Abstract
Objective
It is not well‐known how women receiving counselling consultation about fertility preservation (FP) in the Netherlands perceive the information provision about and referral for FP in the oncology setting. The aim of this study was to qualitatively explore women's experiences with the (process of) information provision about the gonadotoxic effects of cancer treatment and about FP and the decision‐making process and to obtain their recommendation for improvements.
Methods
Semi‐structured interviews with female patients with cancer who had received a counselling consultation on FP (at 18–40 years of age).
Results
Thirty‐four interviews were held (response rate 64%). Information provision was considered to be important. Overall, women were satisfied with the timing and the content of the information, but women were less positive about the need to be assertive to get information, and the multiplicity of decisions and actions to be carried out in a very short time frame.
Conclusions
Information provision on gonadotoxic effects of cancer treatment and about FP was overall deemed sufficient, timely and important. Women recommended standardization of the information provision, improvement of communication among clinicians and medical centres, and availability of FP‐specific patient information materials to improve future information provision processes.
Keywords: cancer, decision making, fertility preservation, information provision, oncology, psychosocial
Introduction
Due to improvements in oncologic treatment, survival for women with cancer has increased. Unfortunately, oncologic treatment is associated with decreased fertility or infertility, as a result of direct gonadotoxic effects of treatment or a delay in childbearing until after treatment is complete.1, 2, 3 The risk of treatment‐induced infertility depends on women's age, and type and dosage of the oncologic treatment.4
Infertility or concerns about fertility due to cancer treatment can be very distressing, leading to a decreased quality of life.5, 6, 7, 8, 9 Therefore, interest in fertility preservation (FP) has risen. Currently, the techniques available include embryo and oocyte cryopreservation, ovarian tissue cryopreservation and ovarian suppression or transposition. Except for embryo cryopreservation, FP techniques are still experimental.
Despite an increasing number of studies, and guidelines from the Netherlands,10 Europe11 and the United States of America4, 12 demonstrating the need for discussion of fertility‐related issues with patients with cancer, only about 30–75% of the female patients with cancer of fertile age report having discussed these issues with the oncologist.13, 14, 15, 16, 17 Furthermore, the information provision and the process of referral are often inadequate,15, 18, 19 and not all women are satisfied with all aspects of the information provision.20, 21
Sufficient and clear information is necessary to enable effective patient decision making. Involvement of patients in decision making is especially important in deciding on treatments with possible long‐term consequences for quality of life, such as gonadotoxic and FP treatments. It has been found that not receiving sufficient information about FP, not seeing a fertility specialist, and deciding to ‘wait and see’ (expectant management) were related to more regret and decisional conflict.22, 23 Furthermore, receiving counselling about reproductive loss and pursuing FP has been found to be associated with less regret, greater physical QOL and trends of greater psychological QOL.24
At this moment, it is not known whether the information women in the Netherlands receive about FP is sufficient for them to engage in decision making with their physicians. Therefore, it is necessary to explore patient's experiences with the current information provision about FP and with the decision‐making process.
This study describes the experiences of women who had received at least one counselling consultation on FP in relation to the procedure of information provision and decision making about FP, and their recommendations for improvement of these processes. Research questions were as follows:
What are women's experiences with the information provided to them in the past about gonadotoxic effects of oncologic therapy and about FP?
What are women's experiences with the process of information provision and decision making about FP?
How do women think the information provision and decision‐making processes can be improved?
Methods
Sample
Since July 2002, techniques have been available at the Leiden University Medical Centre (LUMC) to cryopreserve ovarian tissue, and since October 2005 to cryopreserve embryos on oncologic indication. From 2002 to 2007, these techniques (FP) were discussed with 61 women at risk of gonadotoxic effects of oncologic treatment. Women were eligible for this study when they had had at least one counselling consultation about FP between 2002 and 2007, as registered in a LUMC database for FP, were between 18 and 40 years of age at the time of the counselling and had sufficient knowledge of the Dutch language. Eligible women were approached by means of a personal invitation letter, signed by a team of gynaecologists. Two weeks after the letter was sent, they were contacted by phone to make an appointment for the interview. Our study was approved by the Medical Ethical Committee of the LUMC.
Data collection
Data was collected by means of retrospective semi‐structured interviews between November 2007 and April 2008. The topic list for the interviews is presented in Box 1.
Box 1. Topic list.
Demographic characteristics
Date of birth/partner status/parity/pregnancies/menses/oncologic treatment/desire for children (yes/no/maybe)
Information provision about treatment induced infertility & fertility preservation (FP)
Can you describe when and by whom the information provision about FP was initiated? What is your opinion about the moment chosen to inform you? What is your opinion about the information received? What is your opinion about the conversation, and the way the information was given to you? What effect did receiving information have on you? How important did you think receiving information about FP was at that time? How important was the possibility of losing your fertility compared to the diagnosis of cancer for you at that time vs now?
Improvements for future patient information procedure about FP
What did you miss in the information provision about FP? Which patients should be informed about FP? What type of physician would be best to inform patients about FP?
Who should make the decision whether or not to undergo FP (patient, physician, both)?
Do you have recommendations for future information provision?
