Abstract
Objectives
Newborn bloodspot screening (NBS) panels have expanded to include conditions for which treatment effects are less certain, creating debate about population‐based screening criteria. We investigated Canadian public expectations and values regarding the types of conditions that should be included in NBS and whether parents should provide consent.
Methods
Eight focus groups (FG; n = 60) included education, deliberative discussion and pre‐/post‐questionnaires. Data were analysed quantitatively and qualitatively.
Results
Quantitatively, the majority supported NBS for serious disorders for which treatment is not available (95–98, 82%). A majority endorsed screening without explicit consent (77–88%) for treatable disorders, but 62% supported unpressured choice for screening for untreatable disorders. Qualitatively, participants valued treatment‐related benefits for infants and informational benefits for families. Concern for anxiety, stigma and unwanted knowledge depended upon disease context and strength of countervailing benefits.
Conclusions
Anticipated benefits of expanded infant screening were prioritized over harms, with information provision perceived as a mechanism for mitigating harms and enabling choice. However, we urge caution around the potential for public enthusiasm to foster unlimited uptake of infant screening technologies.
Keywords: expanded newborn screening, mixed methods, public engagement, public expectations
Background
The confluence of increasingly sophisticated medical screening technologies,1, 2 the broadening of disease definitions to include mild manifestations of disease3, 4 and advocacy to shift the goals of screening to attend to health and non‐health outcomes1, 2, 5, 6, 7, 8 fuel a lively debate in the context of newborn bloodspot screening (NBS).1, 6, 9, 10, 11, 12, 13, 14, 15 Traditionally, candidate conditions for NBS were those for which the natural history of disease was clear and effective treatment was available.16 Recently proposed screening logic considers a much broader range of diseases as well as health and non‐health outcomes. Proponents of this viewpoint expand the notion of primary screening benefits to include information, support, surveillance or intervention for the index individual or family members1, 6 and endorse the use of screening as a way to mobilize research infrastructure to better define diseases and treatments.5, 9 Current debates about NBS logic invoke longstanding questions about the role of parental choice,17, 18 distinguishing between conditions of such severity and treatability that a mandatory or routinized approach to screening is appropriate and conditions where the lack of assured clinical benefit suggests consideration for parental choice.19, 20
In the Canadian context, the number of disorders for which screening is offered ranges from 5 to 38, depending on the jurisdiction. All jurisdictions offer screening for congenital hypothyroidism and phenylketonuria (PKU); screening for these two conditions is mandatory in only one province.21 Otherwise, screening is recommended as standard of care but is not mandatory. In July 2010, the Canadian College of Medical Geneticists recommended that all provinces screen newborns for cystic fibrosis (CF)22; currently, 4 of 10 provinces have implemented this recommendation.21 While one Canadian jurisdiction included Duchenne muscular dystrophy (DMD) in its NBS panel beginning in 1986,23 pilot data suggested DMD newborn screening did not achieve its goal to decrease the number of repeat cases of DMD within families or its overall population frequency, calling into question the viability of NBS for DMD.24 Presently, NBS for DMD exists only in Belgium25, 26 (Prof. Angus Clarke, personal communication, 13 November 2012), but emerging evidence related to molecular‐based therapies for DMD27 has refocused attention on optimizing screening strategies in the newborn period.28
Given this variability, it is unsurprising that debate continues regarding the expanded scope of newborn screening; this includes debate around the evidence of benefits and harms,29, 30 expectations and values,10 and policy directions to be pursued.2, 9, 11, 12, 13, 14, 15, 29, 30 Evidence specific to expectations and values is particularly limited; what exists focuses on the experiences of invested stakeholders, typically parents of affected children and involved health professionals. Studies reflecting the attitudes of invested parents generally endorse screening for the target disorder.31, 32, 33 Kerruish et al., however, identified reduced enthusiasm for disease susceptibility screening through qualitative interviews with parents who received NBS for type I diabetes.34 Also qualitatively, Lipstein et al.35 found that, compared with parents attending genetics clinics, parents attending primary care clinics were more likely to support optional screening and conveyed varied preferences for screening depending on disease characteristics, efficacy of treatment and personal experiences. With respect to health‐care provider values, a lack of consensus about expanded NBS persists. While general paediatricians report relative comfort with routinized screening for a broad range of conditions, genetic counsellors and specialist paediatricians are more likely to endorse voluntary screening for conditions that challenge traditional screening principles.36, 37, 38
In sum, parent and provider values related to NBS are neither resolved nor reflective of the full spectrum of stakeholders. Rather, a broader public is owed consultation as the values they hold are fundamental to technology assessment and uptake.39 This is especially evident in the cancer screening context, where public enthusiasm for and premature diffusion of screening technologies40 may complicate efforts to manage phenomena such as lead time, length and over‐diagnosis biases.41 To inform the gap in our understanding of public expectations and values specific to the scope of NBS, we queried the Canadian public on the types of conditions that should be included in NBS and whether parents should provide consent for screening.
