Abstract
Objective
To examine the impact of concordant and discordant comorbidities on patients' assessments of providers' adherence to diabetes‐specific care guidelines and quality of chronic illness care.
Research design and methods
A population‐based survey of 3761 adults with type 2 diabetes, living in Queensland, Australia was conducted in 2008. Based on self‐reports, participants were grouped into four mutually exclusive comorbid categories: none, concordant only, discordant only and both concordant and discordant. Outcome measures included patient‐reported providers' adherence to guideline‐recommended care and the Patient Assessment of Chronic Illness Care (PACIC), which measures care according to the Chronic Care Model. Analyses using the former measure included logistic regressions, and the latter measure included univariate analysis of variance, both unadjusted and adjusted for sampling region, gender, age, educational attainment, diabetes duration and treatment status.
Results
Having concordant comorbidities increased the odds of patient‐reported providers' adherence for 7 of the 11 guideline‐recommended care activities in unadjusted analyses. However, the effect remained significant for only two provider activities (reviews of medication and/or complications and blood pressure examinations) when adjusted. A similar pattern was found for the both concordant and discordant comorbidity category. The presence of discordant comorbidities influenced only one provider activity (blood pressure examinations). No association between comorbidity type and the overall PACIC score was found.
Conclusions
Comorbidity type is associated with diabetes‐specific care, but does not seem to influence broader aspects of chronic illness care directly. Providers need to place more emphasis on care activities which are not comorbidity‐specific and thus transferable across different chronic conditions.
Keywords: chronic illness care, comorbidity, diabetes, guideline adherence, patient perspectives, quality of care
Introduction
Ageing of populations has contributed to increasing chronic disease burden. With increasing age, prevalence of comorbidity (the simultaneous presence of one or more additional conditions) also increases, further compounding the chronic disease burden.1, 2, 3 Effective management of chronic diseases like diabetes requires an understanding of the effect of comorbidity on chronic illness care. This can then inform the design and assessment of chronic disease management interventions.4
For most people living with diabetes, comorbidity is common and complicates the provision of high quality care.5 Research into the relationship between comorbidity and quality of diabetes care has been contradictory. While some research has indicated that comorbidities may lower the care received by the patient,6, 7 others suggest that the burden of comorbidities has no effect on the quality of care8 or indeed can improve the care provided.9, 10, 11 Differences in the type of comorbidities have been suggested as a factor contributing to the inconsistency in reported findings.12 Thus, the presence of discordant comorbidities (those that do not share the same pathogenesis or treatment approach as diabetes, e.g. cancers, arthritis) or comorbidities with a higher symptom burden than diabetes are the ones likely to have a negative impact on the management of diabetes,13 while concordant comorbidities (e.g. heart disease, renal disease) may improve the quality of diabetes care.14, 15 Another observation has been that specific comorbidities which expose patients to the health system may also have an important role in promoting diabetes care.12
Piette and Kerr12 recommended further research on the impact of comorbidity types on self‐management support and coordinated care through patient‐centred measures, proposing the Patient Assessment of Chronic Illness Care (PACIC)16 as one potentially useful measure. Similarly, Glasgow et al.16 suggested investigating the relationship between the PACIC and comorbidity measures using more than just a comorbidity count. The PACIC measures the extent to which patients report receiving care that is consistent with the dimensions of the Chronic Care Model (CCM). The CCM is a comprehensive, evidence‐based framework for improving care processes for those with chronic diseases such as diabetes. The CCM emphasizes the enhancement of diabetes care through the use of evidence‐based, planned, coordinated multidisciplinary care involving five basic elements: (i) reorganization of practice systems and provider roles; (ii) improved patient self‐management support; (iii) increased access to decision support; (iv) greater availability and use of clinical information and (v) better use of community resources.17 A growing body of evidence indicates that this more proactive and integrated approach to the management and treatment of diabetes results in improved quality of life and health outcomes for patients with diabetes.18, 19 The present study thus aims to assess whether patients with concordant and/or discordant comorbidities receive better quality of diabetes care from their perspectives. In addition, patients' reports of their health‐care providers' adherence to recommended diabetes‐specific care will be examined in relation to concordant and/or discordant nature of comorbidities. We also aim to assess the impact of specific comorbidities on patient‐assessed quality of care.
