Abstract
Context
Treatment burden, the burden associated with the treatment and management of chronic illness, has not yet been well articulated.
Objective
Using Rodgers' (1989, Journal of Advanced Nursing, 14, 330–335) method of concept analysis, this review describes the ways in which treatment burden has been conceptualized to define the concept and to develop a framework for understanding its attributes, antecedents and consequences.
Methods
Leading databases were searched electronically between the years 2002 and 2011. To ensure the review focused on actual observations of the concept of interest, articles that did not measure treatment burden (either qualitatively or quantitatively) were excluded. An inductive approach was used to identify themes related to the concept of treatment burden.
Main results
Thirty articles, identified from 1557 abstracts, were included in the review. The attributes of treatment burden include burden as a dynamic process, as a multidimensional concept, and comprising of both subjective and objective elements. Prominent predisposing factors (antecedents) include the person's age and gender, their family circumstances, possible comorbidity, high use of medications, characteristics of treatment and their relationship with their health‐care provider. The most dominant consequences are poor health and well‐being, non‐adherence to treatment, ineffective resource use and burden on significant others. Furthermore, many of these consequences can also become antecedents, reflecting the cyclic and dynamic nature of treatment burden.
Conclusion
The findings underscore the need for researchers and health‐care professionals to engage in collaborative discussions and make cooperative efforts to help alleviate treatment burden and tailor treatment regimens to the realities of people's daily lives.
Keywords: chronic illness, concept analysis, health professional, medication burden, treatment burden
Introduction
Chronic diseases are the leading cause of death in the world, largely associated with 63% of the 57 million deaths that occurred in 2008.1 The majority of these deaths are attributed to cardiovascular diseases, cancers, chronic respiratory diseases and diabetes.1 Although the burden associated with chronic illness is well documented, the burden associated with the treatment and management of chronic illness has not been well defined. Related terms such as disease burden and symptom burden have been well articulated,2, 3 but the definition of treatment burden has remained elusive and confusing. Although treatment burden is often inseparable from disease burden, it is not based on the natural history of the disease, but on the need to treat the disease in order to change its course or ameliorate its effects. Treatment burden is, therefore, an important concept that is distinct from disease burden, symptom burden and other related terms.
Treatment of chronic illness comes in many forms including surgery, physical therapy, psychological therapy and radiotherapy. However, one of the most common treatment forms is the use of medication. In Australia, as in many developed nations, the use of medications represents one of the largest components of health expenditure; accounting for 13% of the total health expenditure in 2006–07.4 There were 262 million prescriptions filled in 2008,4 many of which were used to treat chronic illness. The prevention and treatment of chronic illness, especially when involving multiple medications, can become burdensome.
Few validated instruments have been developed to assess the experiences of treatment burden on patients.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 In some cases however, the concept of treatment burden has been included as one domain within a multidimensional instrument designed to assess health‐related quality of life or treatment satisfaction.5, 6, 7, 8, 9, 10, 11, 12, 17, 18 These measures reflect wide variation in terms of the dimensions of treatment burden and its definition. One team of researchers explored the experience of treatment burden with the purpose of identifying its core components.19 These researchers identified four main tasks that contributed to the sense of burden, namely learning about treatments and their consequences, engaging with others and mobilizing support, adhering to treatment and lifestyle changes and monitoring treatments. Although the most useful conceptualization of treatment burden in the literature, this analysis was not intended to provide a concept analysis. Instead, it was focused on the utility of an existing theoretical framework as a tool for identifying burden. Thus, it is important to conduct further empirical investigation of this concept to enhance our knowledge and draw coherent conclusions about its prevalence and impact.
The lack of a clear conceptual model of treatment burden has contributed to our inability to measure its impact or identify people most at risk, thereby obscuring the health professional's role in assisting people to alleviate this burden. It has generated confusion and misinterpretation that detracts from appropriate and timely management or prevention. A crucial first step in assessing treatment burden and articulating the role of health professionals is defining the concept itself and developing a framework for understanding its occurrence and impact.
The purpose of this review is to comprehensively analyse the concept of treatment burden to enable informed recommendations for health professionals who wish to alleviate its impact. This analysis aims to outline the ways in which treatment burden has been conceptualized and operationalized, by identifying and discussing the critical attributes of treatment burden, exploring the factors that can lead to treatment burden (antecedents) and highlighting its consequences. Antecedents are predisposing events that occur prior to the concept, whereas consequences are events that occur as a result of the concept.20 Furthermore, attributes are at the heart of a concept, providing insight into its occurrence.20 A thorough knowledge of the attributes, antecedents and consequences of treatment burden is important from a health practice perspective because without such knowledge, health professionals will not be able to provide services that alleviate such burden among people with chronic illness.
Methods
Rodgers'21 evolutionary method of concept analysis was used to comprehensively analyse the concept of treatment burden. This particular method is well suited to the concept of treatment burden because of its changing and dynamic nature (e.g. the emergence of new health technologies leading to possible burden). The evolutionary view of concept analysis indicates that concepts are influenced by contextual factors and may change over time.22
Data sources and search strategy
A systematic search was conducted using the terms ‘treatment burden’, ‘burden of treatment’, ‘medication burden’ and ‘burden of medication’ as keywords in the following databases: Medline, PsychINFO, Cinahl, Cochrane, Scopus, Health Reference Centre (HRC), PsychEXTRA, Informit, the System for Information on Grey Literature in Europe (SIGLE) and National Technical Information Service USA (NTIS). A sensitivity analysis conducted prior to the search suggested that these key search terms encompassed most of the research within the field. In particular, the sensitivity analysis confirmed that medication was the most prominent form of treatment for chronic illness, necessitating the inclusion of this search term. Although the use of broader terminology may have identified other bodies of literature, restricting the search to these specific terms ensured that the overlap with other forms of burden was minimized.
