Abstract
Background
There is growing attention towards increasing patient and service user engagement (PSUE) in biomedical and health services research. Existing variations in language and design inhibit reporting and indexing, which are crucial to comparative effectiveness in determining best practices.
Objective
This paper utilizes a systematic review and environmental scan to derive an evidence‐based framework for PSUE.
Design
A metanarrative systematic review and environmental scan/manual search using scientific databases and other search engines, along with feedback from a patient advisory group (PAG).
Eligible sources
English‐language studies, commentaries, grey literature and other sources (including systematic and non‐systematic reviews) pertaining to patient and public involvement in biomedical and health services research.
Data extracted
Study description (e.g. participant demographics, research setting) and design, if applicable; frameworks, conceptualizations or planning schemes for PSUE‐related endeavours; and methods for PSUE initiation and gathering patients'/service users' input or contributions.
Results
Overall, 202 sources were included and met eligibility criteria; 41 of these presented some framework or conceptualization of PSUE. Sources were synthesized into a two‐part framework for PSUE: (i) integral PSUE components include patient and service user initiation, reciprocal relationships, colearning and re‐assessment and feedback, (ii) sources describe PSUE at several research stages, within three larger phases: preparatory, execution and translational.
Discussion and Conclusions
Efforts at developing a solid evidence base on PSUE are limited by the non‐standard and non‐empirical nature of much of the literature. Our proposed two‐part framework provides a standard structure and language for reporting and indexing to support comparative effectiveness and optimize PSUE.
Keywords: patient and public involvement, patient engagement, systematic review
There is a large and growing impetus among patients, politicians, clinicians and researchers towards increasing the engagement of patients and other layperson/non‐professional service users in health‐ and health‐care‐related research.1, 2, 3, 4, 5, 6 Attempts to expand this engagement of lay (i.e. non‐clinician/non‐professional researcher) users of health services have emphasized patients and caregivers,5, 7, 8, 9, 10, 11 but also broader categorizations and terms such as consumers/users of health‐care services, community members or public/citizens.1, 10, 12, 13, 14, 15 Given the range of these terms, we will use patient and service user engagement (PSUE) in research to emphasize patients while referring to these endeavours broadly.
Increasing PSUE generally follows two aims: a moral/ethical drive to empower lay participants in an otherwise expert‐dominated endeavour and ensure civically responsible research5, 16, 17; and ‘consequentialist’ reasoning for optimizing the validity, design, applicability or dissemination of the research itself and the effectiveness of resulting interventions.18, 19, 20, 21
However, the promise of PSUE to ensure equitability and robust findings remains unrealized. Despite policy and funding directives (e.g. INVOLVE in the UK22, 23; PCORI in the US), and some evidence that PSUE can improve research,12, 19, 24, 25, 26, 27, 28, 29 challenges persist. In particular, there remains some doubt as to whether current approaches represent merely ‘tokenistic’ efforts30 or truly incorporate patient and service user contributions. Moreover, some groups are less likely than others to have their voices included.31 In addition, there is limited evidence on PSUE's tangible benefits.26, 29, 31 This is exacerbated by an absence of standard approaches to conducting and reporting PSUE32, 33 – when it is reported at all34 – which limits the potential for indexing, knowledge synthesis or comparative effectiveness for determining best practices. In sum, the reasoning for PSUE is compelling and multifaceted, but the extent to which existing approaches actually ensure inclusion, and whether there are consistent benefits in doing so, remains unclear because of a lack of standard framework or language.
Utilizing a systematic review, environmental scan and manual search of peer‐reviewed literature and other sources regarding PSUE in biomedical and health services research, this paper synthesizes a standardized, evidence‐based framework for understanding, reporting and assessing PSUE to jump‐start a reliable and comparative evidence base. An important percentage of previous work on this topic has consisted of individual studies (usually at single sites and with limited resources) or commentaries. Few studies (incorporated and reviewed here as well) have been performed with necessary rigour or an eye towards systematically assessing and organizing existing evidence into a broadly applicable framework. Doing so is vital to assessing PSUE in existing research and building towards an evidence‐based understanding of best practices.
Methods
Methods consisted of a systematic review, environmental scan and manual search of peer‐reviewed literature and other sources, supplemented with input from a patient advisory group (PAG). The study received Institutional Review Board clearance.
Electronic database search
Eligibility criteria
We included studies of any design, size and patient age or morbidity status, published in English, in which patients, surrogates, caregivers or other service user stakeholders participated in planning or conducting biomedical and health services research (including input on research/funding agendas, outcomes or design, and active engagement in research activities). We also included non‐original or summarized literature (systematic or non‐systematic literature reviews, commentaries, etc.).
Search strategy
An expert reference librarian (PJE) and systematic review methodologist (MHM) collaborated to develop the search strategy. Medical subject heading (MESH) terms and text words were selected based on common indexing practices. Search terms were compiled and tested repeatedly to produce sensitive searches and capture potentially relevant publications. Our search covered biomedical databases and other sources: PubMed/Ovid MEDLINE, Ovid EMBASE; Ovid PsycInfo, Ovid Cochrane (especially Sys Rev, Methods, HTA), EBSCO CINAHL; SCOPUS (to capture potentially relevant sources in the social sciences); Web of Science (multidisciplinary scientific content); and Business Search Premier, Academic Search Premier and Google Scholar. We also reviewed reference lists from eligible studies, conducted additional MEDLINE searches using the PubMed‐'related articles' feature for eligible studies and employed SciSearch for publications that cited eligible studies.
Environmental scan and manual search
The goal of the environmental scan was to identify relevant information in sources not published in the biomedical bibliographic databases. We were interested in the actors and stakeholders involved, key events, documentation (white papers, position papers, proceedings) and trends (descriptions of upcoming and on‐going activities). We used search engines Scirus and Sciverse, which contain scientific journal content, scientists' homepages, courseware, pre‐print server material, patents and institutional repository and Website information, along with Google and Bing. The manual search covered Websites recommended by topic experts including the project team, external advisors and the PCORI methodology working group. We extracted data from relevant links including title, source, author, URL, content description and main conclusions.
Study selection
We collated initial references in citation files using Endnote software, removed duplicates and screened titles and abstracts against eligibility criteria using DistillerSR software. Team members reviewed studies in duplicate until adequate agreement (Kappa > 0.80) was achieved. Disagreements during initial screening were automatically included. Potentially eligible studies then received full text review following a similar procedure. Disagreements in full text screening were reconciled by discussion, consensus or arbitration by third reviewer. We exclusively used electronic file formats (Portable Document Format/PDF) to reduce costs and paper use.