Research design and methods
Participants
Data reported here are taken from the baseline survey of a longitudinal, prospective cohort study, the Living with Diabetes Study (LWDS) conducted in 2008. Participants were recruited from the National Diabetes Services Scheme (NDSS), an Australian government initiative that delivers diabetes‐related products at subsidized prices to registrants and covers 80–90% of the Australian population diagnosed with diabetes.20 A sample of 14 439 adult NDSS registrants living in Queensland, Australia, was invited to participate. Oversampling occurred in an outer metropolitan, a developing suburban and a coastal agricultural area of policy interest. A detailed description of the study has been reported elsewhere.21
Completed self‐report questionnaires were returned by 3951 participants, yielding a participation rate at baseline of 29%. Ninety five per cent (n = 3761) of participants were verified as having type 2 diabetes using NDSS registration data and are included the analyses presented here. Analyses using aggregated NDSS data showed that individuals who accepted the invitation to participate were largely similar to those who did not, although individuals were more likely to participate if they were aged over 60, and Indigenous Australians were less likely to participate.22 Ethics approval for the study was granted by the University of Queensland's Behavioural and Social Sciences Ethical Review Committee.
Principal measures
Comorbidity
Diabetes concordant (heart disease, stroke, renal disease, neuropathy, eye complications, erectile dysfunction, poor peripheral circulation, foot ulcers, gangrene) and discordant (mental health conditions, asthma, cancers, osteoporosis, arthritis, chronic obstructive pulmonary disease, Alzheimer's/dementia, substance use disorder) comorbid conditions were identified based on self‐report. Respondents were asked to indicate which of the listed conditions they had ever been told they had by a doctor or nurse. Patients were grouped into mutually exclusive comorbidity categories: (i) no comorbid conditions; (ii) concordant comorbid conditions only; (iii) discordant comorbid conditions only; (iv) both concordant and discordant comorbid conditions.
Quality of diabetes care
The first measure was the respondents' reports on 11 recommended care quality indicators based on Australian diabetes management guidelines.23 Participants indicated whether a member of their health‐care team had undertaken each of the activities in the preceding 12 months. A summary measure for providers' adherence to care activities in the guidelines was computed by simply adding the responses on 11 care activities undertaken. The second measure was the Patient Assessment of Chronic Illness Care (PACIC), which measures the extent to which patients report receiving care (for their diabetes in the preceding 6 months) that is consistent with the dimensions of the CCM. A recent assessment of 31 measures of quality of care designed for use in people with chronic illness found the PACIC to be the most appropriate instrument, as determined by its psychometric properties and perceived applicability and relevance.24 The PACIC consists of 20 items, each of which is scored on a 5‐response scale: 1 (none of the time) being the lowest point on the scale and 5 (always) being the highest. The overall PACIC score is the average of the scores for all items answered. Higher scores indicate higher quality of care.
Statistical analyses
Descriptive analyses provided frequencies, means and standard deviations on participant characteristics and other health‐care‐related variables, including the receipt of multidisciplinary care. Chi‐square analyses were used to assess the association between comorbidity type and categorical covariates. Logistic regression was used to analyse the effect of comorbidity type on the patient‐reported health‐care providers' adherence to recommended reviews, examinations and tests, firstly in an unadjusted model and then a multivariable model. Covariates in the adjusted model included sampling region, gender, age, educational attainment, duration of living with diabetes and treatment status (insulin, oral medications or neither).
For analyses involving the overall PACIC score, data were included only for the 86% of diabetes patients who answered 11 or more of the 20 PACIC items. Differences in the overall PACIC score among participants with various types of comorbidities were investigated through analysis of variance (anova); firstly in an unadjusted model and then in a multivariable model using the same covariates as outlined above. The relationship between multidisciplinary care and either the PACIC or the summary measure of providers' adherence to guideline‐recommended care was determined by using anova. We found, as expected, that having concordant and/or discordant comorbidities was associated with more visits to specialists/providers, and this in turn led to getting more guideline‐recommended care and higher PACIC scores (data not shown). All these findings are consistent with multidisciplinary care being an intermediate variable (in the pathway between comorbidity type and PACIC/guideline adherence), and hence, it was not included in the adjusted model. All analyses were conducted using spss statistical software (IBM® SPSS® Statistics 20.0, Chicago, IL, USA).