To ensure a contemporary exploration of this concept, the search was limited to articles published from 2002 to 2011 with human subjects and a focus on the major chronic illnesses that have been named as priorities in Australia: asthma, diabetes, cardiovascular disease, musculoskeletal illness, cancer and mental health. According to the World Health Organization, these conditions contribute a significant burden in terms of mortality and/or morbidity globally.1 After this search, 1157 abstracts were identified.
These abstracts were reviewed by two members of the research team. Abstracts without a substantial focus on treatment burden were excluded, along with those that emphasized disease or symptom burden. If both researchers were uncertain about whether the abstract met the inclusion criteria or were not in agreement, the full article was retrieved and reviewed. As a result of this process, 170 articles were thoroughly reviewed by two researchers, leading to the exclusion of a further 140 articles (Fig. 1). Further exclusions were applied to ensure the review focused on articles that actually measured a person's experience of treatment burden. Specifically, articles that did not measure treatment burden (either qualitatively or quantitatively) were excluded (i.e. opinion pieces or theoretical articles). Thirty articles met the inclusion criteria and were included in the concept analysis (Table 1). Most of these studies were conducted in the United States of America (USA) and included self‐report survey questionnaires to assess the level of treatment burden among patients and their carers. A number of inter‐related themes were identified from the review relating to the antecedents, attributes and consequences of treatment burden.
Figure 1.
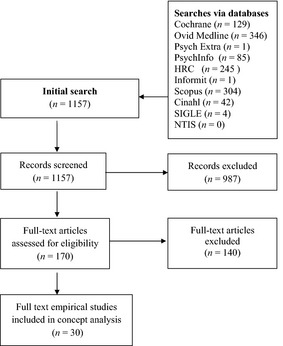
Selection process for concept analysis
Table 1.
Articles included in the concept analysis
| Reference | Country | Participants | Data collection methods |
|---|---|---|---|
| Anderson et al.5 | USA | n = 170 and n = 402, people with diabetes | Mixed methods |
| Fried et al.51 | USA | n = 125, 65 years or older with a limited life expectancy | Quantitative survey |
| Kim et al.27 | USA | n = 1083, male and female with schizophrenia | Quantitative survey |
| Brod et al.8 | Multination | n = 17,488 with type 2 diabetes | Quantitative survey |
| Gallacher et al.19 | UK | n = 47 patients with chronic heart failure | Qualitative interview |
| Henry et al.40 | USA | n = 15,532 people with cancer | Quantitative survey |
| Campbell et al.9 | UK | n = 19 with asthma for focus groups, n = 131 for questionnaire | Mixed methods |
| Vijan et al.37 | USA | n = 1,653 Veteran patients with type 2 diabetes | Quantitative survey |
| Benner et al.45 | USA | n = 5759 patients with initiated antihypertensive lipid‐lowering therapy | Quantitative survey |
| Bernhard et al.13 | Switzerland/Italy | n = 249 patients in a trial for the prophylaxis for delayed emesis | Quantitative survey and diary cards |
| Ribi et al.16 | Switzerland | n = 373 women with early or advanced breast cancer | Quantitative survey |
| Yoon et al.47 | USA | n = 1,219 women with breast cancer | Quantitative survey |
| Ow33 | Singapore | n = 22 parents from 20 families with children with childhood cancer | Quantitative survey |
| Zucca et al.35 | Australia | n = 1410 adults with cancer | Quantitative survey |
| Graves et al.24 | USA | n = 101 primary caregivers of children with asthma | Quantitative survey |
| Olinder et al.48 | Sweden | n = 90 adolescents aged 12–18 years with diabetes | Diary records and survey |
| Haugstvedt et al.41 | Norway | n = 103 (mothers) and n = 97 (fathers) of 115 children with type 1 diabetes | Quantitative survey and medical records |
| Thomas31 | USA/France | n = 1500 patients with schizophrenia (Study 1) and n = 284 (Study 2) | Quantitative survey |
| Wysoci & Gavin44 | USA | n = 190 adult couple caregivers of a child with a chronic condition | Quantitative survey |
| Martire et al.42 | USA | n = 244 dyads with a major depressive disorder | Quantitative survey |
| Longo et al.34 | Canada | n = 282 patients with cancer | Quantitative survey |
| Rodbard et al.26 | USA | n = 3551 individuals with type 2 diabetes mellitus | Quantitative survey |
| Brod et al.23 | Australia/NZ | n = 299 male or female with type 1 diabetes | Quantitative survey |
| Fiese et al.25 | USA | n = 153 families of a child with asthma. | Quantitative survey |
| Nicholl et al.36 | USA | n = 970 with recent schizophrenia and 2996 with ongoing schizophrenia. | Medical claims |
| Tija et al.32 | USA | n = 457 women (60–65 years) eligible for breast cancer chemotherapy | Quantitative survey |
| de Kraker et al.43 | Multination | n = 410 patients between ages 6 months and 18 years with Wilms' tumour | Medical outcomes |
| Gutierrez‐Maldonado et al.28 | Chile | n = 45 caregivers of people with schizophrenia | Quantitative survey |
| Moss & Crane29 | USA | n = 86 older women with post‐myocardial infarction | Quantitative survey |
| Ziaian et al.38 | Australia | n = 160 children aged 10–16 years with a chronic illness | Quantitative survey |
Data extraction and analysis
An initial coding framework was developed based on the questions in Table 2. These questions reflected Rodgers' method of concept analysis, which involves a multidisciplinary literature review to identify the common attributes, antecedents and consequences of the concept.3
Table 2.