Methods
Design
A mixed‐methods, public engagement study examined citizens' views regarding the scope of NBS, secondary use of stored samples and the role of parental choice. In this paper, we report the data on scope of screening and parental choice. Views on storage and secondary use of samples are reported elsewhere.42
Recruitment
Research ethics boards at the University of Toronto and McGill University approved this study. Focus group participants were purposively recruited in 2009 through Internet advertisements on community‐based websites, print advertisements in community magazines and pamphlets circulated to community‐based agencies serving families in the Greater Toronto and Montreal areas. In addition to web and print‐based advertisements, members of the research team visited community centres to describe the project and invite participation. A community‐based approach was used to generate a sample representative of socio‐economic, ethnic, age and family life diversity. Adults able to consent and converse in English were included; no other exclusion or inclusion criteria were applied.
Data collection
Focus groups
Team‐developed background information was provided in a pamphlet one week prior to the focus group discussion and through an expert presentation immediately before the discussion. Intended to inform our participant population, the educational material exceeded that which is routinely provided to parents prior to NBS. It described key principles of screening and characteristics of three paradigmatic conditions: (i) the case of PKU, a serious disorder that, with treatment, leads to essentially normal development, (ii) the case of CF, a disorder for which treatment in the newborn period reduces symptom severity but a chronic, life‐limiting condition remains, and (iii) the case of DMD, a severe muscle‐wasting disorder for which early medical intervention does not improve health outcomes and survival is rare beyond age 30. Deliberative focus group discussions explored citizens' views on (i) the types of disorders that should be included in NBS, including consideration of the aforementioned disease paradigms and (ii) parental choice with respect to screening. Discussions were guided by showcards that used a story with ‘reveals’ and were followed by deliberative questions (Appendix S1).
Questionnaire and administration
The team‐developed questionnaire was based on a review of the literature31, 32, 33, 34, 35 and was pre‐tested with 10 individuals using cognitive interviews. Pre‐test participants were recruited from the administrative teams at the same community‐based agencies later used for study recruitment. The questionnaire was modified following pre‐test interviews to maximize comprehension, readability and face and content validity. The questionnaire assessed participants' (i) knowledge of NBS using nine true/false questions (knowledge score: 0–9) and (ii) attitudes related to the types of disorders that should be included in NBS and the role of parental choice using five‐point Likert scales (i.e. strongly disagree to strongly agree). Questionnaires were administered immediately before and after the focus group discussions, to explore changes in knowledge and attitudes as a result of deliberation. Unlike in the focus group discussions where disease examples were explicitly named (i.e. PKU, CF, DMD), the questionnaire characterized them paradigmatically, reflecting variation along a spectrum of treatability.
Data analysis
We converted five‐point Likert scale response items into three categorical variables (i.e. strongly agree, agree vs. strongly disagree, disagree vs. neutral). We calculated the proportion of respondents in each category with respect to (i) screening for each of the three types of disorders and (ii) approaches to parental choice. Data were managed and analysed using spss 18.0.0.43
Focus group discussions were cofacilitated by two members of the research team and a note‐taker. The discussions were recorded digitally. Audiotapes were transcribed verbatim and analysed using a modified grounded theory approach,44 based on the principles of constant comparison45 and qualitative description.46, 47 Participants' views on the scope of NBS, the benefits and harms of expansion and the role of parental choice were compared within and across transcripts. More granular coding within each of these thematic categories better defined each concept. Thematic analysis was triangulated with quantitative data to better understand participants' views.
Results
Study participants
Sixty individuals participated in eight focus group discussions: five were conducted in Toronto (n = 36), and three in Montreal (n = 24). The two French focus group discussions were transcribed verbatim and then translated into English. Most participants were female (60%), single/separated/divorced or widowed (60%), and had at least some college or university education (87%). Of the total, 27% of participants were 18–29 years old, 43% were 30–49, and 30% were >50 years old; 43% had children.
