Abstract
Objective
To investigate whether the introduction of non‐invasive pre‐natal testing for Down's syndrome (DS) has the potential to undermine informed choice.
Participants
Three hundred and ninety‐three health professionals; 523 pregnant women.
Methods
A cross‐sectional questionnaire study across nine maternity units and three conferences in the UK designed to assess opinions regarding test delivery and how information should be communicated to women when offered Down's syndrome screening (DSS) or diagnosis using invasive (IDT) or non‐invasive testing (NIPT).
Results
Both pregnant women and health professionals in the NIPT and DSS groups were less likely than the IDT group to consider that testing should take place at a return visit or that obtaining written consent was necessary, and more likely to think testing should be carried out routinely. Compared to health professionals, pregnant women expressed a stronger preference for testing to occur on the same day as pre‐test counselling (P = 0.000) and for invasive testing to be offered routinely (P = 0.000). They were also more likely to indicate written consent as necessary for DSS (P = 0.000) and NIPT (P < 0.05).
Conclusions
Health professionals and pregnant women view the consenting process differently across antenatal test types. These differences suggest that informed choice may be undermined with the introduction of NIPT for DS into clinical practice. To maintain high standards of care, effective professional training programmes and practice guidelines are needed which prioritize informed consent and take into account the views and needs of service users.
Keywords: Down's syndrome, informed choice, invasive pre‐natal diagnosis, non‐invasive prenatal testing, screening, service delivery
Introduction
In many developed countries, pregnant women are offered screened to provide an estimate of the risk that an individual pregnancy will be affected by Down's syndrome (DS). Women identified as ‘high risk’ are then offered definitive diagnosis through invasive diagnostic testing (IDT) such as amniocentesis or chorionic villus sampling (CVS), both of which require the insertion of a needle into the womb and thus carry a small but significant miscarriage risk of around 1%.1 Guidelines produced by the UK National Screening Committee2 and the Royal College of Obstetricians and Gynaecologists3, 4 state clearly that informed consent must be sought and documented for all pre‐natal screening and testing procedures. Additionally, women should not feel pressurised to accept testing but feel free to choose and be given time to discuss their options, ideally having had information about DS and the screening tests at least 24 h before being asked to make decisions to facilitate informed choice.2
Will the ethical principles that underpin these processes shift with the introduction of non‐invasive pre‐natal testing (NIPT) based on analysis of cell‐free foetal DNA (cffDNA) in maternal blood? Rapid advancements in the development and potential clinical application of NIPT since the discovery of cffDNA in the late 1990's5 have permitted pre‐natal diagnosis of selected genetic conditions without the risk of miscarriage by analysis of a maternal blood sample. Currently in the UK, NIPT is available for foetal sex determination in women at high risk of sex‐linked disorders,6 foetal rhesus D typing in Rhesus negative mothers at high risk of haemolytic disease of the newborn7 and the diagnosis of some monogenic disorders8 including Achondroplasia9 and Thanatophoric dysplasia.10 In some parts of Europe, foetal Rhesus D typing is routinely offered to Rhesus D‐negative mothers to direct antenatal immunoprophylaxis with anti‐D immunoglobulin.11 Following the publication of several large scale demonstration projects,12 NIPT for DS is now available through the private sector in the USA, China and Europe,13 increasing the pressure for rapid implementation into routine clinical practice in the public sector. Within the UK, NIPT for DS is available in the private sector, with samples sent to the USA for analysis. However, implementation into the National Health Service (NHS) will require further evaluation in low risk populations. Furthermore, ethical integrity in implementation at a population level would mean taking into account the views and needs of service users and providers. Quality education for health professionals should also be based on thorough evidencing of these viewpoints.
The potential for routine implementation of this technology has raised ethical concerns.14, 15 IDT carries procedural risks and requires more specialised skills, and therefore tends to take place in specialist units. In the current UK NHS care pathway, referral for IDT usually follows a two‐staged process: screening followed by a ‘high risk’ result, which is accompanied by discussion and decision making before a referral is made. As and when NIPT is introduced, it is unclear whether it will be offered routinely to all women (replacing current screening) or as a replacement to IDT (to those identified as ‘high risk’) in the same two‐staged process.16 In the longer term, given the continued occurrence of false positives, invasive tests will still be necessary to confirm positive results.17, 18 Nevertheless, its simplicity may quickly see it established as a routine aspect of standard pre‐natal care.19, 20 There is recognition that this could undermine informed choice: a fundamental part of ethical clinical practice21 defined as autonomous decision making based on an individual's knowledge and personal values.22
Research shows that people are more likely to make informed decisions when pre‐natal tests are seen as optional rather than routine.23, 24 When offered as part of routine pre‐natal care, tests are often viewed as a recommendation and are therefore more difficult for mothers (wishing to be seen as responsible) to reject.25 Through the provision of adequate pre‐test counselling and informed consent procedures, health‐care services help to ensure that women fully understand the procedure and its implications, and are aware of their right to decline: only through the provision of choice can parents ‘take responsibility for their own reproductive decisions’21(p32). As argued by de Jong and colleagues, facilitating meaningful reproductive choices should be the very purpose of making such tests available.14
Previous research has highlighted the potential for NIPT for DS to be viewed differently from invasive procedures and as more similar to screening (DSS), with subsequently less emphasis placed on the informed consent process.19 However, the study was based on a small sample of health professionals, mostly obstetricians. In order to understand how the introduction of NIPT may impact on professional practice, the views of a larger number of clinicians with the role of providing pre‐test counselling and support drawn from all relevant disciplines is required. Understanding the attitudes of a wider range of health professionals towards the delivery of pre‐natal tests is important when we consider that these attitudes have the potential to affect their professional practice.26 Greater numbers were also considered vital to be able to generalise findings within the UK. Thus far, there have been only a relatively small number of studies exploring the views of service users towards NIPT:27 these mainly relate to the use of NIPT for foetal sex determination or the diagnosis of monogenetic disorders in women at high risk of genetic conditions28, 29 or attitudes towards NIPT for DS in women in the US.30 As such, the inclusion of pregnant women was considered an important addition to this study.