Decision‐making on FP
Who made the decision? What were considerations in decision‐making? How did this decision made you feel (effect)? Were you sufficiently informed to make a decision? Did you discuss your decision with others, who? What did you think about the attitude of your physician in the issue of FP? Would you make the same decision now?
Demographic characteristics were both obtained during the interview (Box 1) and by medical record searches (type of malignancy, type of cryopreservation).
All interviews were conducted at the women's homes or at the LUMC (depending on the women's preference) by a researcher not involved in the treatment or counselling of the women (EJ), one interview was conducted by a clinician (LL). Both interviewers had acquired their interview skills during medical training. They were not involved in the treatment of the women they had interviewed.
Data analysis
All interviews were audiotaped, transcribed and content coded. Qualitative data were analysed using Nvivo® software. For the qualitative analysis, we relied on the steps identified as the Framework Approach,25 indicating identification of themes (a framework) using our a priori coding scheme as a framework (based on the structuring of the interview questions; Box 1). Respondents were anonymized in the analysis. The first fifteen interviews were deductively content coded by two independent researchers thus building an a priori code book (MG and RB). At that point, no new codes emerged, and one researcher continued coding the other interviews using the a priori code book (MG). Additionally, specific subthemes and subcodes were allocated to the initial coding. Subthemes were double coded in all interviews (RB, MG) to ensure reliability. Dissimilarities in coding were continuously discussed and adapted based on consensus, to find the code that best described the experiences of the respondents. The definite coding scheme with al its subcodes was checked with the other project members. Interpretations of the data were discussed first by two researchers (MG, RB) and secondly in the project group.
To compare responders and non‐responders, a non‐response analysis was conducted on data regarding demographic or medical characteristics, using Statistical Package for the Social Sciences (SPSS), version 16.0 (IBM, Amsterdam, the Netherlands).
Results
Fifty‐three women were eligible and invited for the study (Fig. 1). Thirteen women (25%) refused to participate, six did not respond to the invitation (11%). Reasons for refusal were no interest in participating in the study (n = 5), lack of time (n = 3) or unknown (n = 5). Eventually, thirty‐four interviews (response rate = 64%) were held with an average duration of 51 min. (SD = 17; range, 23–88 min.). Mean time since the counselling session was 24 months (SD = 13). Significantly more women in the response group (n = 28, 82%) were diagnosed with breast cancer (χ2(2,53) = 11.23; P = 0.001), than in the non‐response group (n = 11, 58%). Otherwise, no significant differences were found between responders and non‐responders in demographic and medical characteristics.
Figure 1.
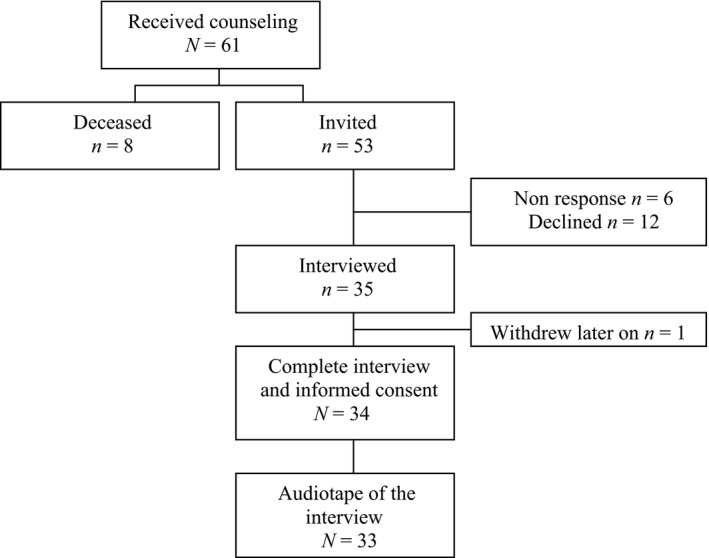
Flow chart of the in‐ and exclusion of participants.
Characteristics of the participants
Sixty‐two per cent of the women (n = 21) had had either embryo (n = 9, 26%) or ovarian tissue (n = 12, 35%) cryopreservation (Table 1). The remainder had chosen to ‘wait and see’ (n = 13, 38%). The majority of the women had been diagnosed with breast cancer (n = 28, 82%). Other diagnoses were Hodgkin (n = 2) and non‐Hodgkin lymphoma (n = 2), and metastasized myxoid liposarcoma (n = 1). Women had been treated with chemotherapy, local or total body irradiation, surgery, stem cell transplantation or a combination of these. One respondent had not received any treatment, because of a pregnancy. No differences were found in women's evaluation of the process of information provision or decision making between those who were diagnosed at different years or with different types of cancer.
Table 1.