Review questions for data extraction
| Number | Review questions |
|---|---|
| 1 | What are the key attributes of the concept? |
| 2 | Which factors (antecedents) are proposed to precede treatment burden? |
| 3 | What are the consequences of treatment burden? |
Five researchers independently extracted data from the selected articles in accordance with the review questions in Table 2 and coded the data within the initial framework, expanding the subthemes as required. Each coding framework was then reviewed by two researchers for recurring themes, which were placed into categories (attributes, antecedents, and consequences).
Results
Attributes of treatment burden
The defining attributes of treatment burden were chosen due to their frequent occurrence in the literature. Treatment burden emerged as a dynamic multidimensional concept that comprised of both subjective and objective elements.
A dynamic process
Treatment burden changed over time23 in response to disease severity and control24, 25 and the development of comorbidities.26, 27 It is possible that that either familiarity with, or acceptance of, treatment lessens the experience of burden through a process of adjustment. In patients with diabetes, longer duration of disease was associated with less burden.8 Despite changing over time, some degree of burden appeared to persist.28 Treatment burden also had a cyclic aspect, with many of the consequences of treatment burden also being antecedents. For example, increased burden could result in non‐adherence to medication which may then lead to further burden.29
A multidimensional concept
Treatment burden emerged as a multidimensional concept, including physical, financial, temporal, and psychosocial time demands.
Physical side‐effects30 were a significant source of treatment burden,13, 24, 29, 31 arising in particular from medications or drug interactions.29 Side‐effects could occur at any stage of treatment. For example, they could be more acute at the commencement of therapy, they could be ongoing or they may result from cumulative toxicity in the later stages of treatment.29 Side‐effects varied in nature and severity, from minor hypoglycaemic events and weight gain,8 to those that are so unpleasant that the possibility of experiencing them frightened some patients.8 Experiencing more than one side‐effect contributed to overall treatment burden.13 Some side‐effects may be preventable especially those related to the use of medications that were no longer required.32
The cost of treatment could be high,24 contributing to overall treatment burden.28, 29, 33 Even when treatment was subsidized, the out‐of‐pocket costs could be intolerable.34 The cost of travel was one of the greatest out‐of‐pocket expenses, especially for patients from non‐metropolitan areas,35 although this was often obscured by the rhetoric of ‘free’ hospital care. Inpatient treatment was also associated with financial burden.36 Personal medical costs competed with other living expenses, such as, food, clothing and housing.26
The time required to plan and organize travel for treatment,37 receive treatment,19, 35, 38 learn about treatments and their potential outcomes,19 monitor treatment37, 39 and manage side‐effects40 was related to treatment burden. As well as consuming financial resources, travel required a great deal of time, especially for patients who lived in outer regional areas.35 A substantial time burden for family and carers as well as for patients themselves was evident.40 One study reported families of children with chronic illnesses may already be under considerable time pressure, and the increased use of long‐term home‐based care for these children adds to this burden over time.38
The psychological and social aspects of treatment burden were closely linked, often because additional support is needed to manage treatment28 or day‐to‐day tasks or both. Some patients had difficulties managing home responsibilities as a consequence of treatment,33, 35, 40 and often, household and personal tasks, normally completed by the patient, were carried out by other family members.34 Parents needed to accompany, administer medication to and monitor a child receiving treatment. However, many families seemed to adapt well to treatment tasks.41 When children attended school, the burden of administering medications was likely to be borne by the child or a staff member, which may explain why some children experience more burden than their parents.38 Older adults generally relied on family for assistance and support.42 Family members or carers also at times needed to accompany or support patients receiving invasive or lengthy treatments such as cancer treatment.40 The impact of treatment on significant others added to the burden experienced by the patient.43 Family members could perceive themselves as being incompetent or not able to care for the patient due to a lack of personal or economic resources.28 These perceptions could also add to the patient's sense of burden.28 Thus, there is a need for adequate information to be provided by health‐care professionals about treatment and its consequences in order to address these perceptions.33
Treatment tasks that interfered with daily activities24 or lifestyle5, 41, 43also contributed to treatment burden.36, 41 For instance, it was inconvenient to transport treatment equipment or medications (e.g. insulin injections or metered dose inhalers) and embarrassing to use (medication) in public.9 More invasive treatments such as dialysis were even more inconvenient and restrictive.5, 9 Interestingly, new technologies (e.g. new forms of blood glucose monitoring) did not emerge as a significant source of distress or burden,44 but this area requires further investigation.