Data extraction
Data extraction from included studies followed a standardized form developed from the protocol, created in DistillerSR and tested on a small sample (n = 10) of included studies to ensure sufficient quality and performance. Data extracted included study description (e.g. participant demographics, research setting), methods for patient/service user selection or initiation into research and for obtaining their input or contributions, and frameworks or conceptualizations used for the approach.
Patient advisory group
Finally, we presented our project aims, initial findings and recommendations to a PAG35 and asked for feedback on terminology, usefulness and applicability (located at the end of the Results). Notably, among the terms for PSUE participants (consumers, representatives, etc.), they felt that ‘patient’ or ‘informant’ was most useful, but found most terms generally confusing. For clarity and inclusivity – and to avoid confusion with ‘participants’ in more conventional researcher‐driven models (e.g. participants as data points in a study) while also avoiding non‐relevant connotations of ‘informant’ – we will simply use patient and service user representatives (PSURs) to refer to layperson patients, service users or their surrogates who are engaged in the research process itself.
Analysis
Due to our study objectives and heterogeneity of sources, we did not conduct a quantitative meta‐analysis. Rather, we followed a metanarrative approach.11 The present analysis focused on assessing sources, which included frameworks or conceptualizations of PSUE processes, or which described specific stages of PSUE, and then synthesizing a systematically inclusive framework.
Results
Search and selection results: framework for PSUE
Our search identified 5560 possibly relevant citations, of which 202 met eligibility criteria (study selection is described in Fig. 1); see full bibliography in Data S1. Of these 202 sources, 41 described some framework, conceptualization or planning scheme for the parts of the PSUE process. Of these 41 sources, most were not accompanied by original evidence, but rather largely consisted of non‐systematic literature reviews, commentaries, etc. Instead, the 161 other eligible sources provided a great deal of detail (including research examples). We synthesized the frameworks and supporting sources into a two‐part framework, outlined here as an organizing structure, comprised of (i) the integral components of PSUE, and (ii) the phases and stages of PSUE in research.
Figure 1.

Study selection process.
Components of PSUE
Two reviewers coded the components of PSUE described in studies into discrete categories and recorded their inter‐relations. We found four essential components: patient and service user initiation, building reciprocal relationships, colearning and re‐assessment and feedback (Fig. 2). Functionally, these components were described as having a circular, bidirectional relationship, comprising consecutive feedback loops in researcher–PSUR collaboration. We were unable to identify one particular component as being the most important, but this proposed framework describes a process in which changes or decisions related to each component may inform and influence the others. Thus, ideally, work on these components might be performed continually throughout projects until saturation – that is, when no additional information is being shared and when both parties agree to move forward – although finite time and resources will likely place limits on groups' abilities to do this. Some studies employed only described one component; others incorporated several. We describe the components below.
Figure 2.
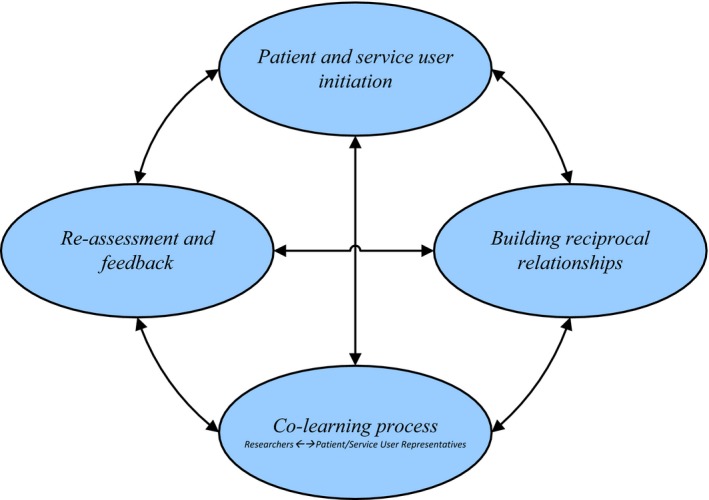
Components of patient and public involvement in research.
Component 1. Patient and service user initiation
Ten sources included information on patient and service user initiation, meaning the entry of lay patients and service users into the research process – whether through researchers' engagement efforts or PSURs' own interests and actions. Three overarching factors are keys to initiation of PSURs' efforts in PSUE processes. First, studies demonstrated the importance of engaging PSURs as early as possible in the process so that they can steer agendas and outcomes and provide a values context, which will improve study design and applicability, ensure a relevant perspective and prevent patients and service users from being relegated to disempowered ‘subjects’ with no impact.36, 37, 38, 39, 40, 41, 42, 43, 44, 45 Second, PSURs should consist of individuals or communities for whom the outcomes are of interest,46, 47 but must also display at least notable characteristics, particularly participatory behaviour.45 Third, there must be potential for PSURs to have an active role – including their ability to engage, agreement on expectations and the possibility for a sense of equality between parties.48 Presumably, these three factors would be best represented by the many instances in which PSURs initiate and lead research themselves.17, 49 However, a review on PSUR‐controlled research did note several concerns, including inequality in the interactions between PSURs themselves, added stress for PSURs, and the perception that such research will be less valued than conventional researcher‐controlled research.49
Component 2. Building reciprocal relationships
Thirty studies highlighted this component. Many stated that, from the very beginning, researchers should see PSURs as equal partners and consider them as a reliable component of the team, rather than simply an additional variable or complication.36, 37, 38, 39, 40, 41, 43, 44 Both parties have to clearly know their roles and the importance of those roles independent from each other. Thus, reciprocal cultural competence is a vital feature of PSUE,50 and partnerships should include a mutual understanding of partners' needs, capacities and goals,51 with conflicts solved promptly and explicitly. After all, PSURs' suboptimal experiences (e.g. abandonment after data collection) could affect future relationships with researchers.52
Component 3. Colearning process
Colearning received attention in nine studies. PSURs may need some research expertise to prevent researchers from dominating agendas and opinions.53 Thus, PSURs may require education/training about content or methodology to carry out a productive dialogue or conduct research themselves.16 Colearning also may increase PSURs' confidence, promoting more active engagement and reducing the risk of tokenistic PSUE. More broadly, PSUE must provide opportunities to all team members to acquire new knowledge and skills; researchers could begin by training themselves so that projects better adhere to participatory principles.54 Additionally, researcher education could improve awareness regarding the realities that PSURs face and about relevant social dynamics.16 This may also improve protocols, phrasing/language in measurement and applicability of results.45
Component 4. Re‐assessment and feedback
The final component, supported by two studies, entails evaluating the PSUE process, further clarifying PSURs' and researchers' roles and expectations and potentially modifying the other as needed. Doing so continuously not only helps ensure PSUR empowerment, but also can reveal potential facilitators and barriers.55, 56 Thus, the execution of this component will improve the robustness of the research project in question and future attempts at PSUE.