The aim of the current study was to estimate the potential for informed choice within pre‐natal testing to be undermined by the introduction of NIPT for DS, to inform educational and training standards. Viewpoints about pre‐test counselling requirements for NIPT, IDT and DSS formed the focus of the survey. The views of a variety of health professionals and pregnant women were collected and compared.
Materials and methods
This was a cross‐sectional questionnaire study with a between‐subjects design. The National Research Ethics Service Committee South Central – Berkshire (11/SC/0180) approved the study.
Participants
There were two groups of participants: (i) maternity health professionals including midwives, obstetricians, genetic counsellors, clinical geneticists and sonographers (Table S1) and (ii) pregnant women (Table 1, Table S2). Recruitment took place in nine NHS maternity clinics across England. Additional health professionals were recruited at three relevant UK conferences. Exclusions were health professionals not currently practising in the UK and women under the age of 18 and/or not currently pregnant.
Table 1.
Pregnancy‐specific demographics across test types [frequency followed by % of total who responded (in brackets)]
| Total | DSS | IPD | NIPT | P‐value | |
|---|---|---|---|---|---|
| Mean gestation ‐ weeks (SD) and range | 26.5 (9.2) 5–41 | 27 (9.1) 5–41 | 26.8 (9.2) 8–41 | 25.7 (9.3) 8–41 | 0.347 |
| First pregnancy | 170 (33.7) | 53 (31.5) | 67 (39.9) | 50 (29.6) | 0.105 |
| Had fertility treatment | 64 (12.7) | 25 (14.9) | 19 (11.4) | 20 (12) | 0.589 |
| Suffered pregnancy loss in the past | 166 (33.7) | 47 (28.8) | 54 (32.5) | 65 (39.9) | 0.100 |
| Been offered screening in this/previous pregnancy: | |||||
| Yes | 453 (86.6) | 150 (86.7) | 151 (86.3) | 152 (86.9) | 0.995 |
| No | 45 (8.6) | 15 (8.7) | 16 (9.1) | 14 (8) | |
| Not answered | 25 (4.8) | 8 (4.6) | 8 (4.6) | 9 (5.1) | |
| Undergone screening in this/previous pregnancy: | |||||
| Yes | 295 (56.4) | 106 (61.3) | 87 (49.7) | 102 (58.3) | 0.187 |
| No | 181 (34.6) | 55 (31.8) | 71 (46) | 55 (31.4) | |
| Not answered | 47 (9.0) | 12 (6.9) | 17 (9.7) | 18 (10.3) | |
| Undergone IPD (%) in this or previous pregnancy: | |||||
| Yes | 31 (5.9) | 12 (6.9) | 10 (5.7) | 9 (5.1) | 0.721 |
| No | 334 (63.9) | 115 (66.5) | 111 (63.4) | 108 (61.7) | |
| Not answered | 158 (30.2) | 46 (26.6) | 54 (30.9) | 58 (33.1) | |
Materials and questionnaire design
This study employed experimental vignettes describing clinical scenarios adapted from a previous study investigating health professionals' attitudes.16 There were three possible vignettes, each based on a different test type: IDT, NIPT or DSS (Fig. S1). Each vignette was followed by the same questions (see Measures below). The wording of the vignettes and the questions was informed by current clinical guidelines.2, 3, 4 Vignettes were reviewed extensively by the research team which included representatives from Antenatal Results and Choices (ARC) and Genetics Alliance UK, obstetricians, midwives, psychologists, geneticists and genetic counsellors before being piloted on a small sample (20 health professionals and 20 pregnant women). The wording of materials was finalised using the feedback.
Similar materials were developed for health professionals and service users. Health professionals were asked to imagine themselves in discussion with a pregnant woman attending a pre‐natal screening clinic (at a point in time when all pregnant women were routinely offered pre‐natal screening and/or IDT for DS). Pregnant women were asked to imagine themselves as the woman attending the clinic.
Measures
Attitudes to aspects of counselling and the care pathway believed to reflect informed choice were evaluated by asking participants to respond to the following questions about the test presented:
Pre‐test information: Table 2 lists the eight information topics that participants were asked to tick if considered important for pre‐test counselling. They were also asked to list the ‘top three topics’ they viewed as most important.
Timing of testing: participants were asked to choose whether the test should be performed on the same day as the consultation or at a return visit, which would theoretically allow the woman time for reflection and discussion with her support network.
Signed consent: participants were asked how important it was for women undergoing the test to sign a consent form using a 4‐point scale ranging from 1 (definitely yes) to 4 (definitely not). Space was left for participants to explain their answers.
Access: participants were asked if the test should be offered routinely to all pregnant women or only to women at high risk of DS. They were also given the chance to tick ‘other’ to express other preferences.
Table 2.