Quantitative findings
Participants' mean knowledge significantly increased from the beginning to the end of the focus group discussions [6.87 (SD: 2.68) (pre) to 7.80 (SD: 2.12) (post), p < 0.0001].42 Findings reported herein reflect participants' views following the focus group discussions.
Participant views regarding NBS for various types of conditions
The vast majority of participants agreed (agreed or strongly agreed) that NBS should identify serious disorders where, with treatment, the child is likely to develop normally (98.3%) and where treatment is available to reduce symptom severity (94.8%). A substantial majority (82.5%) also agreed with NBS for conditions where no treatment exists (Table 1).
Table 1.
Attitudes towards the scope of newborn screening programmes
| Q: ‘I think newborn screening should try to find babies with serious disorders…’ | Total N (%) |
|---|---|
| … that can be treated and, with treatment, the child is likely to develop normally (n = 58) | |
| Agreea | 57 (98.3) |
| Neutral | 0 (0.0) |
| Disagreeb | 1 (1.7) |
| … where treatment will reduce the severity of the symptoms, but the child will still have symptoms of the disorder (n = 58) | |
| Agreea | 55 (94.8) |
| Neutral | 3 (5.2) |
| Disagreeb | 0 (0.0) |
| … that cannot be treated but parents learn sooner about the disorder – before symptoms begin to show (n = 57) | |
| Agreea | 47 (82.5) |
| Neutral | 6 (10.5) |
| Disagreeb | 4 (7.0) |
Respondents checking ‘strongly agree’ and ‘agree’ to these questions were included in this category.
Respondents checking ‘strongly disagree’ and ‘disagree’ to these questions were included in this category.
Participant views regarding parental choice for NBS
With respect to ‘disorders 1 and 2’ (e.g. PKU and CF), large majorities agreed that parents should be ‘strongly encouraged’ (88 and 84%, respectively) or ‘required’ (79 and 77%, respectively) to have their babies screened, and only about half agreed that parents should be ‘able to choose without pressure’ (54 and 52%, respectively). When the condition identified could not be treated (‘disorder 3’, e.g. DMD), fewer respondents agreed that parents should be ‘strongly encouraged’ (74%) or ‘required’ (60%) to have their babies screened, and more respondents agreed that parents should be ‘able to choose without pressure’ (62%; Table 2).
Table 2.
Attitudes towards consent for newborn screening for disorders 1, 2 and 3
| Q: ‘I think parents should be…’ | Disorder 1 Total N (%) | Disorder 2 Total N (%) | Disorder 3 Total N (%) |
|---|---|---|---|
| …required to have their baby screened (n = 58) | |||
| Agreea | 46 (79.0) | 44 (77.0) | 34 (60.0) |
| Neutral | 1 (2.0) | 1 (2.0) | 4 (7.0) |
| Disagreeb | 11 (19) | 12 (21.0) | 19 (33.0) |
| …strongly encouraged to have their baby screened (n = 58) | |||
| Agreea | 51 (88.0) | 47 (84.0) | 42 (74.0) |
| Neutral | 4 (7.0) | 4 (7.0) | 9 (16.0) |
| Disagreeb | 3 (5.0) | 5 (9.0) | 6 (11.0) |
| …able to choose without pressure whether to have their baby screened (n = 57) | |||
| Agreea | 31 (54.0) | 29 (52.0) | 36 (62.0) |
| Neutral | 8 (14.0) | 11 (20.0) | 10 (17.0) |
| Disagreeb | 18 (32.0) | 16 (29.0) | 12 (21.0) |
Respondents checking ‘strongly agree’ and ‘agree’ to these questions were included in this category.
Respondents checking ‘strongly disagree’ and ‘disagree’ to these questions were included in this category.
Qualitative findings
Overall, perceived benefits of screening were specific to improved health outcomes for affected children and information for family members. Informational harms were considered, but how they were valued depended upon which benefits could also be achieved. Information provision was viewed as a mechanism for mitigating harm and enabling choice, especially where early detection through screening could not improve health outcomes (Fig. 1).
Figure 1.
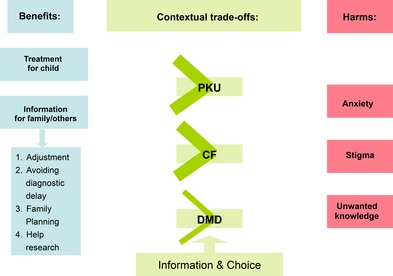
Thematic framework.