Phases of PSUE in research
Available literature describes PSUE across the research process. Broadly, at any stage of research, PSUE represents a potential spectrum of engagement for PSURs, as organized by existing sources outlining this spectrum,57, 58, 59 ranging from the most passive role of being a study participant (a data point) to more engaged roles including tighter collaboration with researchers, to ultimately initiating, conducting, ‘owning’ or ‘leading’ research themselves (Table 1).
Table 1.
Levels of patient and service user engagement in research
Thirty‐seven sources describing frameworks/conceptualizations of PSUE converged into a synthesized framework (Fig. 3), comprised of three broad phases of research (preparatory, execution and translational phases), which are, in turn, comprised of specific stages. Five sources were most complete and useful in providing terminology and structure,53, 60, 61, 62, 63 and so we drew from these most heavily in the terms and examples provided for Fig. 3.
Figure 3.

Phases and stages of patient and service user engagement in research.
Phase I: Preparatory phase
This phase consists of setting research and funding/resource allocation agendas (including identifying and prioritizing key topics and questions), in the service of answering the question: What to research?63 Thirty‐three of 202 studies described PSUE in this phase. Notably, some studies or PSUE efforts have been initiated or primarily conducted by PSURs themselves,64, 65, 66 sometimes due to their dissatisfaction with existing care or weariness of being disempowered research ‘subjects’.67, 68 The preparatory phase includes two stages:
Stage 1: Agenda Setting. Broadly, as revealed in a prior systematic review that summarized studies published through 2008,69 a sizable literature (156 studies) exists in which PSURs have actively taken part in shaping research priorities; PSURs specifically identified important research topics or questions in 148 of those studies.
Stage 2: Funding. PSUE in funding aspects was much more limited than agenda setting. One study reported some involvement in developing a bid or a proposal, and another reported PSURs' input leading to improved coverage of all trial expenses.70, 71 We also found a survey by INVOLVE that evaluated PSUE in commissioning and funding processes.23 The 2007 survey of 32 statutory and voluntary organizations showed that around 80% of surveyed organizations never or hardly ever considered PSUE as a criterion of funding. Also, 50% of organizations never involved PSURs in funding decisions. It is worth noting research conducted by The James Lind Alliance on ‘patient and public involvement’ among 55 clinical research organizations and funders in the UK along with a review of the literature.72 This assessment found that current funding decisions are largely based on judgment about scientific merit, rather relevance and importance of outcomes to PSURs.
Phase II: Execution phase
Study execution includes PSUR feedback or participation in the development of study design and procedures and execution of the protocol (including subject enrolment, delivering the intervention and data collection and analysis).
Stage 1: Study Design and Procedures. Study design includes the selection of primary outcomes and methods, to answer the question: How to do the research project?63 Thirty studies described PSUE in study design and procedures. One notable aspect of PSUE here is the issue of ethical considerations raised by PSURs.37
Stage 2: Recruitment and participation. Forty‐three sources described PSUE pertaining to subjects/participant recruitment. In particular, numerous systematic reviews and studies examined individuals' perceptions of, or experiences in, trials to address low trial enrolment and participation among different populations.73, 74, 75, 76 One systematic review15 found evidence that a critical reason for low trial enrolment could be confusion and difficulties with the concept of randomization: without an understanding of the structure and aim of a trial, the decision to participate will be extremely difficult to make.77 Indeed, there is often a lack of distinction between the goals of research and medical care, and so all parties need assistance to ensure that decisions are consistent with participants' values.78 Thus, PSUE here can ensure clearer communication and better understanding for prospective subjects/participants.
Stage 3: Data Collection. Seven studies addressed PSUE in data collection. A clear need for more attention is needed: one survey taken by Cochrane research groups79 showed that one in three respondents did not include consumers' perspectives in determining the data to be collected in systematic reviews. There was also no apparent consensus regarding the importance of identifying and collecting information on PSUR‐defined outcomes or on integrating such information into their activities. Yet, PSUE in developing self‐report questionnaires/indices can be helpful80, 81, 82 and may produce evidence more consistent with PSURs' concerns and minimize bias towards providers' perspectives. In addition, PSUE allowed PSURs who were uncomfortable with questionnaires to participate – for example, engaging key PSURs as trained research assistants in collecting and analysing data.80
Stage 4: Data Analysis. Twelve included studies, with various research designs, presented relevant data regarding PSUE at this stage. Largely, PSUE in data analysis – including presenting findings and conclusions to participants prior to publication – resulted in an improved ability to contextualize conclusions to PSURs' environments and beliefs, added language and cultural insight and highlighted PSURs' priorities for a more focused analysis.37, 44, 83, 84, 85, 86 Additionally, PSUE at this stage may increase the validity of findings, as the different parties must reach consensus on emerging conclusions, acting as checks and balances on one another's biases.80 In analysis perhaps more than any other stage, PSUE requires sufficient education and training.37, 44, 80 However, PSURs' abilities should not be undervalued: challenges such as serious mental illness80 or lack of resources in developing countries37 have not prevented PSURs from understanding research foundations, giving feedback and strengthening research. Thus, information should be understandable to PSURs while avoiding oversimplification.83
Phase III: Translational phase
This phase consists of post‐analysis activities.