Responses across outcome measures, by test type and participant group
| Total | P‐value | DSS | P‐value | IPD | P‐value | NIPT | P‐value | P‐value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HP | SU | HP | SU | HP | SU | HP | SU | HP | SU | |||||
| % Of pts who identified topics as important | ||||||||||||||
| 1. Description of DS | 88.5 | 75.1 | 0.000b | 85 | 76.3 | 0.060 | 92.6 | 77.1 | 0.000b | 88.4 | 72 | 0.000b | 0.165 | 0.491 |
| 2. Description of procedure | 98.2 | 88.1 | 0.000b | 95.5 | 84.1 | 0.002b | 100 | 92.6 | 0.002b | 99.3 | 87.4 | 0.000b | 0.013 n/v | 0.058a |
| 3. Accuracy | 98.2 | 90.8 | 0.000b | 96.2 | 92.5 | 0.166 | 100 | 89.1 | 0.000b | 98.6 | 90.9 | 0.004b | 0.073 n/v | 0.588 |
| 4. Risk | 82.9 | 92.5 | 0.000b | 77.9 | 89 | 0.008a | 97.5 | 97.7 | 0.914 | 74.6 | 90.9 | 0.000b | 0.000b | 0.005b |
| 5. Testing is a choice | 99.5 | 81 | 0.000b | 99.2 | 83.7 | 0.000b | 100 | 83.4 | 0.000b | 99.3 | 76 | 0.000b | 0.638 n/v | 0.114 |
| 6. What happens after test | 98 | 83 | 0.000b | 96.2 | 80.2 | 0.000b | 98.3 | 83.4 | 0.000b | 99.3 | 85.1 | 0.000b | 0.202 n/v | 0.467 |
| 7. Options if test positive | 94.4 | 84.7 | 0.000b | 94 | 83.1 | 0.004b | 93.4 | 87.4 | 0.095 | 95.6 | 83.4 | 0.001b | 0.719 | 0.462 |
| 8. Provision of written info | 95.9 | 56.7 | 0.000b | 96.2 | 56.4 | 0.000b | 96.7 | 59.4 | 0.000b | 94.9 | 54.3 | 0.000b | 0.753 | 0.621 |
| Timing of test% | 0.000b | 0.000b | 0.000b | 0.000b | 0.000b | 0.000b | ||||||||
| Same day | 23.4 | 56.5 | 29.8 | 64.1 | 10 | 38 | 29.2 | 66.9 | ||||||
| Return visit | 76.7 | 43.5 | 70.2 | 35.9 | 90 | 62 | 70.8 | 33.1 | ||||||
| Written consent% | 0.000b | 0.001b | ||||||||||||
| Definitely yes | 65.3 | 60.9 | 57.9 | 61.3 | 86.8 | 69.2 | 54 | 52.3 | Mean Rank | Mean Rank | ||||
| Probably yes | 16.8 | 31 | 17.3 | 30.1 | 8.3 | 27.9 | 24.1 | 35.1 | IPD 153.2 | IPD 234.9 | ||||
| Probably not | 14.8 | 7.1 | 21.1 | 8.1 | 4.1 | 2.9 | 18.2 | 10.3 | DSS 213 | DSS 259.6 | ||||
| Definitely not | 3.1 | 1 | 3.8 | 0.6 | 0.8 | 0 | 3.6 | 2.3 | NIPT 217.4 | NIPT 285.3 | ||||
| Written consent (Y/N) | 0.000b | 0.000b | 0.371 | 0.030a | 0.000b | 0.004b | ||||||||
| Yes | 82.1 | 91.9 | 75.2 | 91.3 | 95 | 97.1 | 78.1 | 87.4 | ||||||
| No | 17.9 | 8.1 | 24.8 | 8.7 | 5 | 2.9 | 21.9 | 12.6 | ||||||
| How test offered% | 0.014a | 0.012a | 0.000b | 0.480 | 0.000b | 0.000b | ||||||||
| Routinely | 71.6 | 80 | 93 | 83.3 | 22.8 | 65.6 | 92.5 | 90.2 | ||||||
| Only to ‘high risk’ women | 28.4 | 20 | 7 | 16.7 | 77.2 | 34.4 | 7.5 | 9.8 | ||||||
HP, health professional; SU, pregnant women.
n/v = not valid as more than 30% of cells have less than required number to conduct test.
Significant at 0.01 or 0.05 level.
Significant at 0.001 or 0.005 level.
Demographic information was collected from both groups of participants including whether women had experience of pre‐natal screening/testing for DS.
Procedure
An equal number of the vignettes were printed and randomly distributed within each site or conference, and convenience sampling used for both participant groups. Pregnant women were approached by a member of the local research team whilst waiting for routine antenatal appointments and given an information sheet outlining the study. Upon verbal consent to take part, they were asked to read the vignette and complete the questionnaires before handing them back to the researcher. During piloting, health professionals were recruited at work through email to target greater numbers. However, this was found to be too labour‐intensive and response rates were very low. To maximise response rates in the final study, a variety of health professionals were approached face to face when working in clinics or at team meetings. They were additionally recruited through relevant conferences to increase sample numbers and diversity. As above, this invitation included an information sheet, one of the vignettes and a questionnaire. They could either complete the paper version or follow a link to an online questionnaire, which was included at the bottom of the information sheet.
For two of the conferences, questionnaires were placed in delegate packs, with a summary and invitation to participate given at the start of the meeting. One of the research team then collected questionnaires during breaks and at the end of the conference. For the third conference, invitations to take part in the study were given to people who approached the stand, and questionnaires were either returned to the stand that day or posted back in a pre‐paid envelope.
Analysis
Statistical analyses were carried out using spss 17.0 (IBM SPSS Statistics for Windows; IBM Corp, Armonk, NY, USA). Distributions of responses were examined descriptively across groups and tested for significance using Chi‐square statistics for categorical, and anova for continuous variables. A series of univariate analyses (Chi‐Square) were conducted to compare perceptions of counselling requirements across test types: topics considered important to communicate, timing of the test, how the test should be offered and whether written consent was necessary. Ordinal data was analyzed by independent sample Kruskal–Wallis. Data for health professionals and pregnant women were analyzed separately and then compared (across all test types combined as well as for each test type). Any significant differences were examined with further post‐hoc analyses. Effects of demographic details on outcomes were also examined using independent samples t‐test (continuous variables) and Chi‐Square (categorical variables).