Results
Description of the sample
A total of 3761 persons with type 2 diabetes responded to the LWDS survey in 2008. Participants' mean age was 62.5 years (SD = 10.88; range 22 to 94), 55.3% were male, 47.8% did not finish high school, and 29.2% were divorced, widowed, separated or had never married. One‐third reported an annual household income of < $20 000, two‐thirds lived in a major city, and only 1.8% were from an Australian Aboriginal or Torres Strait Islander background. Median time since diagnosis with diabetes was 6 years with the interquartile range of 3 to 10. Among the respondents, 18.2% required insulin (with or without oral medications) and 60.4% oral medication for management of their type 2 diabetes. Half of the respondents had a BMI of 30 or more (obese or morbidly obese). The number of types of providers visited by the participants in the last 12 months was used to determine the extent to which their care was multidisciplinary in nature. As 98% of participants reported visiting general practitioners, they were excluded from the list of provider types in this analysis. Among the remaining types of providers, 35% of the participants reported visiting four to eight provider types, 41% visiting two to three types, 15% visiting only one type and 9% did not report visiting any of the providers. One or more of the listed comorbidities was reported by 82.4% of the participants, with a mean of 2.4 comorbidities (SD = 2.10; range from 0 to 13). Of 3758 participants with the available data, 17.6% (n = 662) reported having none of the listed comorbidities, 18.9% (n = 711) had concordant comorbidity only, 22% (n = 828) had discordant comorbidity only and 41.4% (n = 1557) had both concordant and discordant comorbidities.
Patient‐reported health‐care providers' adherence to guidelines
The proportions of participants by comorbidity type who reported receiving each of the guideline‐recommended activities (reviews/examinations/tests) from their health‐care team in the past 12 months are shown in Table 1. For each activity, at least 50% of patients reported that it was conducted by health‐care providers as recommended. Adherence to guidelines for blood tests and blood pressure checks was common, whereas reviews of lifestyle and self‐management activities were comparatively low.
Table 1.
Percentages of participants who reported receiving guideline‐recommended care in the past 12 months
| Activity (recommended frequency)23 | N | % of respondents in each category: | |||
|---|---|---|---|---|---|
| No comorbidity (%) | Concordant comorbidity only (%) | Discordant comorbidity only (%) | Both concordant and discordant comorbidities (%) | ||
| Review: (once per year) | |||||
| Physical activity | 3425 | 64.7 | 62.4 | 63.1 | 61.0 |
| Diet | 3445 | 53.5 | 49.5 | 55.3 | 54.1 |
| Self‐management regime | 3337 | 47.6 | 51.6 | 51.6 | 48.7 |
| Medications and/or complications | 3513 | 73.5 | 81.7 | 75.4 | 80.8 |
| Self‐tested blood glucose readings (3 monthly) | 3551 | 65.2 | 73.0 | 64.5 | 71.1 |
| Examine | |||||
| Feet (every 6 months) | 3570 | 62.2 | 68.6 | 61.9 | 71.8 |
| Eyes (at least every 2 years) | 3581 | 75.6 | 80.9 | 74.0 | 82.3 |
| Blood pressure (every 6 months) | 3680 | 96.5 | 99.3 | 98.6 | 99.0 |
| Test: (once per year) | |||||
| Cholesterol level (blood lipids) | 3641 | 95.6 | 97.7 | 96.5 | 97.1 |
| HbA1c levels | 3445 | 95.0 | 96.5 | 95.5 | 95.3 |
| Kidney function (blood creatinine/urine protein) | 3438 | 88.4 | 92.9 | 88.7 | 91.6 |
Compared to participants with no comorbidity in unadjusted analyses, those with concordant comorbidities were significantly more likely to report that their health‐care team undertook reviews of medications and/or complications and self‐tested blood glucose readings, conducted examinations of their feet, eyes and blood pressure and tested their cholesterol level and kidney function (Table 2). When adjusted, these associations remained significant only for reviews of medications and/or complications (OR = 1.37; 1.03–1.82) and blood pressure checks (OR = 3.02; 1.10–8.28). With the exception of cholesterol tests, the same pattern of significance was found for diabetic patients who reported both concordant and discordant comorbidities.
Table 2.