Stage 1: Dissemination. Twelve studies addressed dissemination. Although peer‐reviewed publication is the common dissemination target for academics, it is not the most direct way to disseminate findings to laypersons.53, 87 A dissemination approach decided jointly by researchers and PSURs is critical to the success of research partnerships, and this process should be personalized and accessible for individuals' different abilities and preferences even within the same population.40, 53, 60, 88 Such accessibility includes language and terminology according to the target population and purpose of the publication – in most scenarios, jargon and non‐applied information should be avoided, which can be facilitated by PSUE.37, 40, 53, 60, 89, 90, 91 Regular updates, through newsletters, mailings or other modes can improve confidence and buy‐in for research projects among target populations.37, 44 In addition, PSUE may help develop creative dissemination methods, which are more efficient and which may not have been considered by researchers.16, 60, 92 This is important, because adequate dissemination has proven to be helpful for future implementation, and making results known within several strata may facilitate obtaining resources and funding for implementation.53
Stage 2: Implementation . Twenty‐eight sources described PSUE in implementation of findings. Several studies advocated involving PSURs throughout implementation44, 53, 60, 89, 93, 94 and called for adequate PSUR education and support37, 60, 89 to maintain their interest and enthusiasm.95, 96 Involvement of authority figures in the community was also helpful for buy‐in.37, 97 However, PSURs may create a force for implementation by themselves, without researcher involvement.86 PSUE was most helpful in prioritizing the sequence of steps and targeting the methodology of implementation.40, 88, 98 Flexible and creative plans are needed; problems may arise unexpectedly during implementation and should serve as learning opportunities.37, 94, 95
Clinical practice guidelines (CPGs) are a form of PSUE in implementation, which may have the greatest impact on health care and services. One systematic review99 found that the most frequently cited objective for PSUE in developing guidelines was to incorporate PSURs' values or perspectives in CPG recommendations, an aspect emphasized by most modern guideline development schemes (e.g. the Grading Recommendations, Assessment, Development and Evaluation/GRADE100), guideline rigour evaluation tools (e.g. Appraisal of Guidelines for REsearch & Evaluation instrument/AGREE II101) or guideline implementability evaluation tools (GuideLine Implementability Appraisal/GLIA102). Boivin et al.6 surveyed 56 guideline developers and highlighted the role of PSUE and the need for training and education. And, of course, PSUE is currently in progress in systematic reviews,103 which will provide evidence for future CPGs. However, there remains no clear guidance or evidence on processes or outcomes to inform the design of PSUE in CPG development, and one systematic review found that PSUR contributions are frequently not acted upon, raising the spectre of tokenistic engagement.104
Stage 3: Evaluation. Very few studies (only 5) addressed this step and mostly did not provide sufficient details. Authors of these studies advised that the evaluation process should be constant; waiting until the end of the process will make problem‐solving more difficult and resource‐intensive.37, 94 A continuous flow of information from PSURs also demonstrated great value,91, 92 as did having clear, pre‐defined assessment tools.97 Despite the benefits of constantly evaluating relationships between PSURs and research teams, the extent of PSURs' participation needs to be clarified to avoid conflicts and favour the development of future projects.53
Environmental scan
The environmental scan revealed numerous links to relevant Websites, organizations, forums, blogs, videos, associations, workshops, presentations, governmental agencies, abstracts and other unpublished resources spanning both health‐care and non‐health‐care PSUE. Some focused on shared decision making in the clinical context and were less relevant to this review, whereas others discussed participatory action research or community‐based participatory research. The most relevant resources can be roughly categorized into three types:
Disease‐specific social networks (mostly not‐for‐profit, PSUR‐established). Many offered insight on participation in research and guided patients and service users to on‐going trials and investigational treatment. Few provided education; one notable example was The Association of Cancer Online Resources,105 which provided clinical trial FAQ; access to investigational drugs; and guides to trial terminology and finding clinical trials.
Non‐disease‐specific Websites focused on PSUE (most commonly in Europe, specifically the UK, and in Canada). Examples include the National Institutes for Health Research,106 the James Lind Alliance,107 and the PatientPartner project,108 which aim to help PSURs set priorities, have input on proposals and funding, actively participate in studies and collaborate with clinicians, researchers and other stakeholders to strengthen research, create new partnerships and address challenges.
Models for PSUE, which were fairly uncommon. Two examples are ‘A Model Framework for Consumer and Community Participation in Health and Medical Research'63, created by Australia's National Health and Medical Research Council; and the ‘National Health Service Patient Involvement Toolkit.'93 These provided a rationale for PSUE at every step of research, and their findings are incorporated in the frameworks presented in this report.
Patient advisory group feedback
We presented our findings and recommendations to a PAG,35 a committee of community member PSURs with a long history of research engagement. Members provided the following feedback:
The terms informant/patient/surrogate/consumer/customer/representative were fairly confusing and none seemed satisfactory, although patient or informant seemed most intuitive. Associated explanatory text attached to any terms used was deemed necessary.
PAG members understood the purpose of the frameworks presented and valued the need for such frameworks.
PAG members equally rated the importance of the four components of the framework (participant selection, building reciprocal provided, colearning and re‐assessment and feedback).
PAG members rated as most important the overarching recommendation to engage PSURs in all three phases of research, as benefits that would likely outweigh difficulties.
In general, PAG members found the extent of possible engagement in research to be surprising.
PAG members provided some suggestions for wording, graphics and presentation.
Comparison with other systematic reviews
A systematic review by Oliver et al. regarding PSUR (‘consumer’) involvement in research and development agenda setting for the UK's National Health Service109 emphasized processes, outcomes and relevant factors of PSUR participation in identifying and prioritizing research topics (similar to part of the ‘preparatory phase’ in the present framework). Within this area, they presented a multipart framework, focused on (i) the different consumers involved, (ii) who initiated the research, (iii) the degree of PSUR involvement, (iv) forums for communication, (v) decision‐making methods, and (vi) practical issues in implementing PSUE in agenda setting. A second review, from a cancer organization in Australia, focused on ‘consumer involvement’ specifically in ‘cancer control’ (meaning research, but also prevention, early detection, treatment and other components).110 Rather than stages of research, this review emphasized key elements of PSUE, including committed organizations, shared focus and other factors, similar to the first part of our two‐part framework. Another review, by Staley for INVOLVE,111 emphasized ‘public’ involvement (including patients, service users, organizations, community members and others) and focused on the impact of public involvement by different stages of research, similar to the second part of our current framework. This review called for a greater evidence base, noting the largest gaps in research on PSUE impact on funding and impact on certain kinds of analyses. Finally, the PIRICOM study17 examined conceptual/theoretical trends, measurement and impact of PSUE on health‐care and social‐care research. Among other findings, the PIRICOM study concluded that relatively little conceptual or theoretical development exists regarding PSUE, noting that papers that have focused on conceptualization in the past have been based upon reflection or opinion.
These previous reviews have varyingly examined the levels of PSUR involvement, key components necessary for PSUE and/or one or more of the stages at which it occurs. They have also, varyingly, emphasized mainly one phase (e.g. agenda setting) but not others,109 emphasized research stages over key components111 or vice versa110; described conceptions in other papers without synthesizing a broadly applicable framework of their own17; or examined approaches to PSUE alongside its impacts, outcomes or other activities as part of a broader view.17, 111 Our review attempts to capture and synthesize the components and stages presented across previous reviews (along with other studies) with the goal of presenting a comprehensive framework and language that can be directly applied to future studies in reporting PSUE activities.