Both subjective and objective burden
Some elements of treatment burden such as the number of medications,19, 24, 29, 32, 36, 45, 46 and time to administer or monitor treatment,19 could be measured objectively. Patients and carers, however, were not homogenous and therefore could have different perceptions concerning the burden related to similar tasks.38 This subjective aspect of treatment burden was associated with a number of factors13, 24, 43 and therefore may be predictable. However, as it includes elements of an intangible nature, for example, guilt, hopelessness, and fear,33 its quantification could be difficult. Subjective aspects, such as fear of medication supply running out, the meaning attributed to side‐effects,16 beliefs about a medication's effectiveness31, 32 and beliefs about the impact of medication on health and well‐being (e.g. believing that medication is harmful and addictive), were associated with increased levels of treatment burden.9
Antecedents of treatment burden
Although a large number of antecedents of treatment burden were identified, there was minimal information about their particular influence on treatment burden, reflecting the lack of theoretical development within the research field. Antecedents were associated with characteristics of the patient, the disease, the treatment, the family or support network and the health‐care system.
Patient characteristics
Gender seemed to be a key antecedent of burden because men and women experienced treatment burden differently. Women experienced more treatment burden than men and also reported more caregiver burden when their children were sick, possibly as a result of their traditional homemaker roles.13, 40, 41 A strong positive correlation was apparent between unemployment and treatment burden.35, 40 A strong relationship between age and treatment burden also emerged.16, 40, 47 Elderly people seemed to experience more treatment burden than young people, as might be expected given the likelihood of illness and multiple conditions.
Disease conditions
As expected, comorbidity was associated with increased burden.26 The presence of particular comorbidities,27, 36 especially psychological illnesses such as anxiety or depression,40 was associated with high levels of treatment burden.28, 36, 37 Particular chronic conditions such as diabetes37 and schizophrenia36 were associated with greater levels of treatment burden. Finally, functional capacity, poor symptom control24 and longer duration of illness8 could also lead to treatment burden.39
Treatment characteristics
Treatment characteristics, particularly medications,8, 13, 31 were an important antecedent of burden. Using a high number of medications19, 24, 29, 32, 45, 46 emerged as the most common antecedent in the literature. Particular dosage forms (e.g. injections as opposed to oral tablets) were also considered to be burdensome. Finally, changes to medication regimens were also a key antecedent of burden and have been attributed to a lack of continuity of health care.19
Family support and engagement
Availability of extended family networks and support from an appropriate social network could lead to lower treatment burden.33 However, the support and assistance provided by a caregiver could also result in treatment burden for both the patient and the carer.28 One way of reducing burden, particularly for carers, was to introduce an intervention designed to support family members to understand, communicate and participate in treatment decisions.28
Health‐care systems
An aspect of health care that emerged frequently was the health practitioner–patient relationship.29, 37 Failure of health‐care practitioners to provide adequate information regarding treatment was associated with treatment burden.37 Poor communication between patients and health‐care providers about medication adherence was likely to result in the use of multiple medications (polypharmacy), which was associated with treatment burden.29 The location of the health‐care centre also emerged as an antecedent of financial and time burden, caused by long travel distances.35 This issue was further complicated by a lack of financial reimbursement for travel of this kind.35
Consequences of treatment burden
The concept analysis identified a number of consequences of treatment burden including poor adherence, reduced health and well‐being, ineffective use of health resources, reduced employment and low productivity, and negative health impacts on family and carers.
Adherence
One of the most widely cited consequences of treatment burden was non‐adherence to treatment. Non‐adherence19, 27, 37, 48 was then related to sub‐optimal health outcomes,29, 37 including disease relapse,31, 36 decreased quality of life24 and the unscheduled use of more expensive health‐care resources, such as increased emergency department visits and hospitalization.31 Non‐adherence was also associated with increased school absences.24
Non‐adherence was most often linked to treatment burden resulting from medication characteristics, including the number of medications, their frequency of administration,45 side‐effects24 and perceived lack of efficacy.24 As the number of medications being used increased, the rate of non‐adherence associated with the addition of each additional medication decreased.45 Hence, there appeared to be a threshold of treatment burden where additional medications did not add further burden. However, the addition of each additional medication did nevertheless add to the financial cost of treatment.27 This was a concerning finding given that the elderly and welfare recipients were found to reduce their use of medications in response to the introduction of prescription co‐payments and subsequently experienced an increase in serious adverse events.34
Health and well‐being
The health and well‐being consequences of treatment burden were many and varied. Treatment burden affected patient choices about treatment,37 with some patients who were recommended insulin refusing this therapy.37 Such a choice was associated with poor glycemic control.37 However, opting for a less‐efficacious alternative treatment could be a reasonable course of action given that ‘…difficult or demanding treatment regimens may appreciably lower treatment effectiveness, which may be possible to achieve with less burdensome treatment’.5:573 In other words, while a treatment might appear to have superior efficacy in the controlled setting of a clinical trial, in reality, the interaction between treatment regimen and treatment burden could create difficulties. Thus, patients may choose to select a less effective, but less burdensome treatment to suit their daily lives, which ultimately may result in better health outcomes.