Demographic and medical characteristics of the study population (n = 34)
| FP (n = 21) | No FP (n = 13) | Total (n = 34) | |
|---|---|---|---|
| Age at the time of the interview in years, Mdn (range) | 32 (22–37) | 34 (24–41) | 33 (22–41) |
| Age at FP consultation, Mdn (range) | 31 (21–35) | 31 (22–40) | 31 (21–40) |
| Partner (yes), n (%) | 17 (81) | 10 (77) | 27 (79) |
| Type of malignancy, n (%) | |||
| Breast cancer | 18 (86) | 10 (77) | 28 (82) |
| Other malignancies | 2 (10) | 3 (23) | 5 (15) |
| Recurrence malignancy | 1 (5) | 1 (3) | |
| Parity n (%) | |||
| 0 children before diagnosis | 18 (86) | 9 (69) | 27 (79) |
| 1 child before diagnosis | 2 (10) | 4 (31) | 6 (18) |
| Menses during/after therapy, n (%) | |||
| Never absent | 3 (14) | 4 (31) | 7 (21) |
| Absent during therapy, returned afterwards | 10 (48) | 4 (31) | 15 (44) |
| Absent since therapy | 7 (33) | 3 (23) | 10 (27) |
| Pregnancy after treatment, n (%) | 4 (19) | 1 (8) | 5 (15) |
FP = fertility preservation, Mdn = median.
Seventy‐nine per cent of the respondents had no children at the time of the FP consult. Seventy‐four per cent of the respondents had had a desire for children, either at that time (47%, n = 16) or later (27%, n = 9). Five women (15%) had become pregnant spontaneously after therapy, resulting in one miscarriage, one live birth and three on‐going pregnancies at the time of the interview. One woman who was pregnant at the time of the interview had made use of her cryopreserved embryos to become pregnant. No differences were found in responses of women with or without children before diagnosis, except in their opinions about FP (see opinions about FP).
Thirty women (88%) were in total remission at the time of the interview, one (3%) in partial remission and one (3%) had metastases.
Initiation and timing of the information provision
The discussion of possible gonadotoxic side‐effects of cancer treatment and FP options had been initiated by a medical oncologist (n = 16; 49%), the patient herself (n = 10; 30%) a surgeon (n = 3; 9%), a nurse (n = 3; 9%) or a general practitioner (n = 1; 3%). The initial information provision took place at the time of diagnosis (n = 1, 3%), soon after diagnosis but before discussion of the cancer therapy (n = 13; 40%), or during or after discussion of the cancer therapy (n = 18; 55%). Initial information about gonadotoxic effects of chemotherapy often included mentioning of the options to preserve fertility as well. However, for detailed content information about FP, women were referred to a gynaecologist or IVF specialist, if necessary in another medical centre.
The appreciation of the timing of the initial information provision was comparable between women who had been informed at different moments, and by different initiators. Nine women appreciated the moment of the information provision without any criticism:
Quote 1: ‘I liked it [the moment], because it gave me the opportunity to think about it [FP] before my treatment started. [..] If you are told about FP too late, it is probably of no use anymore’. (R13, ovarian tissue cryopreservation, age 21)
Twelve women liked the moment the information was given, but gave comments, such as that the information provision was fairly late (n = 6), that it was too much information at once or that the procedure of information provision and start of the FP or oncologic treatment went very fast (n = 6).
Seven women really disliked the moment, because too much information was given at once (i.e. diagnosis, treatments, side‐effects, fertility issues), or the information was given too late. For the latter, there had been ample time between diagnosis and start of adjuvant chemotherapy to decide and undergo FP, but information provision had been delayed (either because the oncologist was late or referral to the gynaecologist was late), which resulted in fewer or no possibilities for FP:
Quote 2: ‘What I didn't appreciate was that you first see a surgeon, and then you have to decide on your surgery. That took a while for me because they said I had that time, just think about it, so I requested for a second opinion. [..]Then my surgery was in January [about 2 months later] and I heard in the second half of January that I would have chemotherapy. [..] If I had known before, perhaps I would have been able to start an IVF procedure in an earlier menstrual cycle'. (R20, embryo cryopreservation, age 31)
Opinions about the information received
Women were ambivalent about the information they received about FP; they seemed positive, but they mentioned negative characteristics of the information as well. In relation to the evaluation of the information women received, they spoke about the content of the information, informants' characteristics and the importance of the information.
The content of the information
In first instance, 31 women thought the information was sufficient, understandable or of sufficient quantity. Interestingly, later on in the interview, 19 women additionally mentioned some negative aspects of the information. For example, they emphasized issues that remained unclear, the actual little amount of information that was available and/or that they missed information. Issues that needed clarification were for example procedural aspects of IVF and cryopreservation of ovarian tissue (e.g. related to the surgery for ovarian tissue cryopreservation or to aspects of the IVF) side‐effects, the complete range of available FP options, inclusion criteria for FP, alternative options to have children after chemotherapy when FP is not possible (such as adoption) and ethical aspects. For some, the information was already unclear at the moment of deciding about FP, for others (additional) questions came up afterwards (e.g. about transplantation of the ovarian tissue or reimplantation of an embryo).
Quote 3: ‘Well, that was not very clear.. [..] It was clear that there were no possibilities and that I needed other information. But I did not have the information I needed.. [about why an age of 40 was an exclusion criterion]’ (R1, no cryopreservation, age 40)
Two women were mainly negative about the information received, because they received incorrect information. Both were first told that they were eligible for FP (by an oncologist or gynaecologist), but heard later on that they were not. They experienced this as burdensome and of significant (negative) impact.
Informants' characteristics
Many women mentioned the informant (gynaecologist or oncologist) to be likable or the tone of the counselling consultation to be pleasant. Moreover, 10 women appreciated the clinicians' understanding and willingness to help or think along with them; they mentioned clinicians were often open for questions during the consultation or even accessible for questions or advice after the consultation.