Limitations and strengths
The main limitation to this systematic review is the non‐comparative, observational and/or non‐empirical nature of available literature. Therefore, our two‐part framework of the components and stages of PSUE engagement is built from sometimes disconnected, and insufficiently tested or reported, literatures. Additionally, the lack of specific indexing terms in bibliographic databases means that some studies using PSUE may have been missed, and there is a lack of standardized, explicit reporting for PSUE processes. Therefore, standard reporting guidelines for study designs (e.g. the CONSORT statement for randomized trials) can be enhanced by including a template for reporting use of PSUE and at what phases and stages. To overcome these challenges in indexing and reporting, we attempted an environmental scan to supplement the literature search. Heterogeneity of populations, methods and outcomes constitute further limitations to extrapolation of evidence. Publication and reporting biases have also likely affected the conclusions of this report, and their impact could not be estimated.
The strengths of this report include a comprehensive and sensitive search strategy spanning multiple databases and augmented by an environmental scan of unpublished relevant sources and contact with content experts to further capture related studies, Websites and viewpoints. A priori protocols for selecting and appraising evidence were implemented to reduce biased selection of studies. Our review and synthesized framework lay the groundwork for a standardized evidence base using guided indexing procedures and following a synthesized model.
Knowledge gaps and recommendations for research
Based on the proposed model, the following areas of research are needed to develop a complete body of evidence:
PSUE in the phase of study execution and specifically in the areas of data collection and analysis is needed. Available literature focuses on earlier (agenda setting and participant enrolment) and some later stages (translation) of research.
Research comparing the different methods of engagement and obtaining PSUR voice is needed, but this will require standard reporting and measurement.
Peer‐reviewed studies are needed that incorporate and describe PSUE as outlined in our synthesized framework to assess feasibility; it is notable that of the 41 sources which most informed the structure of the synthesized framework, most provided no original evidence to support their framework or conceptualization.
Proper indexing of studies on PSUE will facilitate future synthesis of evidence and advancement of methods and outcomes.
Our review reveals a disconnect in the approaches used by existing studies; the need for a standard framework and language is clear. In synthesizing existing work into such a framework, the present paper also provides such a framework with the broad applicability and cohesive underpinnings necessary to integrate existing knowledge and guide future endeavours.
Funding source
This project was partially funded by the Patient‐Centered Outcomes Research Institute (PCORI); the data were presented, in part, within a larger presentation to PCORI's Methodology Committee in Baltimore, MD, 5 March 2012. The content of that presentation and other projects are available at PCORI's Website (pcori.org).
Supporting information
Data S1. Included sources.
References
- 1. Telford R, Faulkner A. Learning about service user involvement in mental health research. Journal of Mental Health, 2004; 13: 549–559. [Google Scholar]
- 2. Marsden J, Bradburn J; Clinical TCAGf ; Macmillan CLJ . Patient and clinician collaboration in the design of a national randomized breast cancer trial. Health Expectations, 2004; 1: 6–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abma TA, Nierse CJ, Widdershoven GAM. Patients as partners in responsive research: methodological notions for collaborations in mixed research teams. Qualitative Health Research, 2009; 19: 401–415. [DOI] [PubMed] [Google Scholar]
- 4. O'Donnell M, Entwistle V. Consumer involvement in decisions about what health‐related research is funded. Health Policy, 2004; 70: 281–290. [DOI] [PubMed] [Google Scholar]
- 5.patientslikeme. patientslikeme [12/1/2011]. Available at: http://www.patientslikeme.com, accessed 22 May 2013.
- 6. Boivin A, Currie K, Fervers B et al Patient and public involvement in clinical guidelines: international experiences and future perspectives. Quality and Safety in Health Care, 2010; 19: 1–4. [DOI] [PubMed] [Google Scholar]
- 7. Abma TA, Broerse JEW. Patient participation as dialogue: setting research agendas. Health Expectations, 2010; 13: 160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ali K, Roffe C, Crome P. What patients want. Stroke, 2006; 37: 865–871. [DOI] [PubMed] [Google Scholar]
- 9. Tong A, Sainsbury P, Carter SM et al Patients' priorities for health research: focus group study of patients with chronic kidney disease. Nephrology Dialysis Transplantation, 2008; 23: 3206–3214. [DOI] [PubMed] [Google Scholar]
- 10. Shea B, Santesso N, Qualman A et al Consumer‐driven health care: building partnerships in research. Health Expectations, 2005; 8: 352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O, Peacock R. Storylines of research in diffusion of innovation: a meta‐narrative approach to systematic review. Social Science & Medicine, 2005; 61: 417–430. [DOI] [PubMed] [Google Scholar]
- 12. Andejeski Y, Bisceglio IT, Dickersin K et al Quantitative impact of including consumers in the scientific review of breast cancer research proposals. Journal of Women's Health & Gender‐Based Medicine, 2002; 11: 379–388. [DOI] [PubMed] [Google Scholar]
- 13. Faulkner A, Thomas P. User‐led research and evidence‐based medicine. The British Journal of Psychiatry, 2002; 180: 1–3. [DOI] [PubMed] [Google Scholar]
- 14. Gooberman‐Hill R, Horwood J, Calnan M. Citizens' juries in planning research priorities: process, engagement and outcome. Health Expectations, 2008; 11: 272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Donovan JL, Brindle L, Mills N. Capturing users' experiences of participating in cancer trials. European Journal Cancer Care (Engl), 2002; 11: 210–214. [DOI] [PubMed] [Google Scholar]
- 16. White MA, Verhoef MJ. Toward a patient‐centered approach: incorporating principles of participatory action research into clinical studies. Integrative Cancer Therapies, 2005; 4: 21–24. [DOI] [PubMed] [Google Scholar]
- 17. Brett J, Staniszewska S, Mockford C, Seers K, Herron‐Marx S, Bayliss H. The PIRICOM Study: A Systematic Review of the Conceptualisation, Measurement, Impact and Outcomes of Patients and Public Involvement in Health and Social Care Research. Warwick: Royal College of Nursing, University of Warwick, and UK Clinical Research Collaborative, Studies SoHS, 2010. [Google Scholar]
- 18. Thompson J, Barber R, Ward PR et al Health researchers' attitudes towards public involvement in health research. Health Expectations, 2009; 12: 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bogart LM, Uyeda KE. Community‐based participatory research. Health Psychology, 2009; 28: 391–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caron‐Flinterman JF, Broerse JEW, Bunders JFG. The experiential knowledge of patients: a new resource for biomedical research? Social Science & Medicine, 2005; 60: 2575–2584. [DOI] [PubMed] [Google Scholar]
- 21. Nierse CJ, Schipper K, van Zadelhoff E, van de Griendt J, Abma TA. Collaboration and co‐ownership in research: dynamics and dialogues between patient research partners and professional researchers in a research team. Health Expectations, 2011; 15: 242–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Howe A, MacDonald H, Barrett B, Little B. Ensuring public and patient participation in research: a case study in infrastructure development in one UK Research and Development consortium. Primary Health Care Research and Development, 2006; 7: 60–67. Epub 31 Oct 2006. [Google Scholar]
- 23. INVOLVE. July 2012. [cited 2012]. http://www.invo.org.uk/, accessed 22 May 2013.