Results
Participants
A total of 510 health professionals and 548 pregnant women were invited to take part in the study. The response rate for the hospital sites was 78% for health professionals and 98% for pregnant women, ranging from 57 to 100% across sites. The average response rates of conference attendees ranged from 55 to 60%. Of the 537 service user questionnaires collected, 14 were excluded from analysis because they did not meet the study criteria: seven questionnaires were incomplete, five were from women under 18 years of age and two were from women who were not pregnant. From the 400 health professional questionnaires collected, seven were excluded (from one conference) as they were not currently practising their profession in the UK. A total of 393 health professionals and 523 pregnant women formed the useable sample.
The demographic composition of the final sample is shown in Tables S1–S2, and in Table 1. Most health professionals were female and midwives, although those recruited at conferences consisted of more obstetricians (26.8%), clinical geneticists and genetic counsellors (28%). Age and level of experience were variable. The sample as a whole reflects the general UK population in terms of recorded ethnicity (according to 2001 Census data): the majority were born in the UK and Europe, with English as their first language. Most pregnant women were in a current relationship (94.7%) with a wide range of maternal and gestational ages. One third were in their first pregnancy and over 40% were educated to degree level or above. Of 86.6% reported being previously offered DSS, 56.4% having undergone DSS and 5.9% had undergone IPD.
Vignette types were evenly distributed across the participant groups. Demographic variables were evenly distributed across test types, for both groups. Table 2 gives full data and statistical analyses of outcome measures by test type and participant group.
Information topics
For health professionals, 7/8 information topics were seen as equally important across test types (including ‘testing as a choice’): with ‘risk of miscarriage’ as significantly more important only for the IDT condition (P = 0.000). For pregnant women, 6/8 information topics were seen as equally important; they saw ‘risk of miscarriage’ (P < 0.005) and ‘description of procedure’ (P < 0.05) as more important in IDT. Fewer pregnant women than health professionals rated each topic as important in total (except for ‘risk of miscarriage’), with only 56.7% of women compared with 95.9% of health professionals rating ‘provision of written information’ as important. Frequencies that topics were listed in the ‘Top 3’ were added and compared across test types (Table 3).
Table 3.
Frequency that topics were listed in the ‘top 3’ (% included in brackets)
| Topic | DSS | IDT | NIPT | |||
|---|---|---|---|---|---|---|
| HP | SU | HP | SU | HP | SU | |
| Description of DS | 38 (29) | 56 (32.4) | 27 (22.3) | 33 (18.9) | 37 (26.6) | 44 (25.1) |
| Description of procedure | 41 (31) | 91 (52.6) | 54 (44.6) | 104 (59.4) | 42 (30.2) | 83 (47.4) |
| Accuracy | 71 (53.4) | 108 (62.4) | 44 (36.4) | 90 (51.4) | 81 (58.3) | 111 (63.4) |
| Risks | 13 (10) | 110 (63.6) | 76 (62.8) | 143 (81.7) | 20 (14.4) | 104 (59.4) |
| Testing is a choice | 96 (72.2) | 28 (16.2) | 70 (57.9) | 37 (21.1) | 100 (71.9) | 31 (17.7) |
| What happens after | 46 (34.6) | 33 (19.1) | 23 (19) | 34 (19.4) | 48 (34.5) | 45 (25.7) |
| Options if test positive | 60 (45.1) | 68 (39.3) | 39 (32.2) | 59 (33.7) | 54 (38.8) | 89 (50.9) |
| Written information | 31 (23.3) | 9 (5.2) | 24 (19.8) | 4 (2.3) | 27 (19.4) | 6 (3.4) |
Highlighted cells show the ‘top 3’ for each participant group according to test type.
Informed choice measures
The NIPT and DSS participants were less likely than the IDT group to state that testing should take place at a return visit (health professionals: IDT 90 vs. 70.2 and 70.8% DSS and NIPT, respectively, P = 0.000; pregnant women: 62 vs. 35.8 and 33.1%, P = 0.000) (Fig. 1). However, when compared between participant groups, pregnant women expressed a greater preference than health professionals for testing to occur on the same day as pre‐test counselling, across all test types (P = 0.000).
Figure 1.
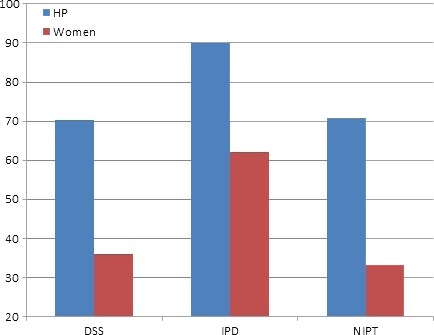
Percentage of participants who believed testing should be performed at a return visit.
The perceived need for written consent also differed between test types, with those in the NIPT and DSS groups being less likely than the IDT groups to state that obtaining written consent was necessary (health professionals: IDT 95 vs. 75.2 and 78.1% DSS and NIPT, respectively, P = 0.000; pregnant women: 97.1 vs. 91.3 and 87.4%, P < 0.005) (Fig. 2). Test type was also found to have a significant effect on perceived need for written consent when responses were analyzed as ordinal data: H(2) = 35.6, P = 0.000 (health professionals); H(2) = 13.1, P = 0.001 (pregnant women). IDT in both participant groups was significantly more likely to be rated as requiring written consent (than NIPT or DSS). However, women considered a greater need for written consent than health professionals, with around 90% of women believing written consent was necessary for DSS (P = 0.000) and NIPT (P < 0.05). Analysis of the open comments written by participants demonstrated a range of opinions: some believed written consent constituted standard practice, required by health professionals and hospitals not only to ‘cover themselves’ legally, but also to ensure that the woman herself understands what she is agreeing to has fully considered all issues involved and to prevent confusion or regret following the procedure.
