Abstract
Background
Genetic carrier screening is increasingly possible for many conditions, but it is important to ensure decisions are informed. The multidimensional measure of informed choice (MMIC) is a quantitative instrument developed to evaluate informed choice in prenatal screening for Down syndrome, measuring knowledge, attitudes and uptake. To apply the MMIC in other screening settings, the knowledge scale must be modified.
Objective
To develop and validate a modified MMIC knowledge scale for use with women undergoing carrier screening for fragile X syndrome (FXS).
Setting and participants
Responses to MMIC items were collected through questionnaires as part of a FXS carrier screening pilot study in a preconception setting in Melbourne, Australia.
Design
Ten knowledge scale items were developed using a modified Delphi technique. Cronbach's alpha and factor analysis were used to validate the new FXS knowledge scale. We summarized the knowledge, attitudes and informed choice status based on the modified MMIC.
Results
Two hundred and eighty‐five women were recruited, 241 eligible questionnaires were complete for analysis. The FXS knowledge scale items measured one salient construct and were internally consistent (alpha = 0.70). 71% (172/241) of participants were classified as having good knowledge, 70% (169/241) had positive attitudes and 27% (65/241) made an informed choice to accept or decline screening.
Discussion and conclusions
We present the development of a knowledge scale as part of a MMIC to evaluate informed choice in population carrier screening for FXS. This can be used as a template by other researchers to develop knowledge scales for other conditions for use in the MMIC.
Keywords: decision‐making, evaluation, fragile X syndrome, informed choice, measurement scales, screening
Introduction
Population‐based genetic carrier screening programmes offer individuals the opportunity to learn information about their risk of having children with genetic conditions and may also provide personal health information. Guidelines state that such programmes are to be voluntary and should aim to promote informed choice regarding the decision to accept or decline screening.1 Informed choice is considered a central tenet of genetic screening programmes and should be prioritized as the key desired outcome ahead of focusing on high test uptake.1 Understanding the purpose and implications of the genetic tests may reduce potential psychosocial harms caused by unexpected outcomes of choosing or declining screening.1, 2
There is not one single definition of informed choice, but all definitions generally include two key features: being informed and acting autonomously.2, 3, 4, 5, 6 Being informed entails understanding the risks and benefits of the options available, allowing individuals to prepare for the potential outcomes of their choice. Acting autonomously entails making a choice independently, without controlling influences.3 The complexities of evaluating informed choice have been recognized,7, 8 and while efforts have been made to evaluate informed choice in prenatal screening for Down syndrome,4, 9, 10 sickle cell and thalassaemia,11, 12, 13 and community cancer screening,14, 15, 16, 17, 18, 19 informed choice in population‐based genetic carrier screening in preconception settings has not been evaluated.
As the potential for specific genetic screening tests grows, it is important to establish both the benefits and harms of screening prior to its implementation,1, 20, 21 including ascertaining whether an informed choice has been made by individuals undergoing screening. The multidimensional measure of informed choice (MMIC),4, 10 is an instrument originally designed for women undergoing prenatal screening for Down syndrome, and it has been applied in several studies.5, 9, 11, 22, 23, 24, 25, 26 The model is based on a specific definition of informed choice; that is, a decision made with good knowledge and in accord with one's values and attitudes towards testing.2, 10, 27 When individuals have a positive attitude towards the test and are tested, or have a negative attitude towards the test and decline testing, their choice is considered value consistent. To measure informed choice, the MMIC uses a knowledge scale and an attitudes scale combined with test behaviour.
The knowledge scale within the MMIC must be developed specifically for the conditions for which screening is being offered, that is, Down syndrome in the original version of the MMIC. The attitudes scale, developed from the Theory of Planned Behaviour,27 is designed to measure the latent construct of an individual's values with questions about attitudes regarding the screening test.4 The responses to both the knowledge and attitude scales are scored and converted into a binary classification of good/poor knowledge and positive/negative attitude towards the test. To date, the MMIC has been developed for use in low literacy populations,12 translated and validated into one other language (Greek)28 and has been used for other conditions,11, 12 mainly in cancer screening.14, 15, 16, 19, 29, 30, 31 Development and validation of the knowledge scales for these differing settings has not been published in detail, nor has the MMIC been applied to population‐based preconception carrier screening, such as fragile X syndrome (FXS).
Fragile X syndrome is the most common cause of inherited intellectual disability, it is an X‐linked condition, caused by a hypermethylated triplet repeat expansion in the 5′ untranslated region of the FMR1 gene.32, 33 The normal FMR1 allele is between six and 44 triplet repeats, whereas an expansion of >200 repeats results in FXS.34 Population carrier screening aims to detect women with a premutation (55–200 repeats) as this mutation can be unstable, expanding to cause FXS when passed to offspring by mothers rather than fathers.32, 35, 36 Therefore, women with a premutation may be at risk of having children with FXS without having symptoms or a family history of the condition.37 Women who are carriers of the premutation may also be at risk of fertility problems and early menopause, a condition known as fragile X‐associated primary ovarian insufficiency (FXPOI).38 FXS carrier screening provides women with the opportunity to learn of their FXS carrier status to inform their family planning. While there is a strong case for implementation of population carrier screening for FXS,39 it is not yet offered widely and guidelines emphasize the need for evaluation in a research setting.37, 40
Fragile X syndrome provides a useful example to explore informed choice in population‐based genetic carrier screening as a carrier result conveys reproductive as well as health risks of the individual. A model of preconception genetic carrier screening for FXS has been piloted in the general community in Melbourne, Victoria,40, 41 and we now report on the development of a specific knowledge scale involving the application of quantitative analyses to these data.