Only few (n = 4) women thought the conversation was unpleasant and mentioned the informant to be distant or not empathetic.
Importance of the information provision
Although the majority of the women focused more on surviving the cancer than on fertility at the time, receiving information on the gonadotoxic effects of chemotherapy and FP in addition to all other information on cancer was valued important for almost all women (n = 27, Quote 4, 5). Receiving information was mentioned to enable women to have a choice in this matter (FP), and therefore in ones own future, which was desired by many respondents. It was suggested that women should be provided with some information, after which they could decide for themselves whether they would like more information.
Some women thought it was merely a secondary issue (oncologic treatment first), or only recently realized how important information about FP had been for them.
Quote 4: ‘Of course I thought it was important to find out that I was going to be infertile. Of course, at least, I think it is not that important compared to surviving the cancer. But when something like this [FP] is being offered to you, I say go for it!’ (R7, ovarian tissue cryopreservation, age 31)
Quote 5: ‘[..] You hear something terrible, but you also hear that there are still possibilities. I liked that balance’ (R2, ovarian tissue cryopreservation, age 35)
Decision making about FP
Women had decided about FP by themselves (n = 15), with their partner (n = 14) or the physician had made the decision for them (n = 5). Some women added that talking with significant others helped them in decision making. When the physician had made the decision, FP had not been possible because of unfulfilled inclusion criteria, like being too old or having a poor prognosis.
Women spoke about their opinions about the FP options, considerations in decision making, effects of decision making and post‐decisional satisfaction.
Opinions about FP
Most women were happy about the availability of possibilities to spare their fertility. Moreover, the options were often associated with positive feelings such as hope, a reason to live, relief, feeling good about trying to preserve fertility and amazement about what is possible nowadays.
Quote 6: ‘It gave me hope that there will be stored something there [in the freezer] that I can use in the future. This gave me so much hope for recovery [of the cancer] that I thought: “we should not miss this opportunity, we have to take this chance’. (R63, ovarian tissue cryopreservation, age 34)
Four other women were merely neutral (n = 2) or more negative about the options (n = 2, both had had one child before diagnosis of cancer) and mentioned as reason the insecurities associated with the success rates of the options.
Quote 7: ‘I have mixed feelings about it, especially because it is no insurance [of your fertility] at all’ (R25, no cryopreservation, age 33)
When no(t all) options were possible, women mentioned either feelings of acceptation (n = 3) or frustration (n = 8; these include the two women who received incorrect information, mentioned before):
Quote 8: ‘There you go.., you see it, tears..’ (R25, no cryopreservation, respondent cries because there were no possibilities to spare her fertility at her diagnosis)
Considerations
For most of the women, the main reason for undergoing FP was to have done everything to ensure future fertility. Several other factors that were taken into consideration were as follows: the necessity of FP (having a small chance of infertility), (un)/willingness to undergo surgery, whether there is time for hormonal stimulation in case of IVF, risk of metastasis with cryopreservation of ovarian tissue, no choice/impossibilities regarding FP (e.g. ineligibility), the experimental character of cryopreservation of ovarian tissue (uncertainties), success rates, ethical aspects, not want to be stuck with embryo's from the current partner and costs or insurance.
One woman mentioned that she made an emotional decision because rationally she had no reasons not to pursue FP, but it did not feel right to her, so she chose not to.
Effects of Decision Making
It was often emphasized that deciding about FP was just one of many decisions to be made. For some, this made it easier to decide on FP because they were already in a decision‐making ‘mode’, for others it made decision making on FP harder (especially in an emotional sense). Some additionally mentioned that the nice thing about this decision is that this was actually one of the few decisions that they could make themselves, next to all decisions related to the cancer treatments. For many women, decision making felt good or peaceful (relaxed):
Quote 9: ‘Looking back, I have the feeling that I made the right decision. It makes me feel good to know what the possibilities are and to make an informed decision. It was not easy, but it felt good, as if we made the right decision for us, yes’. (R20, embryo cryopreservation, age 31).
Only few mentioned a very hard time decision making, feeling preoccupied with it at the time they had to decide or burdensome emotions that came with decision making (n = 6):
Quote 10: ‘I remember I was nonstop talking about it’. (R10, ovarian tissue cryopreservation, age 25)
Post‐decision‐making satisfaction
Of the women who decided about FP by themselves (n = 29), seventeen women who underwent FP (1 unknown) and six women, who decided to wait and see, would still choose the same FP option, irrespective of the procedure:
Quote 11: ‘I would do it again ten times in a row. [..] I was so happy that I was able to do it!’ (R16, embryo cryopreservation, age 34)
Five women experienced post‐decision‐making dissatisfaction. Of these 5 women, 4 women actually underwent FP (1 chose to wait and see). Two women (ovarian tissue cryopreservation) were dissatisfied because they knew or thought they had remained fertile after the oncologic treatment so FP had not been necessary (one was pregnant at the moment of the interview). One woman (cryopreservation of embryos) was dissatisfied because of the side‐effects of the IVF medication. Another woman (ovarian tissue cryopreservation) now knew that by the time her treatment finished, she will be too old to have the pieces of ovarian tissue replaced into the remaining ovary.