Odds ratios (OR) of getting recommended reviews, examinations or tests in the past 12 months
| Concordant comorbidity | Discordant comorbidity | Both concordant and discordant comorbidities | P‐value from Likelihood Ratio Test | |||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | Unadjusted | Adjusteda | Unadjusted | Adjusteda | Unadjusted | Adjusteda | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |||
| Review | ||||||||
| Physical activity | 0.91 (0.72–1.14) | 0.85 (0.67–1.08) | 0.93 (0.75–1.17) | 0.89 (0.71–1.12) | 0.85 (0.70–1.04) | 0.81 (0.65–1.00) | 0.431 | 0.267 |
| Diet | 0.85 (0.68–1.06) | 0.92 (0.72–1.16) | 1.07 (0.87–1.33) | 0.98 (0.79–1.22) | 1.02 (0.85–1.24) | 1.05 (0.85–1.28) | 0.154 | 0.596 |
| Self‐management regime | 1.17 (0.94–1.47) | 1.13 (0.89–1.43) | 1.17 (0.95–1.46) | 1.10 (0.88–1.38) | 1.05 (0.86–1.27) | 0.96 (0.78–1.19) | 0.316 | 0.313 |
| Medications and/or complications | 1.61 (1.23–2.10)*** | 1.37 (1.03–1.82)* | 1.10 (0.87–1.41) | 1.09 (0.85–1.41) | 1.52 (1.22–1.90)*** | 1.35 (1.06–1.72)* | <0.001 | 0.041 |
| Self‐tested blood glucose readings | 1.44 (1.14–1.83)** | 1.15 (0.90–1.49) | 0.97 (0.78–1.21) | 0.95 (0.76–1.20) | 1.31 (1.07–1.60)** | 1.04 (0.84–1.29) | <0.001 | 0.477 |
| Examine | ||||||||
| Feet | 1.33 (1.06–1.67)* | 1.09 (0.86–1.39) | 0.99 (0.79–1.23) | 0.94 (0.75–1.17) | 1.54 (1.27–1.88)*** | 1.22 (0.99–1.51) | <0.001 | 0.051 |
| Eyes | 1.37 (1.05–1.79)* | 1.10 (0.83–1.45) | 0.92 (0.72–1.18) | 0.91 (0.71–1.17) | 1.51 (1.20–1.89)*** | 1.19 (0.94–1.52) | <0.001 | 0.113 |
| Blood pressure | 5.03 (1.90–13.32)** | 3.02 (1.10–8.28)* | 2.68 (1.29–5.53)** | 2.84 (1.35–5.98)** | 3.72 (1.93–7.18)*** | 3.00 (1.44–6.24)** | <0.001 | 0.007 |
| Test | ||||||||
| Cholesterol level (blood lipids) | 1.92 (1.03–3.59)* | 1.55 (0.79–3.01) | 1.28 (0.75–2.18) | 1.33 (0.76–2.32) | 1.53 (0.94–2.48) | 1.29 (0.76–2.19) | 0.169 | 0.592 |
| HbA1c levels | 1.44 (0.83–2.49) | 1.20 (0.67–2.15) | 1.13 (0.69–1.86) | 1.29 (0.77–2.17) | 1.08 (0.70–1.68) | 0.96 (0.60–1.54) | 0.592 | 0.552 |
| Kidney function (blood creatinine or urine protein) | 1.71 (1.16–2.53)** | 1.18 (0.79–1.79) | 1.03 (0.73–1.44) | 1.05 (0.74–1.49) | 1.43 (1.05–1.95)* | 1.05 (0.75–1.46) | 0.006 | 0.873 |
P values of comorbidity group relative to the ‘no comorbidity’ reference group: *P value <0.05; **P value <0.01; ***P value <0.001; 95% CI = 95% confidence interval.
adjusted for sampling region, gender, age, level of education, diabetes duration and treatment status
The only patient‐reported health‐care provider activity associated with having discordant comorbidities was blood pressure examinations, and it was significant in both the unadjusted (OR = 2.68; 1.29–5.53) as well as the adjusted (OR = 2.84; 1.35–5.98) analyses. Diabetic patients in the discordant comorbidity only group were more likely to receive blood pressure examinations than those with no comorbidity, although the effect was weaker than for the concordant comorbidity only group or the both concordant and discordant comorbidity group.