Figure 2.
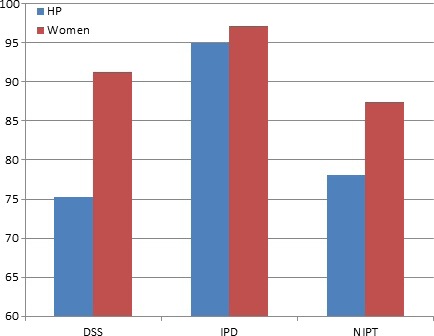
Percentage of participants who believed a written consent form was necessary.
For both health professionals and pregnant women, those in the NIPT and DSS groups were more likely to report that testing should be offered routinely to all pregnant women (health professionals: 22.8% IDT vs. 92.5% NIPT and 93% DSS, P = 0.000; pregnant women: 65.6% IDT vs. 90.2% NIPT and 83.3% DSS, P = 0.000) (Fig. 3). When participant groups were compared, pregnant women were more likely to believe that IDT should to be offered routinely to all pregnant women (P = 0.000). This outcome measure was analyzed as a dichotomy (‘routinely’ or ‘high risk’); however, a small percentage of participants responded ‘other’ in answer to this question on test access: 3.8% of health professionals (15/392) and 2.9% of pregnant women (15/579). Rather than choosing a category, these respondents listed different ways of offering the tests including: only to those who already had a baby with DS, or those who requested the test or to both ‘those at high risk and anyone who requested it’. The majority stated that the tests should be offered to everyone who wanted or requested them, with emphasis on individual choice as opposed to enforced recommendation.
Figure 3.
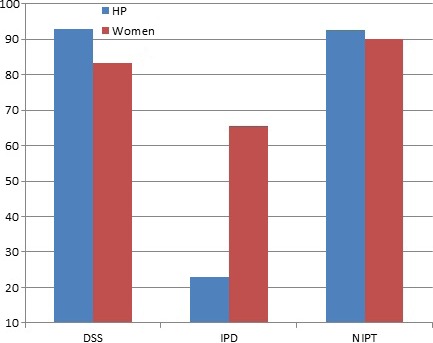
Percentage of participants who believed test should be offered routinely to all pregnant women.
Demographic effects
For the demographic factors, a number of effects were observed. Health professionals' age and years of experience affected views on test access: with more experienced (t(352) = 2.19, P < 0.05, CI 0.259–4.925) and older participants (t(363) = 1.98, P < 0.05, CI 0.013–4.412) more likely to report that tests should be offered routinely to all women. Interestingly, males were more likely to report a preference for same day testing (40.5 vs. 21.4% of female health professionals, χ2 = 6.85(1), P < 0.01) and less likely to report that written consent was necessary (69.4 vs. 83.3%, χ2 = 4.28(1), P < 0.05), but there were no significant effects of profession on any of the outcome measures. Those health professionals who were religious were more likely to report that testing should take place at a return visit (56.6 vs. 49.8%, χ2 = 5.10(1), P < 0.05) and those of Christian faith more likely to answer that tests should be offered routinely to all women, compared to those of non‐Christian faith (77.7 vs. 63.8%, χ2 = 8.4(2), P = 0.015).
For service users, the only demographic factors to have an effect were relationship status and education. Those women who were single or not living with their partner were more likely to want same‐day testing (76 and 74.2%, respectively vs. 52.5% (married) and 57.1% (living together), χ2 = 9.6(3), P = 0.022). Lower educational levels (no qualifications/GCSE) were linked to greater likelihood of reporting that tests should be offered routinely to all women (88.6 vs. 76.7% (A level/NVQ) and 77.7% (degree+), χ2 = 7.919(2), P = 0.019).
Discussion
Summary of findings
This UK study provides evidence that both providers and users of maternity services view NIPT for DS differently from invasive tests in terms of timing, written consent requirements and access to testing. We argue that such differences could result in the informed choice process being undermined. Previous research has highlighted that maternity health professionals (mainly obstetricians) are more likely to view the pre‐test counselling requirements for NIPT as similar to screening rather than invasive diagnostic procedures.19 This study usefully expands upon that research to demonstrate that similar attitudes can be seen across a range of health professionals delivering antenatal care across the UK, including midwives, clinical geneticists and genetic counsellors. Importantly, it also demonstrates some similar response patterns amongst the users of maternity services, indicating that care needs to be taken when offering NIPT for DS to ensure informed choice is supported.
Despite the similarities between participant groups, there were notable differences. Pregnant women were more interested in hearing about potential risks involved in the test (regardless of test type), whilst health professionals only reported this as important for IDT. Other research has identified a similar preference for test safety amongst pregnant women.31, 32, 33, 34 This indicates an important difference in the information concerns of service users that needs to be taken into account within pre‐test counselling, with women prioritising the safety of their unborn child. The preference for same‐day testing (across tests) suggests an increased likelihood that, within pre‐natal testing for DS, women will choose the more convenient option, allowing less time for discussion and careful consideration of the test. Hewison and Bryant conclude that when faced with decisions that are intrinsically stressful, people often take the option that appears easier (over the decision led by their values).35 Single mothers and those not living with partners may have a greater burden of care; the fact that ‘same day’ preference for testing was more pronounced amongst this group should perhaps come as no surprise.
There was a preference amongst women for all tests including invasive procedures to be made available routinely to all, but a small proportion who left comments emphasized the importance of individual choice within this. Women were also more likely to report a need for written consent, although it is difficult to know if they were stating what they thought was expected of them, or saw written consent as an important element of decision making. Further exploratory research is needed to fully understand and explain the differences noted here.