Treatment‐related benefits for affected infants
Participants emphasized the importance of treatment‐related benefits, especially in the context of infants.
… when it comes to newborn… that's what drove me over here,… newborn screening is for life. We've got to give [the child] the best so that the years ahead will be productive and healthy, and happy. [002]
Where treatment leads to essentially normal child development (e.g. PKU), NBS was unanimously endorsed. The time‐sensitivity and dramatic impact of early treatment were key rationales for this view. Participants also endorsed screening for disorders where treatment reduces symptom severity (e.g. CF), even though early treatment did not carry the same urgency.
It's a different outcome obviously … for PKU, you're going to dramatically change the outcome of the disease, but I still think it's important to test – there's still benefits from knowing early that your kid has CF. [001]
Information is also highly valued, but this is for parents and families
When treatment impact was less pronounced, informational benefits assumed more prominence. Overall, these were perceived to be most relevant for family members.
For PKU and CF, that knowledge is mostly for the children's benefit, whereas DMD's more for parents. So, they can adjust their own thinking and the way they want to handle the situation. [003]
Participants operationalized informational benefits in a variety of ways. They felt that diagnostic information generated through NBS would enable families to (i) become educated about the illness and ‘come to terms with’ [005] the idea of illness in a young child and (ii) avoid diagnostic delay:
It's better to know as early as possible because, why wait down the road when … you're going to have to go through more trouble taking the baby back to and from the doctor. [004]
Further, information could be used (iii) ‘for family planning … regardless of what disease it was’ [006] and (iv) ‘to contribute to further treatments and further cures … years from now’ [007].
Navigating harms
Respondents acknowledged several potential harms: (i) anxiety, (ii) stigma and (iii) the burden of unwanted information. While unwelcome, harms were more easily dismissed when screening identified newborns at risk of treatable disorders. Where treatment was lacking, these harms assumed more prominent, but participants anticipated that harms could be mitigated through the provision of information or parental choice.
Participants acknowledged the potential for anxiety related to receiving a false‐positive result, but felt this was a small price to pay, given the alternative of delayed treatment for an affected baby.
… from a community approach, the negative of me having this experience where I'm worried for a week… is less significant than parents not finding out their children have this. [008]
Participants also acknowledged the potential for labelling, such that a child might be disadvantaged by the parent's knowledge of their condition, above and beyond any limitations imposed by the condition itself.
If you have a sick kid or if you have a healthy kid, the relationship is going to be different… the parent of the sick kid would be more protective and shelter the kid off from the world, whereas…the healthy kid may have friends, play outside… [009]
Beyond the case of infants diagnosed with a disease, some participants felt that parents or children identified to be carriers through NBS could experience stigma.
My friend was found that she was a carrier for sickle cell… she had this thing in her mind throughout her life. … she was always second guessing, you know… I like this guy… should I make him test his blood… it became a whole exercise in… prescreening people… [010]
Further, some respondents suggested that diagnostic information that was not medically actionable might be unwanted and burdensome. About DMD:
It's not comforting … it's useless information. I will try to go into different countries and find out, you know, some alternative medicine, but if there's nothing there, then to me it's useless information. You should not have even told me… [009]
Information provision prior to NBS
Respondents expressed enthusiasm for information prior to NBS, but had different views about how fulsome it should be. Some called for a detailed overview of the conditions, ‘parents need to be informed about every single part of the screening’ [011], while others worried that this might cause undue anxiety, ‘I cannot handle it… [telling me] twenty, thirty diseases my child could get…’ [002].
In addition, while some participants saw challenges with providing parental education, ‘sure you've got packages for everyone, but how many read them’ [012], more supported the view that education was rather easy, ‘I mean 9 months is a long time’ [006]. Minimizing information provision challenges, many participants suggested that most of the responsibility for being informed fell to parents.
I think it's the responsibility of the health care providers to give the opportunity for parents to find out more, … say, here's a pamphlet, go to these websites… but if [parents] get that phone call and they didn't read that information, then that's their own fault. [014]
Respondents saw a role for information in mitigating the potential harms of screening. For example, ensuring access to information about NBS prior to the heel prick was seen as an important way to limit the potential harm of a false‐positive result.