An investigation of the covariates that were included in the adjusted model revealed that both age and treatment status were significantly associated with receiving reviews of medications and/or complications and self‐tested blood glucose readings as well as examinations of feet, eyes and blood pressure and tests of cholesterol and kidney function (P < 0.001 to 0.05). For every 10 year increase in age, adherence to guidelines was between 1.10 and 1.53 times more likely. Insulin‐requiring patients were 1.38 to 3.43 times, and those on oral medications were 1.46 to 2.28 times more likely to report that their health‐care team undertook these activities. In addition, those requiring insulin were 1.98 (1.14–3.43) times and those on medications were 2.24 (1.54–3.26) times more likely to get HbA1c tests although getting HbA1c tests was not associated with comorbidity type. Duration of diagnosis with diabetes had a significant effect on receiving two of the activities. Patients with longer diabetes duration were more likely to receive recommended eye examinations from their health‐care provider (OR = 1.19; 1.04–1.37), but were less likely to have their self‐tested blood glucose readings reviewed (OR = 0.90; 0.81–0.99). Gender had a significant effect on receiving dietary review and kidney function tests, with men less likely to get the former (OR = 0.76; 0.66–0.88) and more likely to get the latter (OR = 1.47; 1.14–1.90). Educational attainment had an effect only on getting physical activity reviews, with the university graduates getting more reviews than those with trade/apprenticeship (OR = 0.72; 0.54–0.94), certificate/diploma (OR = 0.68; 0.52–0.90) and year 12 education or equivalent (OR = 0.74; 0.56–0.98).
Because age, treatment status and disease duration are markers of disease severity and progression, they are therefore likely to be associated with the presence of concordant comorbidity and may act as confounders. Indeed, we tested these associations using chi‐square analysis and showed that the older participants, those who were insulin‐requiring or taking oral medications to control their diabetes and those with longer duration of diabetes were more likely to report having concordant comorbidities (all P < 0.001). The effects of these covariates are apparent when comparing the unadjusted with the adjusted models in Table 2 and may account for the attenuation of the association between type of comorbidity and patient‐reported providers' adherence to guidelines.
Patient‐assessed quality of care measured by the PACIC
We found no significant association between the overall PACIC score and comorbidity type in either the unadjusted (P = 0.745) or the adjusted model (P = 0.727) (Table 3).
Table 3.
Relationship between patient‐assessed quality of care and comorbidity type
| Overall PACIC score | ||||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | |||||||
| N | Mean ± SD | SE | P‐value | N | Mean | SE | P‐value | |
| Comorbidity types | ||||||||
| No comorbidity | 565 | 2.41 ± 1.08 | 0.04 | 0.745 | 553 | 2.35 | 0.06 | 0.727 |
| Concordant comorbidity only | 599 | 2.37 ± 1.06 | 0.04 | 578 | 2.36 | 0.06 | ||
| Discordant comorbidity only | 700 | 2.36 ± 1.01 | 0.04 | 683 | 2.29 | 0.06 | ||
| Both concordant & discordant comorbidities | 1356 | 2.35 ± 1.05 | 0.03 | 1291 | 2.32 | 0.05 | ||
adjusted for sampling region, gender, age, level of education, diabetes duration and treatment status.
We also investigated each comorbidity independently. Both unadjusted and adjusted analyses showed similar results on significance of most differences. Among participants with discordant comorbidities, adjusted analysis of the effect of specific comorbidities on the overall PACIC score revealed that participants with a history of asthma reported significantly better quality of care (mean = 2.41; 95% CI = 2.22–2.40) than those who did not (mean = 2.31; 95% CI = 2.29–2.53) with the mean difference of −0.1 (−0.20 to −0.004) and significance level of P = 0.041 (Fig. 1). Although considerable differences in overall PACIC scores between those who had Alzheimer's disease, dementia, schizophrenia, substance use disorder or malignant melanoma and those who did not were observed, the differences were not statistically significant, most probably due to the insufficient number of participants with those conditions. The adjusted analysis showed a trend for patients with a history of osteoporosis to report better care (P = 0.055) with the mean difference of −0.15 (−0.31 to 0.003).
Figure 1.
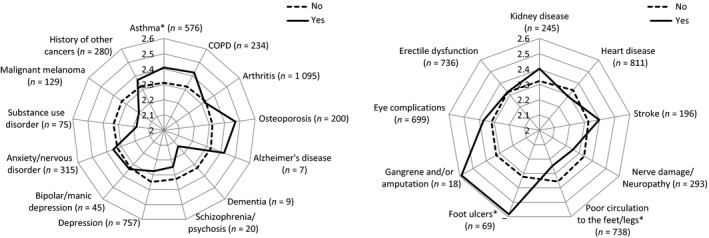
Mean overall PACIC score according to the presence or absence of specific discordant and concordant comorbidities. Yes = have ever been told by a doctor or nurse that the participant has the condition; COPD = chronic obstructive pulmonary disease; Analysis included only men for erectile dysfunction. *P < 0.05 Adjusted for sampling region, gender, age, level of education, diabetes duration and treatment status. n = the number of participants with the condition.