Process of information provision and decision making
The majority of respondents were, in general, satisfied with the procedure of information provision and decision making. However, there seemed to be room for improvements. Typical procedural aspects that were mentioned by many respondents were the assertiveness necessary to receive information in the first place, the amount of information one receives, in combination with the speed at which multiple decisions had to be made in a short time frame (timing), and the multiple medical centres that need to be visited to get information about FP, because only few centres are specialized in FP‐issues.
Assertiveness
Many women had to be assertive in some way to get the topic fertility on the physician's agenda or to get information they required about FP (n = 15). Women had to be assertive either to get initial information about FP, to receive additional information, to be referred, or to get the right treatments (schedules, hormones, etc.). Only few women mentioned specific resulting emotions (anger, frustration). However, from the way women expressed themselves, it emerged that they were unhappy.
Quote 12: ‘You had to be very assertive [..], I thought that was poor. Not all information is [publicly] available, and at that moment you think about different things [than fertility]. Yes, I think many people have missed opportunities as a result of poor information provision.’ (R11, embryo cryopreservation, age 31)
Amount of information and number of decisions, in relation to timing
For many women (n = 12), the process of information provision and decision making about FP went very fast, or the combination of cancer, information about FP and the need for decision making was very much at the same time. This speed at which much information is given and multiple decisions had to be made between cancer diagnosis and start of the cancer treatment was often negatively evaluated. Sometimes, women therefore compared the process to ‘being on an on‐going train’ or ‘in a rotating mill’.
Multiple medical centres
Twenty women commented on the fact that they had to go to a different medical centre to receive detailed information about FP because this information was only available at specialized medical centres in the Netherlands or Belgium (for this study: LUMC, RdGG or a medical centre in Belgium). Half of the women had no problems with visiting multiple centres to receive adequate information about FP, for example, because they were prepared to make this offer to receive the best available information about FP. The other half of the women were more negative about the multiple locations because of poor communication between the centres (Quote 13) with unclear or even wrong information as a consequence, the need to tell their story over and over again, and administrative hassle such as having to register as a patient in each hospital and inconvenience with regard to travel expenses.
Quote 13: Because there were two hospitals, I noticed [..] that the communication was really poor. I often had to give additional details and then they needed consent, they had to fill in forms and did not have the right information. The hormone levels I had to request myself with the gynaecologist because things were too separated between the centres. I understand that it is privacy, but this was very inconvenient. (R2, ovarian tissue cryopreservation, age 35).
Recommendations regarding the process of information provision and DM
With regard to the question who should decide about FP, many women preferred some form of shared decision making between physicians and them (n = 7), or at least emphasized the importance of the provision of reliable information by a physician, after which women can decide for themselves (n = 13).
Three women suggested that only women with a good prognosis should be informed about FP. The majority of the women (n = 26) reported that all (eligible) women should be informed, regardless of their prognosis (quote 14) and that all available information should be given.
Quote 14: ‘Hope makes one feel alive. And a prognosis.., well, there are women who defeat the prognosis!!’ (R23, embryo cryopreservation, age 27, in reaction to whether or not women with a poor prognosis should receive information about FP)
Three women, who did not receive the information face to face, mentioned providing face‐to‐face information as an improvement. Many others preferred to receive information they could take home, either before the consultation with the fertility specialist to prepare themselves for it or after the consultation to be able to read it again. Brochures, websites and checklists (both for patients and clinicians) were mentioned. Further, better communication between clinicians were mentioned, more information about FP, and referral addresses for clinicians to enable them to better inform their patients, attention for FP in social media and implementation of information provision about FP as structural part in the medical trajectory between diagnosis and start of cancer treatment.
Discussion
This study describes women's experiences with information provision about gonadotoxic effects of oncologic treatment and FP, and with decision making about FP, and presents women's recommendations for improvement of information provision and decision making. The conclusions that can be drawn are that information provision on both topics was overall deemed sufficient, timely and important for the majority of women. However, women often had to be assertive, visit multiple medical centres and process much information in a very short time frame. As improvements, women suggested standardization of the information provision, better communication between clinicians or medical centres and availability of FP‐specific patient information materials.
The results of the current study have to be interpreted with caution in view of the study design and method used. First, results will have been subject to selection bias as the study population consisted of women who attended counselling consultation about FP. These will likely be more positive about FP than other women who turned down the offer for counselling or who missed the opportunity. As we had no information on whether eligible women who did not attend counselling had been offered counselling, we felt it unethical to approach all women of fertile age. Further, findings may have been affected by recall bias, as the study reports on women's feelings and thoughts on a past procedure (0.6–4.1 years ago). Most women were in remission at the time of the interview, and some had given birth to a healthy child or were pregnant at the time of the interview. Additionally, more responders than non‐responders were diagnosed with breast cancer. However, in both groups more than half of the diagnoses were breast cancer, which can be explained by the higher prevalence of breast cancer than other diagnoses in women between 18 and 40 years of age.26 The interviewers had no specific training in conducting qualitative interviews other than what was learned during their medical training. Although the attention given to communication, shared decision making and asking further is fairly good in the medical training in the Netherlands, it would have been better when the interviewers had also been specifically trained to qualitative interviewing. The possible lack of specific interview skills may have led to going less deeply into specific answers given by the respondents, which in turn may have led to less depth in the interviews.