I think that knowledge about [the process] is key, knowing that I might get a call… How are [parents] going to feel when they do get the call and they have no idea what's happening? [011]
Finally, while many advocated that information was important to achieving a basic awareness of screening, ‘even though [consent] is implied, parents should know ahead of time’ [015], others advocated that information was key to enabling choice about whether to pursue screening at all, particularly where treatment is lacking.
….I think the information is the thing that is supposed to be required, not the testing… my main concern is that parents are not given the information, all of a sudden they get a call saying, oh, your child might have DMD. [013]
Discussion
Newborn bloodspot screening expansions have posed challenges to the traditional warrant for population screening, by including conditions for which the natural history of disease is poorly understood and where evidence of clinical effectiveness is limited. While some have questioned these developments,11, 12, 13, 14, 15 others have called for a reframing of traditional screening criteria1, 2, 4, 5, 6 to prioritize what were typically valued as secondary benefits (i.e. psychosocial or family benefits) and to enable opportunities for clinical improvement, in contrast to previous requirements for evidence of clinical improvement. Debates over values implicate society at large and increase the need for well‐designed public engagement exercises to enhance transparency and elucidate expectations and values.
Our quantitative data indicate that a majority of respondents endorsed infant screening, irrespective of whether health outcomes might be improved by early detection and treatment. In addition, a majority of citizens was comfortable with routinized (i.e. no explicit consent) screening, although a majority also supported choice where early intervention did not improve clinical outcomes.
Our qualitative data suggest that different interpretations of benefit underpin these attitudes: participants were motivated to positively value different potential benefits of screening, regardless of treatment effect. In part, this was because NBS is directed at children, a vulnerable group owed maximum opportunity. Among these benefits, improved health outcomes in the index child and anticipatory guidance for family members were of central importance. Unlike treatment‐related benefits, however, informational benefits were seen to accrue to parents and family members. These benefits achieved prominence where treatment‐related benefits could not be realized. Informational harms were acknowledged, but how they were perceived differed depending on which benefits could also be achieved. Anxiety related to false‐positive results, for example, was acknowledged as harmful, but seen as a small price to pay to meet the needs of affected children. Other information‐related harms (e.g. stigma, unwanted knowledge) assumed significance when the potential for treatment‐related benefits was reduced.
Finally, our qualitative data suggest support for increased parental discretion where early detection through screening does not lead to improved health outcomes, as parental choice was valued more highly in such contexts. Even in the absence of the need for parental choice, respondents were optimistic about the value of providing information to parents prior to screening and assumed that parents would and could take responsibility for self‐education. These expectations and values may underpin respondents' comfort with routinized screening.
These findings suggest considerable public appetite for expanded NBS and substantial enthusiasm for maintaining a ‘public health emergency’8 mindset while doing so. This appears to be motivated by expectations of extensive benefits and limited harms, positive valuation of both treatment‐ and information‐related benefits and a high tolerance for potential burdens. Our findings are consistent with supportive attitudes of patient groups and advocates32, 33 as well as with some provider groups.36, 37, 38 They are inconsistent, however, with the argument that NBS now operates in a manner akin to a public health service, involving trade‐offs among non‐comparable outcomes and warranting a preference‐sensitive consent process.10, 17, 18, 19, 20 Further, our respondents anticipate harm mitigation through pre‐screening education, despite evidence of considerable barriers facing its delivery.48
Public expectations are motivated by value‐based judgements about the nature of ‘the good’ as well as trade‐offs among diverse benefits and harms. Public expectations are also driven by beliefs about what is possible and about the likelihood and gravity of any harms.49 As has been shown for cancer screening in particular, public expectations are sometimes driven by especially high hopes40; whether such hopes are more or less exaggerated in the child health context is unknown, but warrants exploration. Thus, we urge caution around the potential for this enthusiasm to foster unlimited uptake of infant screening technologies. Direct‐to‐consumer advertisements for home‐based NBS kits or early adoption of exome sequencing in this context,50, 51 for example, may be fuelled by such unbridled enthusiasm.
Limitations
Data collection strategies varied in approach with respect to characterizing the disease paradigms of interest. Specifically, the knowledge questionnaire did not explicitly name the three example diseases, whereas the deliberative discussions did. Our intention was to ascertain broad reflections on disease paradigms quantitatively and more nuanced, disease‐specific views qualitatively. We did not quantitatively account for the possibility that participants' personal life‐experiences may have shaped their impressions of these particular conditions; those experiences were certainly – and intentionally – brought to bear qualitatively. In addition, despite our intentions to orient participants towards collective interests, an individualist mindset prevailed. Finally, the impact of over‐diagnosis and other complex screening biases52, 53 is not well understood in the context of NBS54, 55, 56 so were not considered in this study.