For patients with concordant comorbidities, a paradoxical finding was observed. Participants with a history of foot ulcers reported significantly higher overall PACIC scores (than those without) with a mean difference of −0.26 (−0.51 to −0.01); P = 0.041, while those with poor circulation to the feet or legs reported significantly lower scores with a mean difference of 0.11 (0.02 to 0.20); P = 0.017. Although there was a sizable difference in the overall PACIC score between participants with and without a history of gangrene and/or amputation, the difference was not significant, again probably due to the small number of participants with this condition.
Discussion
This is one of only a few studies investigating the relationship between concordant and discordant comorbidities and diabetes care based on patient assessments. It is also the only study of this kind to use the PACIC as an outcome measure. We have shown that, in unadjusted analyses, patients with type 2 diabetes having concordant comorbidities have an increased likelihood of receiving guideline‐recommended care, but not chronic illness care in general (as assessed by the PACIC measure). However, the former associations for the most part did not hold in multivariable models that controlled for covariates.
Adjustment for age, treatment status and diabetes duration resulted in a reduction in the strength of association between having one or more concordant comorbidities and patient‐reported adherence to guidelines. The adjusted associations actually became non‐significant for five guideline‐recommended activities. These results lead to two major conclusions. First, health‐care providers are strongly influenced by their patient's age and treatment status when making care decisions. Second, health‐care providers do not view comorbidities in isolation when giving care. Thus, several characteristics such as being elderly, having concordant comorbidities, or requiring insulin or oral medications were all found to influence patient‐reported care positively. It is likely therefore that diabetic patients with concordant comorbidities are more likely to report receiving foot and eye examinations simply because providers adjust the level of care to the severity of the patients' needs. Other studies have also found that insulin‐treated or older patients are more likely to report getting eye examinations and/or foot examinations.25, 26, 27, 28
Previous studies that have reported diabetes care outcomes for patients with concordant/discordant comorbidities have not examined providers' support for self‐care.13, 14, 15 We examined the latter and found that concordant comorbidities were not associated with greater reporting of guideline‐recommended reviews on self‐care behaviours (physical activity, diet and self‐management). Patients also reported that such reviews were the least likely to occur. This could reflect a lower priority in the minds of health‐care providers about such discussions with patients even though these should be fundamental elements of the patient‐centred approach to hyperglycaemia.29 Alternatively, it could be that both patient and provider faced with multiple conditions may find that their priorities need to be shifted towards managing the complexities of medications, investigations and care coordination. Nevertheless, the higher the number of comorbidities regardless of their concordant/discordant nature, the lower the self‐management ability of patients.30 This highlights the importance of intensifying the provision of lifestyle and self‐management reviews to those with any comorbidity regardless of its type.
Our finding that type of comorbidity did not influence patient‐assessed quality of care as measured by the PACIC is consistent with previous research. For example, other research groups have reported that neither patient satisfaction nor patient's subjective ratings of the quality of their care differ based on comorbidity type.31 Furthermore, several studies have reported that there is little association between the overall PACIC score and a count of the number of comorbidities.16, 32 So while comorbidity type influences providers' adherence to guidelines, comorbidities (whether counted or categorized according to their concordant/discordant nature) do not influence overall care as measured by the PACIC. There were nevertheless some specific associations between the PACIC and certain individual comorbidities: one discordant (asthma) and two concordant (foot ulcers and poor circulation to the feet/legs) conditions. All these reinforce the notion that the concordant/discordant nature of the comorbidities may have less effect on care than does their tendency to bring the patient in contact with health services,12 especially so in terms of a generalized assessment of chronic care. Having a chronic disease like asthma may result in patients being more familiar with the health‐care environment, such as team care and management plans.