Interestingly, the themes we have found were very similar to unstructured open comments from respondents in a quantitative study about improvements in the referral processes of oncologists and in the counselling consultation by the FP specialist.21 In our study, as much as one‐third of the women initiated the topic themselves or that they at least had to be quite assertive to get the information they needed (irrespective of the year they were diagnosed). Yet, women were satisfied with the information received, although for some improved information could have lead to better expectations regarding the FP treatments and more knowledge about other ways to fulfil a pregnancy in the future. Furthermore, some women thought that too much information was provided at the same time. Therefore, the information should not always be given all at once, and ideally tailored to the individual in an individual consultation with a fertility specialist.15 Generally, women were also satisfied with the timing of the information provision. However, it was emphasized that early information provision is necessary to enable women to decide about FP and to undergo FP treatment.21, 27, 28
Consistent with other research,8, 29 some women were more preoccupied with surviving (the majority), others were focusing on life after cancer. Interestingly, both groups thought information provision about FP was important. Therefore, women should be able to decide for themselves what they want in FP. Moreover, they should not be pushed into a decision in favour of FP, and all possibilities (including ‘wait and see’) and impossibilities should be clarified.30
Similar to other studies, this study found that a majority of women thought all women should be informed about FP.5, 7, 31 In practice, this is currently not the case. One explanation may be that some physicians feel hesitant about informing women with a poor prognosis or advanced disease.19, 32 On the contrary, in our study only a few women thought women with a poor prognosis should not be informed about FP. Furthermore, women think medical personnel should have more knowledge about FP and referral addresses, to be able to better inform patients. Lack of knowledge has indeed been identified as a barrier to informing (and referring) women.32, 33 Attention should be paid to the communication between medical centres or specialists as well. Other suggestions were to increase attention for FP in social media and to make sure information provision about FP is a structural part in the patient trajectory.
Unsurprisingly, the majority of women had a favourable opinion about FP. Other retrospective surveys on adolescents and women with a diagnosis of cancer have also found that women have a positive attitude towards FP.34, 35 Two women with a more negative opinion about FP, both already had a child at diagnosis, and, consistently with their opinion, chose to wait and see. Additionally, in deciding whether or not to choose for FP, most women mentioned rational considerations that were congruent with the option they chose. Although we are not sure whether women had sufficient knowledge to decide, our data indicate that the first requirements for informed decision making were met (attitude and values, here considerations, were congruent with the decision).36 However, some women decided more intuitively with emotion as their primary guide.37
Most women who underwent FP and all women who decided to ‘wait and see’ were still satisfied with the decision made, 2 or more years post‐decision making. Other qualitative research has found that decisional conflict and regret resulted mostly from deciding not to receive FP treatment (i.e. wait and see).23, 38 These different results may be due to differences in counselling consultations on FP. Other studies found that receiving counselling about FP and pursing in FP is associated with less regret24 and that use of a web‐based decision aid leads to reduced decisional conflict and reduced regret at 1‐year post‐decision making.39
Lastly, an often mentioned recommendation was to develop patient brochures, checklists or a website with information about FP.15, 40 Women value additional information to read prior to, or after, the counselling consultation with a gynaecologist or IVF specialist. A quantitative study by Hill et al. also found that women required relevant information both before and after the counselling consultation.21 Balthazar et al. (2012) found that women's knowledge about FP after a counselling consultation only is still limited, and therefore recommended development of educational material.41 Nowadays, web‐based information is also used more often, as an adjunct to the information that is handed out by the physician.42
Future quantitative research should focus on the exact effects of the (perceived) amount of information and satisfaction on decision making processes, and outcomes of decision making in the light of relevant existing decision‐making theories, such as informed or shared decision making, which also take into account knowledge, attitudes and value congruence and are measured with validated quantitative measures.
Based on the results of this article, we recommend healthcare providers to inform all eligible women about FP in a timely manner. The amount and timing of information should be adjusted to the patients' individual preferences. It appears that, in the case of breast cancer, often enough time is available between diagnosis and start of adjuvant treatment to underwent one (or more) cycles for cryopreservation of embryos or a surgery for cryopreservation of ovarian tissue. If information is provided soon after diagnosis, this time can be used optimally for FP. Some women value information to read at home before or after the consultation so better patient information should be developed. Internationally, many websites and some decision aid (DA) websites about FP have been developed (see overview Kelvin et al.).43 Like many DAs on other topics, the DA website myoncofertility has been found to improve decision‐making outcomes, compared to brochures.39, 44 Web‐based information is accessible at any moment in the trajectory and seems a viable format for this population.45, 46 Therefore, we think a Dutch web‐based DA about FP could be a valuable addition to current information provision. Because few Dutch patients have sufficient knowledge of the English language to consult existing (DA) websites, and not all patient information is the same internationally, a Dutch Decision Aid website should be developed as well. The information gathered through these interviews has therefore been used to develop patient information brochures and a web‐based decision aid about FP, which will soon be evaluated.
Source of funding
This study was supported by DSW health and social care insurer, Schiedam, the Netherlands.
Conflict of interest
None declared.
Acknowledgements
The authors would like to thank the participating women for sharing their personal experiences.