Treatment burden was associated with a number of negative health outcomes including specific symptoms,16, 29 recurrence of disease,28, 36, 43 decline in health,29, 36 reduced survival,43 decreased treatment satisfaction23 and reduced quality of life.25, 40 Treatment‐related side‐effects were often found to have a marked impact on quality of life,16 which was also affected by perceived treatment burden, disease severity25 and disruption of lifestyle.48 Finally, as well as increasing the risk of adverse outcomes, which was mediated by non‐adherence, greater prescription co‐payment burden was associated with increased self‐reported psychological distress and attempted suicide in patients with schizophrenia.27
Resource use
Ineffective use of resources has been attributed to treatment burden.19 Unfortunately, a reduction in scheduled care (i.e. non‐adherence) may result in a demand for unscheduled care (i.e. hospital admission), creating avoidable resource use.24, 31 Perceived financial burden caused by prescription co‐payments resulted in the increased self‐reported use of unscheduled care, such as emergency room visits and hospitalization.27 Polypharmacy complicated therapy and health‐care delivery,29 which added to unnecessary use of resources. Moreover, polypharmacy was accompanied by an increased risk that medications included in the treatment regimen were unnecessary and therefore a waste of resources.32
Employment
The burden of treatment had a marked impact on the patient's ability to attend work and maintain productivity. In a cancer clinic, patients who were still employed were absent for an average of 12.6 days during the month.34 Absences from work were related to prescription co‐payment burden,27 the need to travel35 and side‐effects,34, 40 including fatigue associated with chemotherapy. The latter resulted in the loss of 4.2 sick or vacation days per month.40 In some instances, patients needed to change employment status in order to manage treatment burden.34, 40 Caregivers also needed to take time off work to care for cancer patients.34 Unfortunately, work absences could lead to feelings of guilt among patients about burdening their co‐workers and lost productivity,40 which added to burden.
Family and carers
Treatment burden was related to carer burden and fatigue,43 causing patients to forgo caregiver support.34 The distress caused by treatment burden was found to flow in both directions in that seeing a significant other (i.e. patient or carer) suffer could lead to further burden and distress,42 especially in the case of parents.25, 41 There was evidence that effective treatment of a loved one resulted in significant benefits for carers.42
Discussion
This concept analysis provides a much needed theoretical framework for understanding the dynamic, multidimensional and cyclic nature of treatment burden. We found that treatment burden is a dynamic process, evolving with the emergence of new treatment options and symptoms. It persisted over time, but perhaps reached a subjective threshold beyond which perceptions of burden no longer increase. The dimensions of treatment burden include undesirable physical effects of treatment (side‐effects), the economic burden imposed by treatment (financial burden), time required to obtain, administer and manage treatment (time burden), and the psychosocial aspects of burden including the impact on family and lifestyle (personal burden). Treatment burden has both subjective and objective elements including number of medications, time to administer and monitor treatment (objective) vs. feelings of guilt, hopelessness and fear relating to treatment (subjective). A range of antecedents and consequences were identified, although many of the consequences could also become antecedents, reflecting the cyclic nature of treatment burden. The attributes, antecedents and consequences emerging from the review of treatment burden are summarized in Fig. 2, which also highlights the cyclic nature of the concept. As an outcome of this concept analysis, we define treatment burden as a person's subjective and objective overall estimation of the dynamic and multidimensional burden that their treatment regimen for chronic illness has imposed on them and on their family members. It is influenced by a person's characteristics, disease duration/severity, treatment circumstances, level of family support and engagement and also the overall health‐care systems, in which the person obtains treatment.
Figure 2.
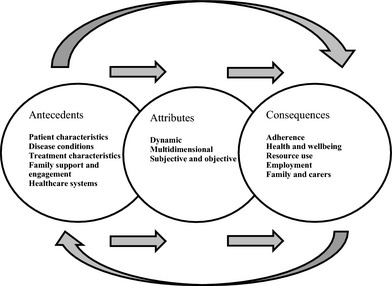
Attributes, antecedents and consequences of treatment burden.
The health consequences of treatment burden are particularly concerning given that treatment burden has been associated with specific symptoms, recurrence of disease, decline in health, reduced survival, decreased treatment satisfaction and reduced quality of life. There is a clear need to implement services that help alleviate the burden of treatment experienced by patients in order to improve their health and well‐being. Another alarming consequence of treatment burden, particularly from a public health perspective, was the ineffective use of resources. In an era of cost efficiency, poor use of health resources is particularly undesirable. By addressing poor adherence and consequently, treatment burden, it may be possible to identify ways of minimizing the use of more costly resources such as hospital admissions. The World Health Organization indicates that poor adherence to the treatment of chronic illness is a global problem averaging almost 50% in developed countries.49 Strategies that increase adherence are urgently needed if we are to optimize health outcomes. However, these strategies must take into account the subjective aspect of treatment burden and its important cyclic nature.
Research implications
The attributes, antecedents and consequences identified in this review also have clear implications for all health‐care professionals to alleviate the burden of treatment for patients. As the perception of treatment burden could be subjective, strategies to alleviate its impact need to be individualized, reflecting the individual's circumstances and preferences. This review has reinforced the fact that it is not only just health outcomes that are important for patients and their family, but also the way in which how health professionals achieve those outcomes for patients. Further, the dynamic nature of treatment burden means that for any one individual patient, their capacity to manage new and multiple treatments may vary over time. The complexity of this concept can best be managed through individualized and holistic care and ongoing evaluation that is responsive to the needs of each person.
The analysis has highlighted the fact that burden can also be a result of interactions with health‐care professionals. Poor health professional–patient relationships and a lack of adequate information regarding treatment were associated with high levels of treatment burden. As Moss and Crane29 argued, poor communication between patients and health‐care providers about medication use may result in the provision of multiple medications, which could then lead to treatment burden. Health‐care professionals need to develop a relationship that is sensitive to patient's preferences and offer explanations of treatment options that include their potential side‐effects. This type of relationship will enable patients to become more actively involved in decision making and integrate treatment with their daily lives, ultimately improving adherence and treatment outcomes.