Methods
The focus of this study is to describe in detail the process of modifying and testing a knowledge scale, based on data collected as part of a pilot screening study at a reproductive and sexual health service, Family Planning Victoria (FPV).40 Approval for this study was granted by the FPV Human Research Ethics Committee.
Development and content validity of FXS knowledge scale
A bank of 19 knowledge items was initially generated by AMJ, SM and AF based on the information brochure. The content of the brochure was developed by staff at Murdoch Childrens Research Institute and Genetic Health Services Victoria (GHSV) with expertise in genetic counselling, clinical genetics, genetics education and public health genetics. Input was also sought from the Fragile X Alliance Inc, a joint patient and clinical support organization. The information included important concepts for participants to understand when deciding about FXS screening, based on genetic counselling guidelines,37 and discussions in standard pre‐test genetic counselling sessions for FXS at GHSV. The brochure was then piloted and revised following feedback from staff and clients at FPV, as described in the needs assessment phase of the pilot study.40 Thus, the proposed knowledge items reflected the content of the brochure and aimed to assess women's knowledge of four areas: inheritance of FXS (three items); risks and symptoms associated with the various CGG repeat sizes (five items), implications of a carrier result for reproduction (four items) and characteristics and treatment of FXS (seven items). Items were designed with the response options of true, false and unsure.
To refine the knowledge items and establish content validity, a modified Delphi technique of two consultation rounds was used (based on Flouris et al., 2010).42 Round one involved submitting the 19 proposed items to nine expert representatives from the areas of clinical genetics, genetics education, public health genetics, genetic counselling and Fragile X Alliance Inc. The questions were assessed for the extent to which they were important in determining women's knowledge of the four concept areas and how difficult the items would be for women to answer using a 5‐point Likert scale (1 – not at all; 2 – somewhat; 3 – moderately; 4 – very; 5 – extremely).
The second round involved consultation with 10 experts (including the nine experts from round one) to refine the number of questions based on agreement (1 – strongly disagree; 2 – disagree; 3 – neither; 4 – agree; 5 – strongly agree) with first round ratings of degree of importance and difficulty. To select the final items for the knowledge scale, items worded ambiguously or those that were deemed by the expert panel to be too difficult were excluded and items agreed upon to be ‘very or extremely important’ in assessing women's knowledge of FXS and ‘not at all or moderately difficult’ for women to answer were selected. Selection of the number of final items for inclusion was also balanced against the requirement for keeping the questionnaire as brief as possible. Therefore, 10 items met these criteria and were used to form the knowledge scale, with at least two questions per knowledge area: inheritance of FXS (items 1 and 2); implications of results for reproduction (items 3 and 4); risks and symptoms associated with results (items 5, 6 and 7); and characteristics and treatment of FXS (items 8, 9 and 10). For subsequent analyses, each question was scored equally, with one point allocated for each correct answer and zero for an incorrect or an ‘unsure’ response. These items, with their correct answer are outlined in Table 1.
Table 1.
Fragile X syndrome (FXS) knowledge item and properties
| Item no. | FXS knowledge item | Correct answer (True/False) | % Correct (n) N = 241 |
|---|---|---|---|
| 1 | FXS is contagious | False | 94 (226) |
| 2 | FXS is caused by having an altered gene | True | 85 (205) |
| 3 | Women who carry the normal length gene have no chance of having a child with FXS | False | 28 (67) |
| 4 | A carrier genetic test for FXS will tell me if I am at risk of having children with FXS | True | 94 (226) |
| 5 | Some carriers of the medium length gene develop mild symptoms of FXS | True | 52 (125) |
| 6 | The longer the FXS gene, the more likely it is that the person will have symptoms of FXS | True | 75 (180) |
| 7 | FXS is caused by having the short length gene | False | 72 (174) |
| 8 | More men than women are affected by FXS | True | 53 (127) |
| 9 | FXS is the most common inherited cause of intellectual disability | True | 59 (143) |
| 10 | There is no cure for FXS | True | 73 (175) |
Sample
While the full details of the pilot screening study have already been published,40 we briefly describe the methods of data collection here. Women attending a drop‐in clinic at FPV located in metropolitan Melbourne, Australia, were invited to participate in the FXS carrier screening study by a research genetic counsellor. Women aged 18 and over, who were not pregnant and who could read, write and speak English were included in the study. Women attending the clinic in crisis or emergency situations were excluded.