Conclusion
Public values towards expanded NBS are supportive but qualified, calling for further public engagement on how NBS‐related benefits and harms are independently valued and traded off. Ultimately, there is a need for NBS policy that acknowledges public values, as well as robust service delivery to ensure that benefits are realized and harms are minimized.
Source of funding
This study was funded by the Canadian Institutes of Health Research (HGE # 89020). The sponsor was not involved in the design or conduct of the study; in the collection, analysis or interpretation of the data; or in the preparation, review or approval of the manuscript. Sponsor's support for this should not imply endorsement of the conclusions, for which the authors retain sole responsibility.
Conflict of interest
There are no conflicts of interest to declare.
Supporting information
Appendix S1. Discussion showcards and questions
Acknowledgements
We thank study participants for the time and insight they dedicated to this deliberative process.
References
- 1. Baily MA, Murray TH. (eds). Ethics and Newborn Genetic Screening: New Technologies, New Challenges. Baltimore: Johns Hopkins UP, 2009. [Google Scholar]
- 2. Watson M, Mann M, Lloyd‐Puryear M, Rinaldo P, Howell R, Group ACoMGNSE . Newborn screening: towrad a uniform screenign panel and system–executive summary. Pediatrics, 2006; 117: S296–S307. [DOI] [PubMed] [Google Scholar]
- 3. Lilley M, Christian S, Hume S et al Newborn screening for cystic fibrosis in Alberta: two years of experience. Paediatrics and Child Health, 2010; 15: 590–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wagener J, Zemanick E, Sontag M. Newborn screening for cystic fibrosis. Current Opinion in Pediatrics, 2012; 24: 329–335. [DOI] [PubMed] [Google Scholar]
- 5. Calonge N, Green N, Rinaldo P et al Committee report: method for evaluating conditions nominated for population‐based screening of newborns and children. Genetics in Medicine, 2010; 12: 153–159. [DOI] [PubMed] [Google Scholar]
- 6. Bailey D. Ethical, legal and social concerns about expanded newborn screening: fragile × syndrome as a prototype for emerging issues. Pediatrics, 2008; 121: e693–e704. [DOI] [PubMed] [Google Scholar]
- 7. Raffle A, Gray J. Screening: Evidence and Practice. Oxford: Oxford UP, 2007. [Google Scholar]
- 8. Grosse S, Boyle C, Kenneson A, Khoury M, Wilfond B. From public health emergency to public health service: the implications of evolving criteria for newborn screening panels. Pediatrics, 2006; 117: 923–929. [DOI] [PubMed] [Google Scholar]
- 9. Moyer V, Calonge N, Teutsch S, Botkin J. Expanding newborn screening: process, policy and priorities. Hasting Center Report, 2008; 38: 32–39. [DOI] [PubMed] [Google Scholar]
- 10. Atkins D, Siegel J, Slutsky J. Making policy when the evidence is in dispute. Health Affairs, 2005; 24: 102–113. [DOI] [PubMed] [Google Scholar]
- 11. Botkin J. Newborn screening for fragile × syndrome: do we care what parents think? Pediatrics, 2011; 127: e1593–e1594. [DOI] [PubMed] [Google Scholar]
- 12. Bombard Y, Miller F, Hayeems R, Avard D, Knoppers B. Reconsidering reproductive benefit: a systematic review of guidelines on preconception, prenatal, and newborn screening. European Journal of Human Genetics, 2010; 18: 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Botkin JR, Clayton EW, Fost N et al Newborn screening technology: proceed with caution. Pediatrics, 2006; 117: 1793–1799. [DOI] [PubMed] [Google Scholar]
- 14. Clayton E. Lessons to be learned from the move toward expanded newborn screening In: Baily M, Murray T. (eds) Ethics and Newborn Genetic Screening: New Technologies, New Challenges. Baltimore: Johns Hopkinds UP, 2009: 125–135. [Google Scholar]
- 15. Clayton E. Currents in contemporary ethics. State run newborn screening in the genomic era, or how to avoid drowning when drinking from a fire hose. The Journal of Law Medicine and Ethics, 2010; 38: 697–700. [DOI] [PubMed] [Google Scholar]