References
- 1. Hickey M, Peate M, Saunders CM, Friedlander M. Breast cancer in young women and its impact on reproductive function. Human Reproduction Update, 2009; 15: 323–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meirow D. The effects of radiotherapy and chemotherapy on female reproduction. Human Reproduction Update, 2001; 7: 535–543. [DOI] [PubMed] [Google Scholar]
- 3. Kim SS, Klemp J, Fabian C. Breast cancer and fertility preservation. Fertility and Sterility, 2011; 95: 1535–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee SJ, Schover LR, Partridge AH et al American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. Journal of Clinical Oncology 2006; 24: 2917–2931. [DOI] [PubMed] [Google Scholar]
- 5. Partridge AH, Gelber S, Peppercorn J et al Web‐based survey of fertility issues in young women with breast cancer. Journal of Clinical Oncology, 2004; 22: 4174–4183. [DOI] [PubMed] [Google Scholar]
- 6. Loscalzo MJ, Clark KL. The psychosocial context of cancer‐related infertility. Cancer Treatment and Research, 2007; 138: 180–190. [DOI] [PubMed] [Google Scholar]
- 7. Duffy CM, Allen S. Medical and psychosocial aspects of fertility after cancer. The Cancer Journal, 2009; 15: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thewes B, Butow P, Girgis A, Pendlebury S. The psychosocial needs of breast cancer survivors; a qualitative study of the shared and unique needs of younger versus older survivors. Psycho‐Oncology, 2004; 13: 177–189. [DOI] [PubMed] [Google Scholar]
- 9. Schover LR. Psychosocial aspects of infertility and decisions about reproduction in young cancer survivors: a review. Medical and Pediatric Oncology, 1999; 33: 53–59. [DOI] [PubMed] [Google Scholar]
- 10. [Guideline cryopreservation of ovarian tissue] Dutch Society for Obstetrics and Gynecology (NVOG) and Quality Institute for Health Care CB , Utrecht, the Netherlands, 2007. [Google Scholar]
- 11. Pentheroudakis G, Orecchia R, Hoekstra HJ, Pavlidis N. Cancer, fertility and pregnancy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology 2010; 21 (Suppl. 5): v266–v273.:v266‐v273. [DOI] [PubMed] [Google Scholar]
- 12. American Society for Reproductive Medicine . Child‐rearing ability and the provision of fertility services. Fertility and Sterility, 2004; 82: S208–S211. [DOI] [PubMed] [Google Scholar]
- 13. Duffy CM, Allen SM, Clark MA. Discussions regarding reproductive health for young women with breast cancer undergoing chemotherapy. Journal of Clinical Oncology, 2005; 23: 766–773. [DOI] [PubMed] [Google Scholar]
- 14. Nakayama K, Liu P, Detry M et al Receiving information on fertility‐ and menopause‐related treatment effects among women who undergo hematopoietic stem cell transplantation: changes in perceived importance over time. Biology of Blood and Marrow Transplantation, 2009; 15: 1465–1474. [DOI] [PubMed] [Google Scholar]
- 15. Thewes B, Meiser B, Taylor A et al Fertility‐ and menopause‐related information needs of younger women with a diagnosis of early breast cancer. Journal of Clinical Oncology, 2005; 23: 5155–5165. [DOI] [PubMed] [Google Scholar]
- 16. Schover LR, Rybicki LA, Martin BA, Bringelsen KA. Having children after cancer. A pilot survey of survivors' attitudes and experiences. Cancer, 1999; 86: 697–709. [DOI] [PubMed] [Google Scholar]
- 17. Peate M, Meiser B, Hickey M, Friedlander M. The fertility‐related concerns, needs and preferences of younger women with breast cancer: a systematic review. Breast Cancer Research and Treatment, 2009; 116: 215–223. [DOI] [PubMed] [Google Scholar]
- 18. Jenninga E, Hilders CG, Louwe LA, Peters AA. Female fertility preservation: practical and ethical considerations of an underused procedure. Cancer Journal, 2008; 14: 333–339. [DOI] [PubMed] [Google Scholar]
- 19. Quinn GP, Vadaparampil ST, King L et al Impact of physicians' personal discomfort and patient prognosis on discussion of fertility preservation with young cancer patients. Patient Education and Counseling, 2009; 77: 338–343. [DOI] [PubMed] [Google Scholar]
- 20. Scanlon M, Blaes A, Geller M, Majhail NS, Lindgren B, Haddad T. Patient satisfaction with physician discussions of treatment impact on fertility, menopause and sexual health among pre‐menopausal women with cancer. Journal of Cancer, 2012; 3: 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hill KA, Nadler T, Mandel R et al Experience of young women diagnosed with breast cancer who undergo fertility preservation consultation. Clinical Breast Cancer, 2012; 12: 127–132. [DOI] [PubMed] [Google Scholar]
- 22. Kovanci E, Amato P, Cisneros P, Buster JE, Gershenson DM, Carson SA. Factors influencing decision making in women seeking fertility preservation at a tertiary care center program. Fertility and Sterility, 2006; 86: S314. [Google Scholar]
- 23. Connell S, Patterson C, Newman B. A qualitative analysis of reproductive issues raised by young Australian women with breast cancer. Health Care for Women International, 2006; 27: 94–110. [DOI] [PubMed] [Google Scholar]
- 24. Niemasik EE, Letourneau J, Dohan D et al Patient perceptions of reproductive health counseling at the time of cancer diagnosis: a qualitative study of female California cancer survivors. Journal of Cancer Survivalship, 2012; 6: 324–332. [DOI] [PubMed] [Google Scholar]
- 25. Pope C, Ziebland S, Mays N. Qualitative research in health care. Analysing qualitative data. BMJ, 2000; 320: 114–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dutch Cancer Registry (NKR) . www.ikcnet.nl. Date accessed 18‐04‐2012.