Recruitment
Participants who attended a drop‐in clinic were offered FXS carrier screening and provided with pre‐test counselling as well as the brochure containing information about FXS. If participants chose to undergo screening, they were required to return to the clinic to provide a blood sample at another time to ensure adequate time was provided for decision making. While participants were asked to complete two questionnaires in the study, only responses from questionnaire one (Q1), administered upon recruitment into the study were used for analysis here. Q1 contained questions about intention to be tested, knowledge about FXS, attitudes towards the test, the short form state scale of the State Trait Anxiety Inventory,43 family history, questions about the decision‐making process, and demographics; all questions in Q1 were subjected to the modified Delphi technique with the panel of experts as described previously.
Procedures
Knowledge scale
Cronbach's alpha was calculated for the knowledge items using Q1 responses to quantify their internal consistency and investigate whether there were any redundancies amongst the items. Alpha values of 0.7–0.9 were assumed to indicate good internal consistency.44 Additionally, we used exploratory factor analysis using orthogonal (varimax) rotation to determine whether the knowledge items spanned more than one dimension.45 The data analysis was performed by AGA and OU using stata 12.46
There is no standard definition of what is deemed to be ‘good’ knowledge. It could be argued that a mid‐point score or above (i.e. ≥5 of 10 in this case) constitutes ‘good knowledge’11; 50% correct responses could, however, also be considered simply adequate knowledge.14 In our previous study, we had classified knowledge into tertiles, with 0–3 correct as ‘poor knowledge’, 4–6 correct as ‘moderate knowledge’ and 7–10 correct as ‘good knowledge. Therefore, for the purpose of dichotomising this scale for its application in the MMIC, we have classified good knowledge as correct responses on at least 70% of the items.
Attitudes scale
To assess participants' attitudes towards FXS carrier screening, they were asked to respond to five items adapted from the attitudes scale used by Marteau,4 with the addition of the item ‘worrying/not worrying’. Although this scale was validated in the prenatal setting,10 the scale was submitted to the modified Delphi technique to establish the content validity described earlier, with all items considered extremely important and not difficult to answer. Cronbach's alpha was used to quantify internal consistency in this preconception setting. Further statistical analyses and validation were not applied to the attitudes scale as this is subject to further research.
In Q1, participants were asked to indicate how they felt about FXS screening on a scale of 0–4 on five dimensions (beneficial/harmful; important/unimportant; a bad thing/good thing; pleasant/unpleasant; worrying/not worrying) in response to the prompt ‘For me, having carrier genetic testing for FXS would be…’. In accordance with the original application of the MMIC attitudes responses were summated and the midpoint of the scale (≥11, range, 0–20) was used as the cut‐off to classify participants as having positive attitudes towards FXS carrier screening.10
Value consistency
The value consistency component of the MMIC was calculated in the same way it was calculated in the original application of the MMIC by combining the participants' attitude classification (positive/negative) and test behaviour (accepted or declined screening). Thus, values were classed as consistent when either a positive attitude was combined with uptake of the carrier test or when a negative attitude was combined with declining screening. A positive attitude combined with declining testing or a negative attitude combined with uptake of the test was considered value inconsistent combinations.
MMIC
To determine whether an informed choice had been made, knowledge and attitudes responses were combined with the participants' screening behaviour. Choices were classified according to the MMIC model,10 which defines an informed choice as one made with good knowledge that is also value‐consistent (i.e. took the test with good knowledge and a positive attitude or did not take the test with good knowledge and a negative attitude). Using this model, all other combinations were considered as instances of non‐informed choice.
Informed choice as calculated by the MMIC is sensitive to the cut‐off used to define ‘good knowledge’. To demonstrate this, we present the proportion with informed choice using different knowledge cut‐off scores.
Results
Sample
Two hundred and eighty‐five women were recruited from the drop‐in clinic, and 241 complete questionnaires were used for analysis.40 Of these 241 women, 11% (26/241) had FXS carrier screening. Demographic information is provided in Table 2.
Table 2.
Demographic characteristics of participants attending the drop‐in clinic at Family Planning Victoria
| Characteristic | N = 240a (%) |
|---|---|
| Age range (years) | |
| 18–25 | 111 (46) |
| 26–30 | 53 (22) |
| 31–35 | 37 (15) |
| 36–40 | 24 (10) |
| 41–45 | 9 (4) |
| >45 | 6 (3) |
| Highest level of education | |
| Year 11 or less | 16 (7) |
| Finished secondary school | 55 (23) |
| Trade/apprenticeship | 2 (1) |
| College certificate/diploma/university qualification | 163 (68) |
| Other | 4 (2) |
| Relationship status | |
| Married/De facto/Living with Partner | 73 (30) |
| Divorced/Separated | 11 (5) |
| Partner, not living together | 68 (28) |
| Single | 86 (36) |
| Widowed | 1 (0) |
| Other | 1 (0) |
| Years living in Australia | |
| All my life | 172 (72) |
| Since my childhood | 22 (9) |
| Since my teenage years | 7 (3) |
| Since my adulthood | 39 (16) |
One non‐responder.
Psychometric properties
Knowledge items
The internal consistency of the knowledge items was improved when item 3, which did not relate to other items, was removed, from this and the subsequent analysis. This resulted in an alpha of 0.70 indicating the items are related to each other without redundancy.