Study critique
A key limitation of this study is the assumptions made about the factors that influence informed choice. Allowing time between the consultation and taking the test arguably allows time for reflection and discussion, which should promote a more informed choice. For instance, qualitative research has shown that women prefer to discuss options with significant others, and such discussions are an important and supportive way of sharing responsibility.36 The importance of slowing down the decision‐making process to encourage informed choice is also noted by Hewison and Bryant, who stress the need to take time to digest information and discuss options with partners and family members before decisions are made.35 However, in the absence of robust empirical evidence, the necessity of separating the test from the consultation to facilitate an informed choice remains an assumption. Some women may have thought through their decision before attending the consultation, perhaps due to previous experiences with screening or less direct exposure. Women on the whole appear to prefer same‐day testing, which has been shown to increase test uptake, though the decisions may not always be thoroughly informed.37 What appears to be important is the need to tailor the consultation to the individual woman rather than providing a ‘one size fits all’ approach; and ensuring the decision to take (or reject) the test has been adequately discussed and reflected on.
Written consent remains a requirement for invasive procedures and is recommended as good practice,3 though the extent to which it facilitates informed choice is also debatable. Nevertheless, the act of obtaining written consent is one way to ensure that health professionals pay particular attention to the process. Written consent for NIPT may help the test stand out from routine blood tests and encourage fuller discussion of the implications. This could be important in relation to potential implications when a woman receives results she is unprepared for. Research shows that women are often not aware of the outcomes following screening and that many are unprepared for any negative results found in their first anomaly scan.38, 39, 40, 41 This echoes the incidental finding in the current study that 9% of the women in the current sample failed to recall whether they had undergone screening, and 30% failed to stipulate whether they had undergone IDT (Table 1). When discussion of procedural risks is no longer necessary, pregnant women may not fully consider the implications of taking the test (including termination of pregnancy as a potential outcome).19 Written consent could help remove the danger of presenting NIPT as ‘just another blood test’.33
Women recruited to this study were predominantly White and well‐educated. Attitudes towards and uptake of pre‐natal testing, as well as informed decision making, has been shown to vary across ethnicity.42, 43, 44 Further research that specifically targets hard‐to‐reach and under‐represented populations is urgently required to enrich our understanding of this area of clinical practice.
Future possibilities
The impact of NIPT for DS upon informed choice will depend largely on how it is implemented in practice. Attitudes of health professionals are likely to influence how the tests are offered and this study has shown an influential effect of factors such as gender, personal beliefs and age/experience. This becomes more salient when we consider that a significant minority of women are likely to follow professional recommendations30 and, according to findings from a health technology assessment on genetic pre‐natal screening, current procedures for achieving informed consent are inadequate.45 However, recorded attitudes may not necessarily translate to actual behaviours. In addition, no matter how thorough the pre‐test counselling, it may be that some women will never be prepared to receive pre‐natal test results that indicate a problem, emphasizing a need for robust post‐test support to also be in place.46 Clearly, further research is needed once NIPT for DS is introduced into clinical practice to evaluate how informed consumer decisions and actions are, and to observe and measure actual clinical practices and behaviours in relation to service delivery.
Interventions to safeguard informed choice should target all health‐care professionals involved in maternity care, including managers and commissioners who may be in greater danger of viewing NIPT as a routine blood test and, as a consequence, allocate insufficient resources for high quality pre‐test counselling. Women may not be fully aware of consenting to testing and may not have adequately considered the impact of receiving positive results. Enabling couples to make informed decisions regarding pre‐natal testing requires more than just presentation of information. Flexible and thorough pre‐test counselling is required to ensure that couples are supported to make a decision in line with their values and beliefs, along with the provision of post‐test support if needed. This has significant implications for health‐care practice and for the experience of women using antenatal services. If NIPT becomes a one‐step diagnostic process, it will remove the time for reflection currently available to women via the ‘two‐step’ screening and diagnostic testing process, making the provision of such counselling and support even more essential.47
Regardless of how NIPT for Down's syndrome is integrated into clinical practice, the rapidity in technological developments suggests that this is likely to be imminent. Effective professional education is essential to maintain a high standard of care. This becomes increasingly important and complex with the ever‐expanding availability of technological advancements. Guidelines and educational programmes need to prioritize informed choice and continue to take into account the views and needs of those who will use the technology: the pregnant women and their partners.
Sources of funding
This research was funded by the National Institute for Health Research (NIHR) Programme Grants for Applied Research (RP‐PG‐0707‐10107) and the NIHR Comprehensive Local Research Network. LSC is partially funded by the Great Ormond Street Hospital Children's Charity and the NIHR comprehensive Biomedical Research Centre at Great Ormond Street Hospital. The research funded is independent and the views expressed in the paper are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
Figure S1. Vignettes.
Table S1. Demographics of health professionals across test type [frequency followed by% of total who responded (in brackets)].
Table S2. Demographics of pregnant women across test type [frequency followed by% of total who responded (in brackets)].
Acknowledgements
We are grateful to the women and health professionals who participated in the study and to all the collaborators, in particular Celine Lewis, Kerry Oxenford and Jan Preece, who assisted with participant recruitment.
References
- 1. Tabor A, Alfirevic Z. Update on procedure‐related risks for prenatal diagnosis techniques. Foetal Diagnosis and Therapy, 2010; 27: 1–7. [DOI] [PubMed] [Google Scholar]
- 2. UK National Screening Committee Antenatal screening – Working standards for Down's Syndrome screening 2007: National Down's Syndrome screening programmes for England.