Despite the variety of settings and methods used in the studies included in this concept analysis, treatment burden resulting from medication use emerged as a key theme. This finding is not surprising given that medication is one of the most common forms of treatment for chronic conditions. In Australia, reports indicate that Australians between the ages of 65 and 75 were taking an average of four medications in 2009 and will be taking, on average, six medications by 2019.50 The findings present clear opportunities for health professionals who are prescribing or dispensing medications to engage in greater discussions and improve medication management among patients. Community pharmacists, in particular, are accessible and well placed to support medication management, so that patients with chronic conditions receive the maximum benefit from their treatment.
Interestingly, the tasks of self‐management (e.g. organizing treatment, monitoring symptoms, changing lifestyle) were identified as a major source of treatment burden. Similar to the findings of Gallacher et al.,19 our study revealed a set of tasks associated with learning about treatments, engaging with and organizing the treatment, altering routines and monitoring symptoms and progress. It is ironic that these core tasks of self‐management represent a significant burden for patients despite being seen as a solution for the long‐term management of chronic illness in society. Gallacher et al.,19 were able to clearly distinguish between treatment burden and illness or disease burden, suggesting that our response to chronic illness generates a great deal of distress that is independent of that which might be experienced otherwise. It is not surprising that Gallacher et al. refer to treatment burden as the ‘work’ of chronic disease management. Our study has confirmed the important role this ‘work’ plays in generating a sense of burden. This review identified many negative consequences of treatment burden some of which may result in the continued escalation of burden over time because they also act as antecedents. Breaking this cycle is important, as is the identification of the factors that have the greatest impact on treatment burden and those that can be most easily modified. Clearly, health professionals have a major role alongside patients and their families in alleviating the burden associated with the treatment of chronic illness.
Research limitations
Like any research, this review also has limitations that must be considered. Only research published in the decade between 2002 and 2011 was included in the analysis. These dates were chosen because treatment burden is a relatively new and evolving concept and therefore research conducted prior to this date was deemed inappropriate for the purposes of this review. Furthermore, the articles used in the concept analysis focused on selected chronic illnesses known to be associated with high burden of disease. We acknowledge that there may be high levels of treatment burden associated with other ongoing health conditions. Given the importance of treatment burden for patients and their family and lack of clarification of the concept to date, the insights from this review provide a valuable foundation on which to further develop this concept.
Conclusion
Given the potential negative impacts of treating a chronic illness(s), researchers and health‐care professionals need to engage in collaborative discussions and make cooperative efforts to help alleviate treatment burden in order to optimize health outcomes. Continued research into treatment burden, its definition, assessment and impact, is needed to understand people's burden experience and implement treatment that suits the realities of daily life.
Sources of funding
This project is funded by the Australian Government Department of Health and Ageing as part of the Fifth Community Pharmacy Agreement Research and Development Program managed by The Pharmacy Guild of Australia. Jennifer A. Whitty is supported by a research fellowship from the Queensland Government Department of Employment, Economic Development and Innovation, Queensland Health and Griffith University.
Conflicts of interest
The authors confirm that there is no conflict of interest.
References
- 1. Alwan A, Armstrong T, Cowan M, Riley L. Noncommunicable Diseases Country Profiles 2011. Geneva, Switzerland: World Health Organization, 2011. [Google Scholar]
- 2. Mathers CD, Vos ET, Stevenson CE, Begg SJ. The burden of disease and injury in Australia. Bulletin of the World Health Organization, 2001; 79: 1076–1084. [PMC free article] [PubMed] [Google Scholar]
- 3. Gapstur RL. Symptom burden: a concept analysis and implications for oncology nurses. Oncology Nursing Forum, 2007; 34: 673–680. [DOI] [PubMed] [Google Scholar]
- 4. Australian Institute of Health and Welfare . Australia's Health, 2010. Australia's Health Series No. 12. Cat. No. AUS 122. In: Australian Institute of Health and Welfare, ed. Canberra: Commonwealth of Australia, 2010. [Google Scholar]
- 5. Anderson RT, Skovlund SE, Marrero D et al Development and validation of the insulin treatment satisfaction questionnaire. Clinicial Therapeutics, 2004; 26: 565–578. [DOI] [PubMed] [Google Scholar]
- 6. Béchetoille A, Arnould B, Bron A et al Measurement of health‐related quality of life with glaucoma: validation of the Glau‐QoL © 36‐item questionnaire. Acta Ophthalmologica, 2008; 86: 71–80. [DOI] [PubMed] [Google Scholar]