The scree test for the exploratory factor analysis, showing an elbow between factors 1 and 2 indicated that only the first factor is salient (Fig. 1). The factor, with an eigenvalue of 3.04, accounted for a third of the variability across the nine knowledge items.
Figure 1.
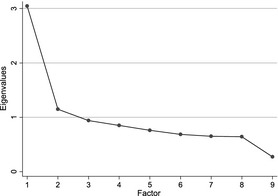
Factor analysis of knowledge items: scree plot of eigenvalues.
As we excluded one item, to dichotomize the scale for MMIC analysis into good/poor knowledge, participants who correctly answered six or more of the nine questions were classified as having good knowledge for the assessment of informed choice.
Attitudes scale
The Cronbach's alpha coefficient of 0.73 indicated the items of the attitudes scale relate well without redundancy.
Measures
The mean knowledge score was 6.6 (2.0 SD) with a median of 7 (IQR = 5–8, range = 0–9); therefore, 71% (172/241) of participants were classified as having good knowledge of FXS, scoring 6 or more. The mean attitudes score was 12.7 (3.7 SD) with a median of 13 (IQR = 10–16, range = 1–20). When classified using the midpoint of the scale of ≥11, 70% (169/241) of participants had positive attitudes towards carrier screening for FXS. When the attitude classifications were combined with test behaviour, 39% (94/241) of participants were classified as having made value‐consistent choices. Thirty‐three per cent (70/215) of those who declined screening and 92% (24/26) of those who had the screening made value‐consistent choices (Table 3).
Table 3.
Informed choice results by multidimensional measure of informed choice (MMIC) classification (N = 241)
| MMIC classification | Knowledge | Attitude | Uptake | Frequency | Percentage |
|---|---|---|---|---|---|
| Informed choice | Gooda | Positiveb | Tested | 15 | 6 |
| Good | Negative | Not tested | 50 | 21 | |
| Uninformed choice | |||||
| Poor knowledge | Poor | Positive | Tested | 9 | 4 |
| Poor | Negative | Not tested | 20 | 8 | |
| Value inconsistent | Good | Positive | Not tested | 105 | 44 |
| Good | Negative | Tested | 2 | 1 | |
| Poor knowledge and value inconsistent | Poor | Positive | Not tested | 40 | 17 |
| Poor | Negative | Tested | 0 | 0 | |
Good knowledge at least 6 of 9.
Positive attitude at least 11 of 20.
MMIC analysis
Using the MMIC classification of informed choice, 27% (65/241) of participants made an informed choice about FXS carrier screening (Table 3). Fifty‐eight per cent (15/26) of participants who had screening, and 23% (50/215) of those who declined screening made an informed choice. Overall, of the participants who did not make an informed choice according to the MMIC model, most were classified as uninformed due to value inconsistencies (61%, 107/176, Table 3). As the cut‐off point for ‘good knowledge’ is raised, the proportion of participants classified as having made an informed choice according to the MMIC decreased (Table 4).
Table 4.
Percentages (n) with ‘good’ knowledge and informed choice according to knowledge score cut‐off
| Knowledge score cut‐off | |||||
|---|---|---|---|---|---|
| 5 or more | 6 or more | 7 or more | 8 or more | 9 | |
| ‘Good’ knowledge, % (n) | 83 (201) | 71 (172) | 63 (151) | 41 (99) | 15 (35) |
| Total informed choice, % (n) | 32 (78) | 27 (65) | 25 (61) | 17 (41) | 7 (17) |
N = 241.
Discussion
We describe the first instance of adapting and applying the MMIC to population‐based preconception genetic carrier screening, in this case FXS, which required developing a specific knowledge scale for this purpose. The strength of our methodology in adapting the MMIC comes from using quantitative methods in the development, exploration and testing of the knowledge scale prior to applying the MMIC. This provides a framework to assist other researchers who wish to develop a knowledge scale for different conditions.
Using a modified Delphi technique, we established the content validity of the knowledge scale items and the factor analysis revealed only one salient factor measured by the items. The internal consistency of the FXS knowledge scale is similar to the original knowledge scale developed for the MMIC (alpha = 0.68,10). The key steps in this process are summarized in Table 5.
Table 5.