- 3. Royal College of Obstetricians and Gynaecologists Green‐top guideline no 8, June 2010: Amniocentesis and chorionic villus sampling. [DOI] [PubMed]
- 4. Royal College of Obstetricians and Gynaecologists Clinical governance advice, Dec 2008: Obtaining valid consent.
- 5. Lo YM, Corbetta N, Chamberlain PF et al Presence of fetal DNA in maternal plasma and serum. Lancet, 1997; 350: 485–487. [DOI] [PubMed] [Google Scholar]
- 6. Hill M, Lewis C, Jenkins L, Allen S, Elles RG, Chitty LS. Implementing noninvasive prenatal fetal sex determination using cell‐free fetal DNA in the United Kingdom. Expert Opinion on Biological Therapy, 2012; 12 (Suppl. 1): S119–S126. [DOI] [PubMed] [Google Scholar]
- 7. Daniels G, Finning K, Martin P, Massey E. Noninvasive prenatal diagnosis of fetal blood group phenotypes: current practice and future prospects. Prenatal Diagnosis, 2009; 29: 101–107. [DOI] [PubMed] [Google Scholar]
- 8. Lench N, Barrett A, Fielding S et al The clinical implementation of non‐invasive prenatal diagnosis for single‐gene disorders: challenges and progress made. Prenatal Diagnosis, 2013; 33: 555–562. [DOI] [PubMed] [Google Scholar]
- 9. Chitty LS, Griffin DR, Meaney C et al New aids for the non‐invasive prenatal diagnosis of achondroplasia: dysmorphic features, charts of fetal size and molecular confirmation using cell‐free fetal DNA in maternal plasma. Ultrasound in Obstetrics and Gynecology, 2011; 37: 283–289. [DOI] [PubMed] [Google Scholar]
- 10. Chitty LS, Khalil A, Barrett AN, Pajkrt E, Griffin DR, Cole TJ. Safe, accurate, prenatal diagnosis of thanatophoric dysplasia using ultrasound and free fetal DNA. Prenatal Diagnosis, 2013; 33: 416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clausen FB, Christiansen M, Steffensen R et al Report of the first nationally implemented clinical routine screening for fetal RHD in D‐ pregnant women to ascertain the requirement for antenatal RhD prophylaxis. Transfusion, 2012; 52: 752–758. [DOI] [PubMed] [Google Scholar]
- 12. Boon EM, Faas BH. Benefits and limitations of whole genome versus targeted approaches for noninvasive prenatal testing for fetal aneuploidies. Prenatal Diagnosis, 2013; 33: 563–568. [DOI] [PubMed] [Google Scholar]
- 13. Agarwal A, Sayres LC, Cho MK, Cook‐Deegan R, Chandrasekharan S. Commercial landscape of noninvasive prenatal testing in the United States. Prenatal Diagnosis, 2013; 33: 521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Jong A, Dondorp WJ, Frints SG, de Die‐Smulders CE, de Wert GM. Advances in prenatal screening: the ethical dimension. Nature Reviews Genetics, 2011; 12: 657–663. [DOI] [PubMed] [Google Scholar]
- 15. Deans Z, Newson A. Ethical considerations for choosing between possible models for using NIPD for aneuploidy detection. Journal of Medical Ethics, 2012; 38: 614–618. [DOI] [PubMed] [Google Scholar]
- 16. Chitty LS, Hill M, White H, Wright D, Morris S. Non‐invasive prenatal testing for aneuploidy ‐ ready for prime time? American Journal of Obstetrics and Gynecology, 2012; 206: 269–275. [DOI] [PubMed] [Google Scholar]
- 17. Pan M, Li FT, Li Y et al Discordant results between fetal karyotyping and non‐invasive prenatal testing by maternal plasma sequencing in a case of uniparental disomy 21 due to trisomic rescue. Prenatal Diagnosis, 2013; 33: 598–601. [DOI] [PubMed] [Google Scholar]
- 18. Lau TK, Jiang FM, Stevenson RJ et al Secondary findings from non‐invasive prenatal testing for common fetal aneuploidies by whole genome sequencing as a clinical service. Prenatal Diagnosis, 2013; 33: 602–608. [DOI] [PubMed] [Google Scholar]
- 19. Van den Heuvel A, Chitty LS, Dormandy E et al Will the introduction of non‐invasive prenatal diagnostic testing erode informed choices? An experimental study of health care professionals. Patient Education and Counseling, 2010; 78: 24–28. [DOI] [PubMed] [Google Scholar]
- 20. PHG Foundation Cell‐free fetal nucleic acids for non‐invasive prenatal diagnosis. Report of the UK expert working group: Executive summary. Cambridge, 2009.