- 27. Thewes B, Meiser B, Rickard J, Friedlander M. The fertility‐ and menopause‐related information needs of younger women with a diagnosis of breast cancer: a qualitative study. Psycho‐Oncology, 2003; 12: 500–511. [DOI] [PubMed] [Google Scholar]
- 28. Lee S, Ozkavukcu S, Heytens E, Moy F, Oktay K. Value of early referral to fertility preservation in young women with breast cancer. Journal of Clinical Oncology, 2010; 28: 4683–4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee RJ, Wakefield A, Foy S, Howell SJ, Wardley AM, Armstrong AC. Facilitating reproductive choices: the impact of health services on the experiences of young women with breast cancer. Psycho‐Oncology, 2011; 20: 1044–1052. [DOI] [PubMed] [Google Scholar]
- 30. Rosen A, Rodriguez‐Wallberg KA, Rosenzweig L. Psychosocial distress in young cancer survivors. Seminars in Oncology Nursing, 2009; 25: 268–277. [DOI] [PubMed] [Google Scholar]
- 31. Surbone A, Petrek JA. Childbearing issues in breast carcinoma survivors. American Cancer Society, 1997; 79: 1271–1278. [PubMed] [Google Scholar]
- 32. Quinn GP, Vadaparampil ST, Bell‐Ellison BA, Gwede CK, Albrecht TL. Patient‐physician communication barriers regarding fertility preservation among newly diagnosed cancer patients. Social Science and Medicine, 2008; 66: 784–789. [DOI] [PubMed] [Google Scholar]
- 33. Vadaparampil ST, Clayton H, Quinn GP, King LM, Nieder M, Wilson C. Pediatric oncology nurses' attitudes related to discussing fertility preservation with pediatric cancer patients and their families. Journal of Pediatric Oncology Nursing, 2007; 24: 255–263. [DOI] [PubMed] [Google Scholar]
- 34. Burns KC, Boudreau C, Panepinto JA. Attitudes regarding fertility preservation in female adolescent cancer patients. Journal of Pediatric Hematology/Oncology, 2006; 28: 350–354. [DOI] [PubMed] [Google Scholar]
- 35. Tschudin S, Bunting L, Abraham J, Gallop‐Evans E, Fiander A, Boivin J. Correlates of fertility issues in an Internet survey of cancer survivors. Journal of Psychosomatic Obstetrics and Gynaecology, 2010; 31: 150–157. [DOI] [PubMed] [Google Scholar]
- 36. Marteau TM, Dormandy E, Michie S. A measure of informed choice. Health Expectations, 2001; 4: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cohn F. Oncofertility and informed consent: addressing beliefs, values, and future decision making. Cancer Treatment and Research, 2010; 156: 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Balthazar U, Fritz MA, Bardsley T, Mersereau JE. Decision making under duress: what predicts decisional conflict among fertility preservation patients? Fertility and Sterility, 2010; 94: S105. [Google Scholar]
- 39. Peate M, Meiser B, Cheah BC et al Making hard choices easier: a prospective, multicentre study to assess the efficacy of a fertility‐related decision aid in young women with early‐stage breast cancer. British Journal of Cancer, 2012; 106: 1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gardino SL, Emanuel LL. Choosing life when facing death: understanding fertility preservation decision‐making for cancer patients. Cancer Treatment and Research, 2010; 156: 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Balthazar U, Deal AM, Fritz MA, Kondapalli LA, Kim JY, Mersereau JE. The current fertility preservation consultation model: are we adequately informing cancer patients of their options? Human Reproduction, 2012; 27: 2413–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Balmer C. The information requirements of people with cancer where to go after the “patient information leaflet”. Cancer Nursing, 2005; 28: 36–44. [DOI] [PubMed] [Google Scholar]
- 43. Kelvin JF, Kroon L, Ogle SK. Fertility preservation for patients with cancer. Clinical Journal of Oncology Nursing, 2012; 16: 205–210. [DOI] [PubMed] [Google Scholar]
- 44. O'Connor AM, Bennett CL, Stacey D et al Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews 2009; CD001431. doi: 10.1002/14651858.CD001431.pub2. [DOI] [PubMed] [Google Scholar]
- 45. Jukkala AM, Azuero A, McNees P, Bates GW, Meneses K. Self‐assessed knowledge of treatment and fertility preservation in young women with breast cancer. Fertility and Sterility, 2010; 94: 2396–2398. [DOI] [PubMed] [Google Scholar]
- 46. Meneses K, McNees P, Azuero A, Jukkala A. Evaluation of the Fertility and Cancer Project (FCP) among young breast cancer survivors. Psycho‐Oncology, 2010; 19: 1112–1115. [DOI] [PubMed] [Google Scholar]