Key steps to developing and validating new knowledge scales for the multidimensional measure of informed choice
| Steps | Techniques used |
|---|---|
| Design informational material for participants | Use content expertise and any relevant guidelines, literature or needs assessment |
| Design knowledge questions | Reflect the informational material and concept areas |
| Select scale items |
Delphi Technique (content validity) Cronbach's alpha (internal consistency) |
| Establish validity and reliability of the scale | Factor analysis (construct validity) |
We were able to demonstrate that the majority of women in the pilot study had good knowledge of FXS when applying the validated knowledge scale. This high level of knowledge may have been influenced by the opportunity to discuss screening with a research genetic counsellor before making a decision about testing in addition to receiving an information brochure. While this level of knowledge is similar to some studies,5, 26, 47 it is somewhat better than other assessments of informed choice in prenatal screening for Down syndrome9, 22, 24 and in community cancer screening.14, 15
When applying the MMIC to our data, only 27% of women had made informed choices according to this model. Many women with positive attitudes towards testing were not classified as making an informed choice because they did not actually have the test. This could perhaps be explained by the design of the pilot study, in which it was necessary for women to return to the clinic to give blood at another time to have testing, based on Human Research Ethics requirements. This may have created an important practical barrier for women with a positive attitude towards screening actually following through and being tested. Consequently, there was a low uptake rate of the test and most ‘uninformed choices’ were categorized because they were apparently value inconsistent; that is, there was a mismatch between participants' attitudes and behaviour rather than they having poor knowledge. The authors of the original MMIC acknowledged that when an individual had a positive attitude yet did not undergo testing, this could indicate a practical barrier to test uptake, as opposed to an ‘uninformed’ choice.4, 10 In this case, returning for the test, or perhaps even the blood test itself, may have been barriers to test uptake resulting in value inconsistent behaviour.
Differences in the decision‐making contexts could also have affected uptake and consequent value inconsistency. In prenatal screening, for which the MMIC was originally designed, pregnant women are under pressure to make very prompt decisions about screening tests. In preconception population carrier screening, however, the same time pressure does not apply and women can decide to have screening at another time in their life.48 In follow‐up interviews with a selection of women who took part in this study, it was noted that some women with positive attitudes to screening opted to defer their decision about testing as they did not consider it relevant to them at this stage of life.41, 48 These women had more time to deliberate and change their mind from their initial decision,41 an important aspect of making an informed choice, but one not directly measured in the MMIC. We can therefore speculate that women may have made an informed choice not to have screening, but were classified by the MMIC model as having made an uninformed choice resulting from their attitudes not matching their behaviour, that is, having ‘value inconsistency’. The conceptualization and use of the attitudes scale in the ‘value consistency’ component of MMIC is problematic in preconception carrier screening, as women can defer the decision to have screening to a more relevant life stage,41 and is therefore a limitation of the MMIC. There is scope to expand how attitudes are evaluated and relate to informed choice such as assessing attitudes towards screening in general, or towards the specific condition and those affected by the condition.41, 49 We recommend future studies investigate the value consistency component of the MMIC, perhaps through incorporating a measure of intention as well as actual behaviour.
Another reason for the low levels of informed choice could be due to the timing of survey administration. The survey was given to participants upon recruitment into the study, not necessarily at the time when making their decision about screening. This period of time between recruitment and the decision about screening, while allowing women the opportunity to deliberate their decision, may have resulted in women changing their minds from their initial response in the questionnaire upon having more time for reflection and discussion with others.41 If this were the case, we would recommend participants complete the measure at the time of making their decision to more accurately measure informed choice. However, a limitation of the MMIC is that it does not capture aspects of deliberation in the decision‐making process.47
There has been further development of the MMIC since this study. van den Berg et al.47 added a deliberation scale to the MMIC to measure informed decision making in prenatal screening for Down syndrome. Throughout the literature, the terms ‘informed choice’ and ‘informed decision making’ are often used interchangeably but are also used to describe different concepts. With the addition of a deliberation scale to the MMIC, the focus shifts to evaluating the decision‐making process, such as weighing the pros and cons and potential outcomes, whereas an informed choice refers to the behavioural outcome of an informed decision.47 Broader definitions of informed decision making have also been used in evaluating prostate cancer screening.50, 51 In population‐based preconception genetic carrier screening, however, further exploration is still needed to develop a consensus definition of informed choice and its evaluation.
Currently for MMIC analysis, there are no established methods or population data to guide us in our decisions to classify good knowledge. Given this, there are two general ways to dichotomize the knowledge scale: based on cut‐off points using the sample distribution of the scale as in the original MMIC or using a percentage of correct items, both of these are arbitrary and neither is ideal. Using the mean or median of the sample distribution, however, will result in a cut‐off, which is relative to the particular sample studied. Instead, we decided that to have good knowledge a score of >70% is necessary. In line with our previous study40 and after excluding one item from the scale (item 3), a score of 6/9 was considered good knowledge. We recognize the level of informed choice is very sensitive to how good knowledge is defined in the analysis. Our exploration of this sensitivity demonstrated that the level of informed choice changed from 32% when the score of 5 of 9 was used to define good knowledge to 7% when the score of 9 of 9 was used to define good knowledge. This change of sensitivity has also been demonstrated in another study where the levels of informed choice were 68 and 91% when using cut‐off points of 6 of 7 and 5 of 7, respectively, to define good knowledge.19 Dichotomizing a continuous knowledge scale can be problematic,52 and defining ‘good’ knowledge is a complex issue, which was not possible to fully resolve in this study.