- 21. Human Genetics Commission Making babies: reproductive decisions and genetic technologies. Jan 2006 www.hgc.gov.uk
- 22. Marteau TM, Dormandy E, Michie S. A measure of informed choice. Health Expectations, 2001; 4: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jacques AM, Sheffield LJ, Halliday JL. Informed choice in women attending private clinics to undergo first‐trimester screening for Down Syndrome. Prenatal Diagnosis, 2005; 25: 656–664. [DOI] [PubMed] [Google Scholar]
- 24. Van den Berg M, Timmermans DR, Ten Kate LP, van Vugt JM, van der Wal G. Are pregnant women making informed choices about prenatal screening? Genetics in Medicine, 2005; 7: 332–338. [DOI] [PubMed] [Google Scholar]
- 25. Williams C, Alderson P, Farsides B. Too many choices? Hospital and community staff reflect on the future of prenatal screening. Social Science and Medicine, 2002; 55: 743–753. [DOI] [PubMed] [Google Scholar]
- 26. Levy V. Protective steering: a grounded theory study of the processes by which midwives facilitate informed choices during pregnancy. Journal of Advanced Nursing, 1999; 29: 104–112. [DOI] [PubMed] [Google Scholar]
- 27. Skirton H, Patch C. Factors affecting the clinical use of non‐invasive prenatal testing: a mixed methods systematic review. Prenatal Diagnosis, 2013; 33: 532–541. [DOI] [PubMed] [Google Scholar]
- 28. Lewis C, Hill M, Skirton H, Chitty LS. Fetal sex determination using cell‐free fetal DNA: service users' experiences of and preferences for service delivery. Prenatal Diagnosis, 2012; 32: 735–741. [DOI] [PubMed] [Google Scholar]
- 29. Lewis C, Hill M, Chitty L. Non‐invasive prenatal diagnosis for single gene disorders: experience of patients. Clinical Genetics, 2013; doi: 10.1111/cge.12179 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 30. Tischler R, Hudgins L, Blumenfeld YJ, Greely HT, Ormond KE. Noninvasive prenatal diagnosis: pregnant women's interest and expected uptake. Prenatal Diagnosis, 2011; 31: 1292–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lewis SM, Cullinane FM, Carlin JB, Halliday JL. Women's and health professionals' preferences for prenatal testing for Down syndrome in Australia. Australian and New Zealand Journal of Obstetrics and Gynaecology, 2006; 46: 205–211. [DOI] [PubMed] [Google Scholar]
- 32. Bishop AJ, Marteau TM, Armstrong D et al Women and health care professionals' preferences for Down's syndrome screening tests: a conjoint analysis. British Journal of Obstetrics and Gynaecology, 2004; 111: 775–779. [DOI] [PubMed] [Google Scholar]
- 33. Lewis C, Hill M, Skirton H, Chitty LS. Non‐invasive prenatal diagnosis for fetal sex determination – benefits and disadvantages from the service users' perspective. European Journal of Human Genetics, 2012; 20: 1127–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hill M, Fisher J, Chitty LS, Morris S. Women's and health professionals' preferences for prenatal tests for Down syndrome: a discrete choice experiment to contrast non‐invasive prenatal diagnosis with current invasive tests. Genetics in Medicine, 2012; 14: 905–913. [DOI] [PubMed] [Google Scholar]
- 35. Hewison J, Bryant L. Informed consent: what should we be doing? In: Kehoe S, Chitty LS, Homfray T. (eds) Reproductive Genetics (RCOG 57th Study Group 2009). London: RCOG Press, 2009: 205–216. [Google Scholar]
- 36. Garcia E, Timmermans DR, van Leeuwen E. Rethinking autonomy in the context of prenatal screening decision‐making. Prenatal Diagnosis, 2008; 28: 115–120. [DOI] [PubMed] [Google Scholar]
- 37. Lorenz RP, Botti JJ, Schmidt CM, Ladda RL. Encouraging patients to undergo prenatal genetic counseling before the day of amniocentesis. Its effect on the use of amniocentesis. Journal of Reproductive Medicine, 1985; 30: 933–935. [PubMed] [Google Scholar]
- 38. Garcia J, Bricker L, Henderson J et al Women's views of pregnancy ultrasound: a systematic review. Birth, 2002; 29: 225–250. [DOI] [PubMed] [Google Scholar]
- 39. French S. Perceptions of routine nuchal translucency screening. British Journal of Midwifery, 2000; 8: 632–638. [Google Scholar]
- 40. Fisher J. First trimester screening: dealing with the fall‐out. Prenatal Diagnosis, 2011; 31: 46–49. [DOI] [PubMed] [Google Scholar]
- 41. Weinans MJ, Kooij L, Muller MA, Bilardo KM, van Lith JM, Tymstra T. Comparison of the impact of screen‐positive results obtained from ultrasound and biomedical screening for Down syndrome in the first trimester: a pilot study. Prenatal Diagnosis, 2004; 24: 347–351. [DOI] [PubMed] [Google Scholar]
- 42. Yu J. A systematic review of issues around antenatal screening and prenatal diagnostic testing for genetic disorders: women of Asian origin in western countries. Health and Social Care in the Community, 2012; 20: 329–346. [DOI] [PubMed] [Google Scholar]
- 43. Ahmed S, Hewison J, Green JM, Cuckle HS, Hirst J, Thornton JG. Decisions about testing and termination of pregnancy for different fetal conditions: a qualitative study of European White and Pakistani mothers of affected children. Journal of Genetic Counseling, 2008; 17: 560–572. [DOI] [PubMed] [Google Scholar]
- 44. Fransen MP, Essink‐Bot ML, Vogel I, Mackenbach JP, Steegers EA, Wildschut HI. Ethnic differences in informed decision‐making about prenatal screening for Down's syndrome. Journal of Epidemiology and Community Health, 2010; 64: 262–268. [DOI] [PubMed] [Google Scholar]
- 45. Green JM, Hewison J, Bekker HL, Bryant LD, Cuckle HS. Psychosocial aspects of genetic screening of pregnant women and newborns: a systematic review. Health Technology Assessment, 2004; 8: 1–109. [DOI] [PubMed] [Google Scholar]
- 46. Fisher J. Supporting patients after disclosure of abnormal first trimester screening results. Current Opinion in Obstetrics and Gynecology, 2012; 24: 109–113. [DOI] [PubMed] [Google Scholar]
- 47. Hill M, Barrett A, Chitty LS. Uses of cell free fetal DNA in maternal circulation. Best Practice & Research Clinical Obstetrics and Gynaecology, 2012; 26: 639–654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Vignettes.
Table S1. Demographics of health professionals across test type [frequency followed by% of total who responded (in brackets)].
Table S2. Demographics of pregnant women across test type [frequency followed by% of total who responded (in brackets)].