- 24. Hanley B, Truesdale A, King A, Elbourne D, Chalmers I. Involving consumers in designing, conducting, and interpreting randomised controlled trials: questionnaire survey. BMJ, 2001; 322: 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boote J, Telford R, Cooper C. Consumer involvement in health research: a review and research agenda. Health Policy, 2002; 61: 213–236. [DOI] [PubMed] [Google Scholar]
- 26. McKevitt C, Fudge N, Wolfe C. What is involvement in research and what does it achieve? Reflections on a pilot study of the personal costs of stroke. Health Expectations, 2010; 13: 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hewlett S, de Wit M, Richards P et al Patients and professionals as research partners: challenges, practicalities, and benefits. Arthritis Care & Research, 2006; 55: 676–680. [DOI] [PubMed] [Google Scholar]
- 28. Whitstock MT. Seeking evidence from medical research consumers as part of the medical research process could improve the uptake of research evidence. Journal of Evaluation in Clinical Practice, 2003; 9: 213–224. [DOI] [PubMed] [Google Scholar]
- 29. Wright D, Foster C, Amir Z, Elliott J, Wilson R. Critical appraisal guidelines for assessing the quality and impact of user involvement in research. Health Expectations, 2010; 13: 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dewar BJ. Beyond tokenistic involvement of older people in research – a framework for future development and understanding. Journal of Clinical Nursing, 2005; 14: 48–53. [DOI] [PubMed] [Google Scholar]
- 31. Hubbard G, Kidd L, Donaghy E, McDonald C, Kearney N. A review of literature about involving people affected by cancer in research, policy and planning and practice. Patient Education and Counseling, 2007; 65: 21–33. [DOI] [PubMed] [Google Scholar]
- 32. Faridi Z, Grunbaum JA, Sajor Grey B, Franks A, Simoes E. Community‐based participatory research: necessary next steps. Preventing Chronic Disease, 2007; 4: A70. [PMC free article] [PubMed] [Google Scholar]
- 33. Bennetts W, Cross W, Bloomer M. Understanding consumer participation in mental health: issues of power and change. International Journal of Mental Health Nursing, 2011; 20: 155–164. [DOI] [PubMed] [Google Scholar]
- 34. Chambers R, Brien LM, Linnell S, Sharp S. Why don't health researchers report consumer involvement? Quality in Primary Care, 2004; 12: 151–157. [Google Scholar]
- 35. Shared Decision Making National Resource Center. Patient Advisory Group: Mayo Clinic; 2012. [08/02/2012]. Available from: http://shareddecisions.mayoclinic.org/stakeholders/diabetes-advisory-group/, accessed 22 May 2013.
- 36. Lindenmeyer A, Hearnshaw H, Sturt J, Ormerod R, Aitchison G. Assessment of the benefits of user involvement in health research from the Warwick Diabetes Care Research User Group: a qualitative case study. Health Expectations, 2007; 10: 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morin SF, Morfit S, Maiorana A et al Building community partnerships: case studies of Community Advisory Boards at research sites in Peru, Zimbabwe, and Thailand. Clinical Trials, 2008; 2: 147–156. [DOI] [PubMed] [Google Scholar]
- 38. May M, Law J. CBPR as community health intervention: institutionalizing CBPR within community based organizations. Progress in Community Health Partnerships, 2008; 2: 145–155. [DOI] [PubMed] [Google Scholar]
- 39. Redwood D, Lanier A, Kemberling M, Klejka J, Sylvester I, Lundgren K. Community‐based participatory research in a large cohort study of chronic diseases among Alaska native adults. Progress in Community Health Partnerships, 2010; 4: 325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Evans S, Corley M, Corrie M, Costley K, Donald C. Evaluating services in partnership with older people: exploring the role of ‘community researchers’. Working with Older People: Community Care Policy & Practice, 2011; 15: 26–33. [Google Scholar]
- 41. Jinks C, Ong BN, Neill TJO. The Keele community knee pain forum: action research to engage with stakeholders about the prevention of knee pain and disability. BMC Musculoskeletal Disorders, 2009; 10: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Swartz LJ, Callahan KA, Butz AM et al Methods and issues in conducting a community‐based environmental randomized trial. Environmental Research, 2004; 95: 156–165. [DOI] [PubMed] [Google Scholar]
- 43. Stewart MK, Colley D, Huff A et al Participatory development and implementation of a community research workshop: experiences from a community‐based participatory research partnership. Progress in Community Health Partnerships, 2009; 3: 165–178. [DOI] [PubMed] [Google Scholar]
- 44. Crowe JL, Keifer MC, Salazar MK. Striving to provide opportunities for farm worker community participation in research. Journal of Agricultural Safety and Health, 2008; 14: 205–219. [DOI] [PubMed] [Google Scholar]
- 45. Decker M, Hemmerling A, Lankoande F. Women front and center: the opportunities of involving women in participatory health research worldwide. Journal of Women's Health (15409996), 2010; 19: 2109–2114. [DOI] [PubMed] [Google Scholar]
- 46. Doyle M, Timonen V. Lessons from a community‐based participatory research project: older people's and researcher's reflections. Research on Aging, 2010; 32: 244–263. [Google Scholar]
- 47. Fawcett SB, Suarez‐Balcazar Y, Balcazar FE et al Conducting intervention research—the design and development process In: Rothman J, Thomas EJ, (eds) Intervention research: Design and development for human service. New York: Haworth Pr, 1994. [Google Scholar]
- 48. Morrow E, Ross F, Grocott P, Bennett J. A model and measure for quality service user involvement in health research. International Journal of Consumer Studies, 2010; 34: 532–539. [Google Scholar]