There are some limitations and biases, which may have influenced this work. First, the study was not designed with the primary aim of establishing the criterion, predictive validity or stability of the knowledge scale items, and therefore not all methods of evaluating validity and reliability were available. Selection of the knowledge questions, and thus what constitutes necessary knowledge for making a decision about FXS carrier screening is a complex task. A number of topics were included in the information brochure but were not necessarily measured in the knowledge scale. Members of the expert panel involved in developing the questions deemed that it was more important to focus on concepts rather than specific numeric details as it was thought that such questions could be too difficult, for example, the brochure contained the carrier frequency but a question asking for the exact value was not included in the knowledge scale. In contrast, a pictorial representation of the different triplet repeat results (short, intermediate, medium and long) without specific repeat numbers were shown in the brochure,40 and this wording was reflected in the questions relating to the implications for the individual's own health and reproductive risk in the scale. Ascertaining the level of information and understanding what women perceive as important, in addition to information that health professionals perceive as important, would be valuable in further developing the knowledge scale. For example, the former issue could be addressed in future research by asking women to reflect on the information provided and explore their information preferences. Lastly, there is the potential for selection bias. Our results may not truly represent those of the general population as women with very negative feelings towards genetic testing may have not participated in the study. Research is also needed to establish whether the MMIC does in fact accurately measure whether women make an informed choice. For example, a prospective study using qualitative methods to explore in‐depth the way in which women make decisions about preconception population carrier screening could inform the development of an evaluation tool that captures the complexities of informed choice which are not currently addressed in the measures available.
Conclusion
We have developed and tested a knowledge scale specific to FXS carrier screening as part of adapting the MMIC. We have good evidence that the knowledge scale is robust, while acknowledging the methodological difficulty of deciding what questions to ask, as well as defining ‘good’ knowledge as a dichotomous variable for the MMIC analysis. We have also suggested that differences between the prenatal and preconception population carrier screening settings may have affected the construct of value consistency, a key component of the MMIC. The MMIC lacks sensitivity and is therefore limited in this setting as there are other factors that contribute to making informed choices not directly measured in the model. We suggest further exploration of the utility of the MMIC in different decision‐making contexts, likely requiring inclusion of measures to capture other factors of decision making, for example, relevance to life stage. The methods described in this article can help other researchers to develop their own knowledge scales for use in the MMIC, and to further develop tools to evaluate informed choice in screening programmes. Population carrier screening aims to facilitate and encourage informed choices, and as genetic screening is offered more widely, the development of such measures are crucial in evaluating informed choice.
Sources of funding
Alice Ames is funded by an Australian Postgraduate Award. This research was supported by the Murdoch Childrens Research Institute, NHMRC Project Grant 607320 and Victorian Government's Operational Infrastructure Support Programme. Rony E Duncan is supported through a fellowship from the Invergowrie Foundation.
Conflict of interest
None to declare.
Acknowledgements
We would like to thank the study team who worked on this pilot study, in particular to Anna Flouris for her work in developing the questionnaire, Erica Brown and Vicky Reddick for their help in recruiting participants. We would also like to thank Melissa Hill for her assistance and supervision.
References
- 1. Godard B, ten Kate L, Evers‐Kiebooms G, Ayme S. Population genetic screening programmes: principles, techniques, practices, and policies. European Journal of Human Genetics, 2003; 11: S49–S87. [DOI] [PubMed] [Google Scholar]
- 2. O'Connor AM, O'Brien‐Pallas L. Decisional conflict (Specify) In: McFarland GK, McFarlane EA. (eds) Nursing Diagnosis & Intervention: Planning for Patient Care, 2nd edn St. Louis, Missouri: Mosby Inc, 1993: 468–478. [Google Scholar]
- 3. Beauchamp TL, Childress JF. Principles of Biomedical Ethics, 3rd edn New York, NY: Oxford University Press, 1989. [Google Scholar]
- 4. Marteau TM, Dormandy E, Michie S. A measure of informed choice. Health Expectations, 2001; 4: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van den Berg M, Timmermans DRM, Ten Kate LP, van Vugt JMG, van der Wal G. Are pregnant women making informed choices about prenatal screening? Genetics in Medicine, 2005; 7: 332–338. [DOI] [PubMed] [Google Scholar]