- 16. Wilson J, Jungner G. Principles and Practice of Screening for Disease. Geneva: World Health Organization, 1968. [Google Scholar]
- 17. Paul D. Contesting consent: the challenge to compulsory neonatal screening for PKU. Perspectives in Biology and Medicine, 1999; 42: 207–219. [DOI] [PubMed] [Google Scholar]
- 18. Gostin L. Privacy: rethinking health information technology and informed consent. Hasting Center Report 2009; (Suppl): 15–17. [PubMed] [Google Scholar]
- 19. Miller F, Hayeems R, Carroll J et al Consent for newborn screening: the attitudes of health care providers. Public Health Genomics, 2010; 13: 181–190. [DOI] [PubMed] [Google Scholar]
- 20. Moody L, Choudhry K. Parental views on informed consent for expanded newborn screening. Health Expectations 2011; 16: 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morrison A, Dowler J. Newborn screening for disorders and abnormalities in Canada Environmental Scan Issue 26. Ottawa: Canadian Agency for Drugs and Technologies in Health, 2011. [Google Scholar]
- 22. Canadian College of Medical Geneticists . CCMG Position Statement: Newborn Screening for Cystic Fibrosis. Ottawa: Canadian College of Medical Geneticists, 2010. [Google Scholar]
- 23. Greenberg C, Jacobs H, Halliday W, Wrogemann K. Three years' experience with neonatal screening for Duchenne/Becker muscular dystrophy: gene analysis, gene expression, and phenotype prediction. American Journal of Medical Genetics, 1991; 39: 68–75. [DOI] [PubMed] [Google Scholar]
- 24. Hildes E, Jacobs H, Cameron A et al Impact of genetic counselling after neonatal screening for Duchenne muscular dystrophy. Journal of Medical Genetics, 1993; 30: 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eyskens F, Philips E. Newborn screening for Duchenne muscular dystrophy. The experience in the province of Antwerp. Neuromuscular Disorders, 2006; 16: 721. [Google Scholar]
- 26. Cyrus A, Street N, Quary S, Kable J, Kenneson A, Fernhoff P. Clinic‐based infant screening for duchenne muscular dystrophy: a feasibility study. PLoS Currents 2012; 4: e4f99c5654147a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cirak S, Arechavala‐Gomeza V, Guglieri M et al Exon skipping and dystrophin restoration in Duchenne muscular dystrophy patients after systemic phosphorodiamidate morpholino oligomer treatment: an open‐label, phase 2, dose‐escalation study. Lancet, 2011; 378: 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mendell J, Shilling C, Leslie N et al Evidence‐based path to newborn screening for Duchenne muscular dystrophy. Annals of Neurology, 2012; 71: 304–313. [DOI] [PubMed] [Google Scholar]
- 29. Bodamer O, Hoffmann G, Lindner M. Expanded newborn screening in Europe 2007. Journal of Inherited Metabolic Disease, 2007; 30: 439–444. [DOI] [PubMed] [Google Scholar]
- 30. Atkinson K, Zuckerman B, Sharfstein J, Levin D, Blatt R, Koh H. A public health response to emerging technology: expansion of the Massachusetts newborn screening program. Public Health Reports, 2001; 116: 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parsons E, Clarke A, Hood K, Lycett E, Bradley D. Newborn screening for Duchenne muscular dystrophy: a psychosocial study. Archives of disease in childhood. Fetal and Neonatal Edition, 2002; 86: F91–F95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Monestrol I, Burucefors A, Sjoberg B, Hjelte L. Parental support for newborn screening for cystic fibrosis. Acta Paediatrica, 2011; 100: 209–215. [DOI] [PubMed] [Google Scholar]
- 33. Hayes I, Collins J, Sahhar M, Wraith J, Delatycki M. Nwborn screening for mucopolysaccharidoses: opinions of patients and their families. Clinical Genetics, 2007; 71: 446–450. [DOI] [PubMed] [Google Scholar]
- 34. Kerruish N. Parents' experiences of newborn screening for genetic susceptibility to type 1 diabetes. Journal of Medical Ethics, 2011; 37: 348–353. [DOI] [PubMed] [Google Scholar]