- 7. Brod M, Hammer M, Kragh N, Lessard S, Bushnell DM. Development and validation of the treatment related impact measure of weight (TRIM‐weight). Health and Quality of Life Outcomes, 2010; 8: 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brod M, Valensi P, Shaban JA, Bushnell DM, Christensen TL. Patient treatment satisfaction after switching to NovoMixReg. 30 (BIAsp 30) in the IMPROVETM study: an analysis of the influence of prior and current treatment factors. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care & Rehabilitation, 2010; 19: 1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campbell JL, Kiebert GM, Partridge MR. Development of the satisfaction with inhaled asthma treatment questionnaire. European Respiratory Journal, 2003; 22: 127–134. [DOI] [PubMed] [Google Scholar]
- 10. Chen H, Cisternas MG, Katz PP et al Evaluating quality of life in patients with asthma and rhinitis: English adaptation of the Rhinasthma Questionnaire. Annals of Allergy, Asthma, & Immunology, 2011; 106: 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Henry B, Aussage P, Grosskopf C, Goehrs JM. Development of the Cystic Fibrosis Questionnaire (CFQ) for assessing quality of life in pediatric and adult patients. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care & Rehabilitation, 2003; 12: 63–76. [DOI] [PubMed] [Google Scholar]
- 12. Modi AC, Quittner AL. Validation of a disease‐specific measure of health‐related quality of life for children with cystic fibrosis. Journal of Pediatric Psychology, 2003; 28: 535–546. [DOI] [PubMed] [Google Scholar]
- 13. Bernhard J, Maibach R, Thurlimann B, Sessa C, Aapro MS, Swiss Group for Clinical Cancer Research . Patients' estimation of overall treatment burden: why not ask the obvious? Journal of Clinical Oncology, 2002; 20: 65–72. [DOI] [PubMed] [Google Scholar]
- 14. Oude Elberink JNG, van der Heide S, Guyatt GH, Dubois AEJ. Analysis of the burden of treatment in patients receiving an EpiPen for yellow jacket anaphylaxis. Journal of Allergy and Clinical Immunology, 2006; 118: 699–704. [DOI] [PubMed] [Google Scholar]
- 15. Wilcox AR, Dragnev MCC, Darcey CJ, Siegel CA. A new tool to measure the burden of Crohn's disease and its treatment: do patient and physician perceptions match? Inflammatory Bowel Diseases, 2010; 16: 645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ribi K, Bernhard J, Bohme C et al Endocrine symptom assessment in women with breast cancer: what a simple “yes” means. Supportive Care in Cancer, 2007; 15: 1349–1356. [DOI] [PubMed] [Google Scholar]
- 17. Bailie GR, Cardone KE, Grabe DW, Hoy CD, Manley HJ, Meola S. Quantifying home medication regimen changes and quality of life in patients receiving nocturnal home hemodialysis. Hemodialysis International, 2011; 15: 234–242. [DOI] [PubMed] [Google Scholar]
- 18. Corona J, Matsumoto H, Roye DP, Vitale MG. Measuring quality of life in children with early onset scoliosis: development and initial validation of the early onset scoliosis questionnaire. Journal of Pediatric Orthopedics, 2011; 31: 180–185. [DOI] [PubMed] [Google Scholar]
- 19. Gallacher K, May CR, Montori VM, Mair FS. Understanding patients' experiences of treatment burden in chronic heart failure using normalization process theory. Annals of Family Medicine, 2011; 9: 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walker LO, Avant KC. Strategies for Theory Construction in Nursing. Upper Saddle River, NJ: Pearson Prentice Hall, 2005. [Google Scholar]
- 21. Rodgers BL. Concepts, analysis and the development of nursing knowledge: the evolutionary cycle. Journal of Advanced Nursing, 1989; 14: 330–335. [DOI] [PubMed] [Google Scholar]
- 22. Rodgers BL, ed. Concept Analysis: An Evolutionary View. Philadelphia, PA: Saunders, 2000. [Google Scholar]
- 23. Brod M, Christensen T, Bushnell D. Maximizing the value of validation findings to better understand treatment satisfaction issues for diabetes. Quality of Life Research, 2007; 16: 1053–1063. [DOI] [PubMed] [Google Scholar]
- 24. Graves MM, Adams CD, Bender JA, Simons S, Portnoy JM. Volitional nonadherence in pediatric asthma: parental report of motivating factors. Current Allergy and Asthma Reports, 2007; 7: 427–432. [DOI] [PubMed] [Google Scholar]
- 25. Fiese BH, Wamboldt FS, Anbar RD. Family asthma management routines: connections to medical adherence and quality of life. Journal of Pediatrics, 2005; 146: 171–176. [DOI] [PubMed] [Google Scholar]
- 26. Rodbard HW, Green AJ, Fox KM, Grandy S, Shield Study Group . Impact of type 2 diabetes mellitus on prescription medication burden and out‐of‐pocket healthcare expenses. Diabetes Research & Clinical Practice, 2010; 87: 360–365. [DOI] [PubMed] [Google Scholar]
- 27. Kim E, Gupta S, Bolge S, Chen CC, Whitehead R, Bates JA. Adherence and outcomes associated with copayment burden in schizophrenia: a cross‐sectional survey. Journal of Medical Economics, 2010; 13: 185–192. [DOI] [PubMed] [Google Scholar]
- 28. Gutierrez‐Maldonado J, Caqueo‐Urizar A. Effectiveness of a psycho‐educational intervention for reducing burden in Latin American families of patients with schizophrenia. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care & Rehabilitation, 2007; 16: 739–747. [DOI] [PubMed] [Google Scholar]