- 6. Bekker H, Thornton JG, Airey CM et al Informed decision making: an annotated bibliography and systematic review. Health Technology Assessment (Winchester, England), 1999; 3: 1–156. [PubMed] [Google Scholar]
- 7. Irwig L, McCaffery K, Salkeld G, Bossuyt P. Informed choice for screening: implications for evaluation. British Medical Journal, 2006; 332: 1148–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jepson RG, Hewison J, Thompson AGH, Weller D. How should we measure informed choice? The case of cancer screening. Journal of Medical Ethics, 2005; 31: 192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dormandy E, Michie S, Hooper R, Marteau TM. Informed choice in antenatal Down syndrome screening: a cluster‐randomised trial of combined versus separate visit testing. Patient Education & Counseling, 2006; 61: 56–64. [DOI] [PubMed] [Google Scholar]
- 10. Michie S, Dormandy E, Marteau TM. The multi‐dimensional measure of informed choice: a validation study. Patient Education & Counseling, 2002; 48: 87–91. [DOI] [PubMed] [Google Scholar]
- 11. Brown K, Dormandy E, Reid E, Gulliford M, Marteau T. Impact on informed choice of offering antenatal sickle cell and thalassaemia screening in primary care: a randomized trial. Journal of Medical Screening, 2011; 18: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dormandy E, Tsui EYL, Marteau TM. Development of a measure of informed choice suitable for use in low literacy populations. Patient Education & Counseling, 2007; 66: 278–295. [DOI] [PubMed] [Google Scholar]
- 13. Dormandy E, Bryan S, Gulliford MC et al Antenatal screening for haemoglobinopathies in primary care: a cohort study and cluster randomised trial to inform a simulation model. The Screening for Haemoglobinopathies in First Trimester (SHIFT) trial. Health Technology Assessment, 2010; 14: 1–160. [DOI] [PubMed] [Google Scholar]
- 14. Smith SK, Trevena L, Simpson JM, Barratt A, Nutbeam D, McCaffery KJ. A decision aid to support informed choices about bowel cancer screening among adults with low education: randomised controlled trial. British Medical Journal, 2010; 341: c5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steckelberg A, Hülfenhaus C, Haastert B, Mühlhauser I. Effect of evidence based risk information on “informed choice” in colorectal cancer screening: randomised controlled trial. British Medical Journal, 2011; 342: d3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van den Bergh KAM, Essink‐Bot ML, van Klaveren RJ, de Koning HJ. Informed participation in a randomised controlled trial of computed tomography screening for lung cancer. European Respiratory Journal, 2009; 34: 711–720. [DOI] [PubMed] [Google Scholar]
- 17. Mullen PD, Allen JD, Glanz K et al Measures used in studies of informed decision making about cancer screening: a systematic review. Annals of Behavioral Medicine, 2006; 32: 188–201. [DOI] [PubMed] [Google Scholar]
- 18. Evans R, Joseph‐Williams N, Edwards A et al Supporting informed decision making for Prostate Specific Antigen (PSA) testing on the web: an online randomized controlled trial. Journal of Medical Internet Research, 2010; 12: e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Korfage IJ, van Ballegooijen M, Wauben B, Habbema JDF, Essink‐Bot M‐L. Informed choice on Pap smear still limited by lack of knowledge on the meaning of false‐positive or false‐negative test results. Patient Education and Counseling, 2011; 85: 214–218. [DOI] [PubMed] [Google Scholar]
- 20. World Health Organisation . Proposed International Guidelines on Ethical Issues in Medical Genetics and Genetic Services. Geneva: World Health Organisation, 1998. [Google Scholar]
- 21. Khoury MJ, McCabe LL, McCabe ERB. Genomic medicine – population screening in the age of genomic medicine. New England Journal of Medicine, 2003; 348: 50–58. [DOI] [PubMed] [Google Scholar]
- 22. Gourounti K, Sandall J. Do pregnant women in Greece make informed choices about antenatal screening for Down's syndrome? A questionnaire survey. Midwifery, 2008; 24: 153–162. [DOI] [PubMed] [Google Scholar]
- 23. Jaques AM, Sheffield LJ, Halliday JL. Informed choice in women attending private clinics to undergo first‐trimester screening for Down syndrome. Prenatal Diagnosis, 2005; 25: 656–664. [DOI] [PubMed] [Google Scholar]
- 24. Rowe HJ, Fisher JRW, Quinlivan JA. Are pregnant Australian women well informed about prenatal genetic screening? A systematic investigation using the multidimensional measure of informed choice [see comment]. Australian & New Zealand Journal of Obstetrics & Gynaecology, 2006; 46: 433–439. [DOI] [PubMed] [Google Scholar]
- 25. Wynter KH, Rowe HJ, Fisher JR, Lee M, Quinlivan JA. Are adolescents' decisions about prenatal screening for Down syndrome informed? A controlled, prospective study. Journal of Pediatric and Adolescent Gynecology, 2011; 24: 29–34. [DOI] [PubMed] [Google Scholar]
- 26. Nagle C, Gunn J, Bell R et al Use of a decision aid for prenatal testing of fetal abnormalities to improve women's informed decision making: a cluster randomised controlled trial ISRCTN22532458. BJOG: An International Journal of Obstetrics and Gynaecology, 2008; 115: 339–347. [DOI] [PubMed] [Google Scholar]
- 27. Ajzen I. The theory of planned behavior. Organizational behavior and human decision processes, 1991; 50: 179–211. [Google Scholar]
- 28. Gourounti K, Sandal J. The validation and translation of multidimensional measure of informed choice in Greek. Midwifery, 2011; 27: 170–173. [DOI] [PubMed] [Google Scholar]