- 35. Lipstein E, Nabi E, Perrin J, Luff D, Browning M, Kuhlthau K. Parents' decision‐making in newborn screening: opinions, choices and informational needs. Pediatrics, 2010; 126: 696–704. [DOI] [PubMed] [Google Scholar]
- 36. Koopmans J, Ross L. Does familiarity breed acceptance? The influence of policy on physicians' attitudes toward newborn screening programs. Pediatrics, 2006; 117: 1477–1485. [DOI] [PubMed] [Google Scholar]
- 37. Hiraki S, Ormond K, Kim K, Ross L. Attitudes of genetic counselors towards expanding newborn screening and offering predictive genetic testing to children. American Journal of Medical Genetics. Part A, 2006; 140A: 2312–2319. [DOI] [PubMed] [Google Scholar]
- 38. Schittek H, Koopmans J, Ross L. Pediatricians' attitudes about screening newborns for infectious diseases. Maternal Child Health Journal, 2010; 14: 174–183. [DOI] [PubMed] [Google Scholar]
- 39. Abelson J, Giacomini M, Lehoux P, Gauvin F. Bringing ‘the public’ into health technology assessment and coverage policy decisions: from principles to practice. Health Policy, 2007; 82: 37–50. [DOI] [PubMed] [Google Scholar]
- 40. Schwartz L, Woloshin S, Fowler F, Welch H. Enthusiasm for cancer screening in the United States. JAMA, 2004; 291: 71–78. [DOI] [PubMed] [Google Scholar]
- 41. Welch H. Screening mammography – a long run for a short slide. New England Journal of Medicine, 2010; 363: 1276–1278. [DOI] [PubMed] [Google Scholar]
- 42. Bombard Y, Miller F, Hayeems R et al Citizens' values regarding research with stored samples from newborn screening. Pediatrics, 2012; 129: 239–247. [DOI] [PubMed] [Google Scholar]
- 43. SPSS Inc . PASW Statistics fro Windows [Program]. Version 18.0 Version. Chicago: SPSS Inc., 2009. [Google Scholar]
- 44. Charmaz K. Constructing Grounded Theory: A Practical Guide Through Qualitative Analysis. London, UK: Sage, 2006. [Google Scholar]
- 45. Boeije H. A purposeful approach to the constant comparative method in the analysis of qualitative interviews. Quality and Quantity, 2002; 36: 391–409. [Google Scholar]
- 46. Thorne S. Interpretive Description. Walnut Creek, CA: Left Coast Press, 2008. [Google Scholar]
- 47. Sandelowski M. Qualitative analysis: what it is and how to begin. Research in Nursing and Health, 1995; 18: 371–375. [DOI] [PubMed] [Google Scholar]
- 48. Hayeems RZ, Miller FA, Little J et al Informing parents about expanded newborn screening: influences on health care provider involvement. Pediatrics, 2009; 124: 950–958. [DOI] [PubMed] [Google Scholar]
- 49. Lehoux P, Daudelin G, Demers‐Payette O, Boivin A. Fostering deliberations about health innovation: what do we want to know from publics? Social Science and Medicine, 2009; 68: 2002–2009. [DOI] [PubMed] [Google Scholar]
- 50. Gollust S, Chandros S, Wilfond B. Limitations of direct‐to‐consumer advertising for clinical genetic testing. JAMA, 2002; 288: 1762–1767. [DOI] [PubMed] [Google Scholar]
- 51. Solomon B, Pineda‐Alvarez D, Bear K, Mullikin J, Evans J, Program NCS. Applying genomic analysis to newborn screening. Molecular Syndromology, 2012; 3: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kramer B, Brawley O. Cancer screening. Hematology/oncology Clinics of North America, 2000; 14: 831–848. [DOI] [PubMed] [Google Scholar]
- 53. Black W. Overdiagnosis: an underrecognized cause of confusion and harm in cancer screening. Journal of the National Cancer Institute, 2000; 92: 1280–1282. [DOI] [PubMed] [Google Scholar]
- 54. Hanley W. Non‐PKU mild hyperphenylalaninemia (MHP) – the dilemma. Molecular Genetics and Metabolism, 2011; 104: 23–26. [DOI] [PubMed] [Google Scholar]
- 55. van Spronsen F. Mild hyperphenylalaninemia: to treat or not to treat. Journal of Inherited Metabolic Disease, 2011; 34: 651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Notes and Queries in Metab‐L . Neonatal screening for MCAD deficiency – how to deal with variant biochemical phenotypes and novel mutations? Molecular Genetics and Metabolism, 2006; 31: 142–145. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Discussion showcards and questions