- 29. Moss L, Crane PB. Exploring polypharmacy in elderly women after myocardial infarction. Journal of Women and Aging, 2010; 22: 22–33. [DOI] [PubMed] [Google Scholar]
- 30. World Health Organization . Diagnosis and Management of Adverse Drug Reactions. Geneva: World Alliance for Patient Safety, 2007. [Google Scholar]
- 31. Thomas P. The stable patient with schizophrenia – from antipsychotic effectiveness to adherence. European Neuropsychopharmacology, 2007; 17: 115–122. [DOI] [PubMed] [Google Scholar]
- 32. Tjia J, Micco E, Armstrong K. Interest in breast cancer chemoprevention among older women. Breast Cancer Research and Treatment, 2008; 108: 435–453. [DOI] [PubMed] [Google Scholar]
- 33. Ow R. Burden of care and childhood cancer: experiences of parents in an Asian context. Health and Social Work, 2003; 28: 232–240. [DOI] [PubMed] [Google Scholar]
- 34. Longo CJ, Fitch M, Deber RB, Williams AP. Financial and family burden associated with cancer treatment in Ontario, Canada. Supportive Care in Cancer, 2006; 14: 1077–1085. [DOI] [PubMed] [Google Scholar]
- 35. Zucca A, Boyes A, Girgis A, Hall A, Newling G. Travelling all over the countryside: travel‐related burden and financial difficulties reported by cancer patients in New South Wales and Victoria. Australian Journal of Rural Health, 2011; 19: 298–305. [DOI] [PubMed] [Google Scholar]
- 36. Nicholl D, Akhras KS, Diels J, Schadrack J. Burden of schizophrenia in recently diagnosed patients: healthcare utilisation and cost perspective. Current Medical Research and Opinion, 2010; 26: 943–955. [DOI] [PubMed] [Google Scholar]
- 37. Vijan S, Hayward RA, Ronis DL, Hofer TP. Brief report: the burden of diabetes therapy: implications for the design of effective patient‐centered treatment regimens. Journal of General Internal Medicine, 2005; 20: 479–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ziaian T, Sawyer MG, Reynolds KE et al Treatment burden and health‐related quality of life of children with diabetes, cystic fibrosis and asthma. Journal of Paediatrics and Child Health, 2006; 42: 596–600. [DOI] [PubMed] [Google Scholar]
- 39. Brod M, Cobden D, Lammert M, Bushnell D, Raskin P. Examining correlates of treatment satisfaction for injectable insulin in type 2 diabetes: lessons learned from a clinical trial comparing biphasic and basal analogues. Health & Quality of Life Outcomes, 2007; 5: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Henry DH, Viswanathan HN, Elkin EP, Traina S, Wade S, Cella D. Symptoms and treatment burden associated with cancer treatment: results from a cross‐sectional national survey in the U.S. Supportive Care in Cancer, 2008; 16: 791–801. [DOI] [PubMed] [Google Scholar]
- 41. Haugstvedt A, Graue M, Rokne B, Wentzel‐Larsen T. Perceived family burden and emotional distress: similarities and differences between mothers and fathers of children with type 1 diabetes in a population‐based study. Pediatric Diabetes, 2011; 12: 107–114. [DOI] [PubMed] [Google Scholar]
- 42. Martire LM, Gildengers AG, Karp JF, Reynolds CF, Schulz R, Whyte EM. Treatment of late‐life depression alleviates caregiver burden. Journal of the American Geriatrics Society, 2010; 58: 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Kraker J, Graf N, Van Tinteren H et al Reduction of postoperative chemotherapy in children with stage I intermediate‐risk and anaplastic Wilms' tumour (SIOP 93–01 trial): a randomised controlled trial. Lancet, 2004; 364: 1229–1235. [DOI] [PubMed] [Google Scholar]
- 44. Wysocki T, Gavin L. Paternal involvement in the management of pediatric chronic diseases: associations with adherence, quality of life, and health status. Journal of Pediatric Psychology, 2006; 31: 501–511. [DOI] [PubMed] [Google Scholar]
- 45. Benner JS, Chapman RH, Petrilla AA, Tang SSK, Rosenberg N, Schwartz JS. Association between prescription burden and medication adherence in patients initiating antihypertensive and lipid‐lowering therapy. American Journal of Health‐System Pharmacy, 2009; 66: 1471–1477. [DOI] [PubMed] [Google Scholar]
- 46. Robertson TA, Cooke CE, Wang J, Shaya FT, Lee HY. Effect of medication burden on persistent use of lipid‐lowering drugs among patients with hypertension. American Journal of Managed Care, 2008; 14: 710–716. [PubMed] [Google Scholar]
- 47. Yoon J, Adams JL, Ganz PA et al Symptoms after breast cancer treatment: are they influenced by patient characteristics? Breast Cancer Research and Treatment, 2008; 108: 69–77. [DOI] [PubMed] [Google Scholar]
- 48. Olinder AL, Kernell A, Smide B. Missed bolus doses: devastating for metabolic control in CSII‐treated adolescents with type 1 diabetes. Pediatric Diabetes, 2009; 10: 142–148. [DOI] [PubMed] [Google Scholar]
- 49. World Health Organization . Adherence to long‐term therapies: evidence for action. 2003. Available at: http://www.who.int/chp/knowledge/publications/adherence_full_report.pdf (accessed 11 May 2012).
- 50. Australian Department of Health and Ageing . Evaluation of the DAA/PMP Programs. 2010. Available at: http://www.health.gov.au/internet/main/publishing.nsf/Content/5B1B138DA00BB9C7CA2578150083984E/$File/DAA%20PMP%20Report.pdf (accessed 11 May 2012).
- 51. Fried TR, Bradley EH, Towle VR. Assessment of patient preferences: integrating treatments and outcomes. The Journals of Gerontology: Series B: Psychological Sciences and Social Sciences, 2002; 57B: S348–S354. [DOI] [PubMed] [Google Scholar]