- 29. Wakefield CE, Meiser B, Homewood J et al A randomized controlled trial of a decision aid for women considering genetic testing for breast and ovarian cancer risk. Breast Cancer Research and Treatment, 2008; 107: 289–301. [DOI] [PubMed] [Google Scholar]
- 30. Wakefield CE, Meiser B, Homewood J et al Randomized trial of a decision aid for individuals considering genetic testing for hereditary nonpolyposis colorectal cancer risk. Cancer, 2008; 113: 956–965. [DOI] [PubMed] [Google Scholar]
- 31. Wakefield CF, Meiser B, Homewood J et al A randomized trial of a breast/ovarian cancer genetic testing decision aid used as a communication aid during genetic counseling. Psycho‐oncology, 2008; 17: 844–854. [DOI] [PubMed] [Google Scholar]
- 32. Fu YH, Kuhl DP, Pizzuti A et al Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell, 1991; 67: 1047–1058. [DOI] [PubMed] [Google Scholar]
- 33. Verkerk AJ, Pieretti M, Sutcliffe JS et al Identification of a gene (FMR‐1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell, 1991; 65: 905–914. [DOI] [PubMed] [Google Scholar]
- 34. Kronquist K, Sherman S, Spector E. Clinical significance of tri‐nucleotide repeats in Fragile X testing: a clarification of American College of Medical Genetics guidelines. Genetics in Medicine, 2008; 10: 845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nolin SL, Lewis FA III, Ye LL et al Familial transmission of the FMR1 CGG repeat. American Journal of Human Genetics, 1996; 59: 1252–1261. [PMC free article] [PubMed] [Google Scholar]
- 36. Berkenstadt M, Ries‐Levavi L, Cuckle H, Peleg L, Barkai G. Preconceptional and prenatal screening for fragile X syndrome: experience with 40,000 tests. Prenatal Diagnosis, 2007; 27: 991–994. [DOI] [PubMed] [Google Scholar]
- 37. McConkie‐Rosell A, Finucane B, Cronister A, Abrams L, Bennett RL, Pettersen BJ. Genetic counseling for fragile X syndrome: updated recommendations of the National Society of Genetic Counselors. Journal of Genetic Counseling, 2005; 14: 249–270. [DOI] [PubMed] [Google Scholar]
- 38. Allingham‐Hawkins DJ, Babul‐Hirji R, Chitayat D et al Fragile X premutation is a significant risk factor for premature ovarian failure: the International Collaborative POF in fragile X study–preliminary data. American Journal of Medical Genetics, 1999; 83: 322–325. [PMC free article] [PubMed] [Google Scholar]
- 39. Hill MK, Archibald AD, Couns GDG, Cohen J, Metcalfe SA. A systematic review of population screening for fragile X syndrome. Genetics in Medicine, 2010; 12: 396–410. [DOI] [PubMed] [Google Scholar]
- 40. Metcalfe S, Jacques A, Archibald A et al A model for offering carrier screening for fragile X syndrome to nonpregnant women: results from a pilot study. Genetics in Medicine, 2008; 10: 525–535. [DOI] [PubMed] [Google Scholar]
- 41. Archibald AD, Jaques AM, Wake S, Collins VR, Cohen J, Metcalfe SA. “It's something I need to consider”: decisions about carrier screening for fragile X syndrome in a population of non‐pregnant women. American Journal of Medical Genetics. Part A, 2009; 149A: 2731–2738. [DOI] [PubMed] [Google Scholar]
- 42. Flouris A, Hawthorne G, Aitken MA, Gaff C, Metcalfe SA. Development of a questionnaire for evaluating genetics education in general practice. Journal of Community Genetics, 2010; 1: 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marteau TM, Bekker H. The development of a six‐item short‐form of the state scale of the Spielberger State‐Trait Anxiety Inventory (STAI). British Journal of Clinical Psychology, 1992; 31: 301–306. [DOI] [PubMed] [Google Scholar]
- 44. Streiner DL, Norman GR. Health Measurement Scales: A Practical Guide to Their Development and Use, 2nd edn Oxford; New York: Oxford University Press, 1995. [Google Scholar]
- 45. Pett MA, Lackey NR, Sullivan JJ. Making Sense of Factor Analysis: The Use of Factor Analysis for Instrument Development in Health Care Research. Thousand Oaks, CA: Sage Publications Inc, 2003; 3–5: 141–143. [Google Scholar]
- 46. StataCorp LP . Stata Statistical Software. College Station. 12.0 ed. Texas: StataCorp LP, 2011. [Google Scholar]
- 47. van den Berg M, Timmermans DRM, ten Kate LP, van Vugt JMG, van der Wal G. Informed decision making in the context of prenatal screening. Patient Education and Counseling, 2006; 63: 110–117. [DOI] [PubMed] [Google Scholar]
- 48. Archibald AD, McClaren BJ. Perceived relevance of genetic carrier screening: observations of the role of health‐related life experiences and stage of life in decision making. Journal of Community Genetics, 2012; 3: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bryant LD, Green JM, Hewison J. The role of attitudes towards the targets of behaviour in predicting and informing prenatal testing choices. Psychology & Health, 2010; 25: 1175–1194. [DOI] [PubMed] [Google Scholar]
- 50. McCormack L, Treiman K, Bann C et al Translating medical evidence to promote informed health care decisions. Health Services Research, 2011; 46: 1200–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Allen JD, Othus MKD, Hart A, Mohllajee AP, Li Y, Bowen D. Do men make informed decisions about prostate cancer screening? Baseline results from the “Take the Wheel” trial. Medical Decision Making, 2011; 31: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dawson NV, Weiss R. Dichotomizing continuous variables in statistical analysis. Medical Decision Making, 2012; 32: 225–226. [DOI] [PubMed] [Google Scholar]