- 49. Turner M, Beresford P. User Controlled Research: Its Meanings and Potential. Final Report 2005 Eastleigh, Hants, UK: Shaping Our Lives and the Centre for Citizen Participation, Brunel University, 2005. [cited 2013 April 1]. Available at: http://www.invo.org.uk/wp-content/uploads/2011/12/UserConRpt081205.pdf, accessed 22 May 2013. [Google Scholar]
- 50. Oscos‐Sanchez MA, Lesser J, Kelly P. Cultural competence: a critical facilitator of success in community‐based participatory action research. Issues in Mental Health Nursing, 2008; 2: 197–200. [DOI] [PubMed] [Google Scholar]
- 51. Ahmed SM, Palermo AGS. Community engagement in research: frameworks for education and peer review. American Journal of Public Health, 2010; 8: 1380–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Karmaliani R, McFarlane J, Asad N et al Applying community‐based participatory research methods to improve maternal and child health in Karachi, Pakistan. Nursing Outlook, 2009; 57: 204–209. [DOI] [PubMed] [Google Scholar]
- 53. Harper GW, Salina DD. Building collaborative partnerships to improve community‐based HIV prevention research: the University‐CBO Collaborative partnership (UCCP) model. Journal of Prevention and Intervention in the Community, 2000; 1: 1–20. [Google Scholar]
- 54. Danley KE, Ellison ML. A Handbook for Participatory Action Research. Boston: Center for Psychiatric Rehabilitation Sargent College of Health and Rehabilitation Sciences Boston University, 2005. [Google Scholar]
- 55. Becker AB, Israel BA, Allen AJ III Instructions for conducting a force field analysis. In: Israel BA, Eng E, Schulz AJ, Parker EA, Satcher D. (eds). Methods in Community‐Based Participatory Research for Health. San Francisco: Jossey‐Bass, 2005. [Google Scholar]
- 56. Barnes M, Bennett G. Frail bodies, courageous voices: older people influencing community care. Health & Social Care in the Community, 1998; 6: 102–111. [DOI] [PubMed] [Google Scholar]
- 57. Oliver SR, Rees RW, Clarke‐Jones L et al A multidimensional conceptual framework for analysing public involvement in health services research. Health Expectations, 2008; 1: 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hall V. Reflections on engaging in participatory research. Evidence Based Midwifery, 2009; 7: 40–45. [Google Scholar]
- 59. Happell B, Roper C. Consumer participation in mental health research: articulating a model to guide practice. Australasian Psychiatry, 2007; 3: 237–241. [DOI] [PubMed] [Google Scholar]
- 60. Gracia J, Blasco JA, Andradas E. A strategy for patient involvement in clinical practice guidelines: methodological approaches. BMJ Quality & Safety, 2011; 20: 779–784. [DOI] [PubMed] [Google Scholar]
- 61. Israel BA, Krieger J, Vlahov D et al Challenges and facilitating factors in sustaining community‐based participatory research partnerships: lessons learned from the Detroit, New York City and Seattle Urban Research Centers. Journal of Urban Health, 2006; 83: 1022–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Saunders C, Crossing S, Girgis A, Butow P, Penman A. Operationalising a model framework for consumer and community participation in health and medical research. Aust New Zealand Health Policy, 2007; 4: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. National Health and Medical Research Council . A Model Framework for Consumer and Community Participation in Health and Medical Research. Canberra: Australian Government, 2005. [Google Scholar]
- 64. Wicks P, Vaughan TE, Massagli MP, Heywood J. Accelerated clinical discovery using self‐reported patient data collected online and a patient‐matching algorithm. Nature Biotechnology, 2011; 29: 411–414. [DOI] [PubMed] [Google Scholar]
- 65. Tweet MS, Gulati R, Aase LA, Hayes SN. Spontaneous coronary artery dissection: a disease‐specific, social networking community‐initiated study. Mayo Clinic Proceedings, 2011; 86: 845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chenoweth L, Kilstoff K. Facilitating positive changes in community dementia management through participatory action research. International Journal of Nursing Practice, 1998; 3: 175–188. [DOI] [PubMed] [Google Scholar]
- 67. Staniszewska S, Jones N, Newburn M, Marshall S. User involvement in the development of a research bid: Barriers, enablers and impacts. Health Expectations, 2007; 10: 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lippman SA, Donini A, Diaz J, Chinaglia M, Reingold A, Kerrigan D. Social‐environmental factors and protective sexual behavior among sex workers: the Encontros intervention in Brazil. American Journal of Public Health, 2010; 100 (Suppl. 1): S216–S223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stewart RJ, Caird J, Oliver K, Oliver S. Patients' and clinicians' research priorities. Health Expectations, 2011; 4: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Edwards V, Wyatt K, Logan S, Britten N. Consulting parents about the design of a randomized controlled trial of osteopathy for children with cerebral palsy. Health Expectations, 2011; 14: 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Staniszewska S, Brett J, Mockford C, Barber R. The GRIPP checklist: strengthening the quality of patient and public involvement reporting in research. International Journal of Technology Assessment [DOI] [PubMed] [Google Scholar]
- 72. Staley K, Hanley B. Scoping research priority setting (and the presence of PPI in priority setting) with UK clinical research organisations and funders, 2008. Report. http://www.lindalliance.org/pdfs/JLA%20Internal%20Reports/TwoCan%20JLA%20report%20March%2009_with%20appendices.pdf, accessed 22 May 2013.
