen C , Osborn R , Huynh PT et al. Taking the pulse of health care systems: experiences of patients with health problems in six countries . Health Affairs 2005. ; Suppl Web Exclusives(W5 ): 509 – 525 . [DOI] [PubMed] [Google Scholar]
- 18. Schoen C , Osborn R , How SKH , Doty MM , Peugh J . In chronic condition: experiences of patients with complex health care needs, in eight countries, 2008 . Health Affairs , 2009. ; 28 : w1 – w16 . [DOI] [PubMed] [Google Scholar]
- 19. Scobie A . Self‐reported medical, medication and laboratory error in eight countries: risk factors for chronically ill adults . International Journal for Quality in Health Care , 2011. ; 23 : 182 – 186 . [DOI] [PubMed] [Google Scholar]
- 20. Archer KJ , Lemeshow S , Hosmer DW . Goodness‐of‐fit tests for logistic regression models when data are collected using a complex sampling design . Computational Statistics and Data Analysis , 2007. ; 51 : 4450 – 4464 . [Google Scholar]
- 21. StataCorp . Stata Statistical Software: Release 11.2 . College Station, TX : Stata Corporation; , 2010. . [Google Scholar]
- 22. Fowler FJ , Epstein A , Weingart SN et al. Adverse events during hospitalization: results of a patient survey . Joint Commission Journal on Quality and Safety , 2008. ; 34 : 583 – 590 . [DOI] [PubMed] [Google Scholar]
- 23. Weingart SN , Pagovich O , Sands DZ et al. What can hospitalized patients tell us about adverse events? Learning from patient‐reported incidents . Journal of General Internal Medicine , 2005. ; 20 : 830 – 836 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. King A , Daniels J , Lim J , Cochrane DD , Taylor A , Ansermino JM . Time to listen: a review of methods to solicit patient reports of adverse events . Quality and Safety in Health Care , 2010. ; 19 : 148 – 157 . [DOI] [PubMed] [Google Scholar]
- 25. Massó Guijarro P , Aranaz Andres JM , Mira JJ , Perdiguero E , Aibar C . Adverse events in hospitals: the patient’s point of view . Quality and Safety in Health Care , 2010. ; 19 : 144 – 147 . [DOI] [PubMed] [Google Scholar]
- 26. Schwappach DLB , Frank O , Hochreutener MA . ‘New perspectives on well‐known issues’: Patients’ experiences and perceptions of safety in Swiss hospitals . Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen , 2011. ; 105 : 542 – 548 . [DOI] [PubMed] [Google Scholar]
- 27. Rathert C , Brandt J , Williams ES . Putting the “patient” in patient safety: a qualitative study of consumer experiences . Health Expectations 2011. ; doi: 10.1111/j.1369‐7625.2011.00685.x . [DOI] [PMC free article] [PubMed] [Google Scholar]