- 73. Lovato LC, Hill K, Hertert S, Hunninghake DB, Probstfield JL. Recruitment for controlled clinical trials: literature summary and annotated bibliography. Controlled Clinical Trials, 1997; 18: 328–352. [DOI] [PubMed] [Google Scholar]
- 74. Pringle M, Churchill R. Randomised controlled trials in general practice. BMJ, 1995; 311: 1382–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tognoni G, Alli C, Avanzini F et al Randomised clinical trials in general practice: lessons from a failure. BMJ, 1991; 303: 969–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hussain‐Gambles M, Leese B, Atkin K, Brown J, Mason S, Tovey P. Involving South Asian patients in clinical trials. Health Technology Assessment, 2004; 8: iii1–109. [DOI] [PubMed] [Google Scholar]
- 77. Snowdon C, Garcia J, Elbourne D. Making sense of randomization; responses of parents of critically ill babies to random allocation of treatment in a clinical trial. Social Science & Medicine, 1997; 45: 1337–1355. [DOI] [PubMed] [Google Scholar]
- 78. Snowdon C, Elbourne D, Garcia J. ‘It was a snap decision': parental and professional perspectives on the speed of decisions about participation in perinatal randomised controlled trials. Social Science & Medicine, 2006; 9: 2279–2290. [DOI] [PubMed] [Google Scholar]
- 79. Kelson MC. Consumer collaboration, patient‐defined outcomes and the preparation of Cochrane Reviews. Health Expectations, 1999; 2: 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Whitley R, Goldman HH. Letters: client involvement in services research. Psychiatric Services, 2005; 56: 1315. [DOI] [PubMed] [Google Scholar]
- 81. Wright JG, Young NL. The patient‐specific index: asking patients what they want. Journal of Bone and Joint Surgery. American Volume, 1997; 79: 974–983. [DOI] [PubMed] [Google Scholar]
- 82. Garcia CM, Gilchrist L, Campesino C, Raymond N, Naughton S, de Patino JG. Using community‐based participatory research to develop a bilingual mental health survey for Latinos. Progress in Community Health Partnerships, 2008; 2: 105–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cashman SB, Adeky S, Allen AJ III et al The power and the promise: working with communities to analyze data, interpret findings, and get to outcomes. American Journal of Public Health, 2008; 98: 1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. McCauley LA, Beltran M, Phillips J, Lasarev M, Sticker D. The Oregon migrant farmworker community: an evolving model for participatory research. Environmental Health Perspectives, 2001; 109 (Suppl. 3): 449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cotterell P. Exploring the value of service user involvement in data analysis: ‘Our interpretation is about what lies below the surface'. Educational Action Research, 2008; 16: 5–17. [Google Scholar]
- 86. Chalmers I. What do I want from health research and researchers when I am a patient? BMJ, 1995; 310: 1315–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Seifer SD, Sisco S. Mining the challenges of CBPR for improvements in urban health. Journal of urban health: bulletin of the New York Academy of Medicine, 2006; 83: 981–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Roe KM, Minkler M, Saunders FF. Combining research, advocacy, and education: the methods of the Grandparent Caregiver Study. Health Education Quarterly, 1995; 22: 458–475. [DOI] [PubMed] [Google Scholar]
- 89. van Wersch A, Eccles M. Involvement of consumers in the development of evidence based clinical guidelines: practical experiences from the North of England evidence based guideline development programme. Quality in health care: QHC, 2001; 10: 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sayers J. Clinical trial registries: a survey of patient advocate group perceptions. Drug Information Journal, 2009; 2: 195–200. [Google Scholar]
- 91. Freysteinson WM. The ethical community consultation model as preparation for nursing research: a case study. Nursing Ethics, 2010; 6: 749–758. [DOI] [PubMed] [Google Scholar]
- 92. van Staa T‐P, Leufkens HG, Zhang B, Smeeth L. A comparison of cost effectiveness using data from randomized trials or actual clinical practice: selective cox‐2 inhibitors as an example. PLoS Medicine, 2009; 6: e1000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Involvement Team: NHS County Durham and Darlington . Patient & Public Involvement Toolkit: a quick guide to support healthcare commissioners. NHS, 2011. Report. Available at: http://www.darlington.gov.uk/PublicMinutes/Health%20and%20Partnerships%20Scrutiny%20Committee/February52014%202012/Appendix%205%20-%20Appendix%205.pdf, accessed 22 May 2013. [Google Scholar]
- 94. Potvin L, Cargo M, McComber AM, Delormier T, Macaulay AC. Implementing participatory intervention and research in communities: lessons from the Kahnawake Schools Diabetes Prevention Project in Canada. Social Science & Medicine, 2003; 56: 1295–1305. [DOI] [PubMed] [Google Scholar]
- 95. Butterfoss FD. Process evaluation for community participation. Annual Review of Public Health, 2006; 27: 323–340. [DOI] [PubMed] [Google Scholar]
- 96. Thurston WE, Vollman AR, Meadows LM, Rutherford E. Public participation for women's health: strange bedfellows or partners in a cause? Health Care for Women International, 2005; 26: 398–421. [DOI] [PubMed] [Google Scholar]
- 97. Thurston WE, MacKean G, Vollman A et al Public participation in regional health policy: a theoretical framework. Health Policy, 2005; 73: 237–252. [DOI] [PubMed] [Google Scholar]
- 98. Ntshanga SP, Ngcobo PS, Mabaso ML. Establishment of a Community Advisory Board (CAB) for tuberculosis control and research in the Inanda, Ntuzuma and KwaMashu (INK) area of KwaZulu‐Natal, South Africa. Health Policy, 2010; 2–3: 211–215. [DOI] [PubMed] [Google Scholar]
- 99. Legare F, Boivin A, van der Weijden T et al Patient and public involvement in clinical practice guidelines: a knowledge synthesis of existing programs. Medical Decision Making, 2011; 31: E45–E74. [DOI] [PubMed] [Google Scholar]
- 100. Guyatt G, Oxman AD, Akl EA et al GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology, 2011; 64: 383–394. [DOI] [PubMed] [Google Scholar]
- 101. Brouwers MC, Kho ME, Browman GP et al AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ, 2010; 182: E839–E842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shiffman RN, Dixon J, Brandt C et al The GuideLine Implementability Appraisal (GLIA): development of an instrument to identify obstacles to guideline implementation. BMC Medical Informatics and Decision Making, 2005; 5: 23. doi: 10.1186/1472‐6947‐5‐23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kreis J, Puhan MA, Schunemann HJ, Dickersin K. Consumer involvement in systematic reviews of comparative effectiveness research. Health Expectations, 2012; doi: 10.1111/j.1369-7625.2011.00722.x [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. van de Bovenkamp HM, Trappenburg MJ. Reconsidering patient participation in guideline development. Health Care Analysis, 2009; 3: 198–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. ACOR .org. Association of Cancer Online Resources, 2012. Available at: http://www.acor.org/pages/faqs, accessed 22 May 2013.
- 106. NHS . National Institute for Health Research, 2012. Available at: http://www.nihr.ac.uk/Pages/default.aspx, accessed 22 May 2013.
- 107. lindalliance.org. The James Lind Alliance, 2012. Available at: http://www.lindalliance.org/, accessed 22 May 2013.
- 108. thepatientpartnerproject.org. The PatientPartner project, 2012. Available at: http://www.thepatientpartnerproject.org/, accessed 22 May 2013.
- 109. Oliver S, Clarke‐Jones L, Rees RW et al Involving consumers in research and development agenda setting for the NHS: developing n evidence‐based approach. Health Technology Assessment, 2004; 8: 1–148. [DOI] [PubMed] [Google Scholar]
- 110. Cancer Australia and Cancer Voices Australia . National Framework for Consumer Involvement in Cancer Control. Canberra, Australia: Cancer Australia, 2011. [Google Scholar]
- 111. Staley K. Exploring Impact: Public involvement in NHS, Public Health and Social Care Research. Eastleigh, UK: INVOLVE‐National Health Service‐National Institute for Health Research, 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Included sources.