Our finding of participants with foot ulcers reporting higher PACIC scores indicates that the clinical importance of this condition might have been recognized by their health‐care providers. On the other hand, our finding of an association between lower CCM‐congruent care and having poor circulation to the feet/legs indicates that generalist providers could often miss elements of chronic care for this condition (especially if there was no ulceration). Thus, for quality improvement, health‐care teams should give adequate attention to specialist involvement in preventative aspects of care, such as support for self‐care.33 However, this finding must be interpreted with caution as it is also possible that patients with symptoms such as leg pain, who were diagnosed as having peripheral vascular disease, may have higher expectations in and report less satisfaction with health care if they did not get as much attention as those with foot ulcers/gangrene did. Further research should examine the impact of varying severity of peripheral vascular disease on quality of care in Australia, using more sophisticated diagnostic data.
Our results suggest that discordant comorbidities do not compete with diabetes care, whether it is measured by patient‐reported providers' guideline adherence or patient‐assessed quality of chronic care. Patients with discordant comorbidities are even more likely to get blood pressure examinations than those with no comorbidity. In addition, having a discordant comorbidity like asthma may result in better patient‐assessed quality of chronic care. We identified two other studies reporting similar results. Voorham et al.14 concluded that discordant comorbidities, even when incident, did not affect the management of hyperglycaemia and hypertension in diabetic patients. Woodard et al.15 reported that having discordant only comorbidities (rather than no comorbidity) increased the odds of achieving glycaemic and lipid control. In contrast, Pentakota et al.13 found that having discordant only comorbidities lowered the care, only when visit frequency was added to the multivariable model. However, their study was limited to a population of a particular health administration system and incident only diabetes. Differences in these findings may be explained by the differences in the prevalence of a particular subtype of discordant comorbidities, such as mental health or chronic respiratory diseases, among the study populations.
This study has both strengths and limitations. One strength of our study is its population‐based sample with sufficient power to detect association, and the ability to incorporate a wide range of variables in order to develop explanatory models for patient‐assessed quality of care. Our study has a broader range of measures of care than other studies investigating the effect of comorbidity type on quality of diabetes care.13, 14, 15 On the other hand, recall bias and under‐reporting of conditions such as dementia, mental health conditions and substance abuse are possible limitations. Although the 29% response rate can be considered a limitation, previous research has shown that bias due to poor participation is not universally seen.34 Similarly, although it is a concern for further reduction in the sample due to missing PACIC items, this is not an issue as 86% of participants who answered 11 or more PACIC items had similar characteristics compared to the whole sample (manuscript under review). In addition, we did not account for the dominant nature of conditions. The small number of participants with some specific comorbid conditions limited these disease‐specific analyses. Furthermore, we only investigated the concordant/discordant nature of comorbidity and not its burden. Finally, we were not able to accurately reflect recommended guidelines for diabetes care in all instances; specifically, the recommended frequency for foot and blood pressure examinations is at least once every 6 months and not annually as measured in our study.
In conclusion, despite comorbidity type being associated with patient‐reported providers' adherence to guideline‐recommended care activities such as foot examinations, it does not seems to be associated with patients' assessments of their care as defined more broadly by the Chronic Care Model. While more studies of this kind may shed light on the pattern of this relationship, this study suggests that more emphasis needs to be placed on care activities, such as support for self‐care, which are shown to be less affected by comorbidity type in this study and are transferrable across different chronic conditions. This requires efficient use of providers' time to actively strengthen general aspects of chronic illness care, which underpin the management of chronic conditions like diabetes.
Authorship
All authors have seen and approved the final version. All authors contributed to the conceptualisation of the manuscript. E.A. analysed the data, and was the lead author in writing the manuscript. M.D and J.D. were responsible for the data acquisition and design of the study and contributed to writing and editing the manuscript. J.C., and G.W. oversaw the data analysis, and reviewed and edited the manuscript. S.A.R.D. critically reviewed and edited the manuscript.
Conflict of interest
All authors (Eindra Aung, Maria Donald, Joseph Coll, Jo Dower, Gail Williams, and Suhail A. R. Doi) declare that there is no conflict of interest.
Source of funding
This research was funded by Queensland Health.
References
- 1. Britt HC, Harrison CM, Miller GC, Knox SA. Prevalence and patterns of multimorbidity in Australia. The Medical Journal of Australia, 2008; 189: 72–77. [DOI] [PubMed] [Google Scholar]
- 2. Uijen AA, van dLEH. Multimorbidity in primary care: prevalence and trend over the last 20 years. The European Journal of General Practice, 2008; 14(Suppl 1): 28–32. [DOI] [PubMed] [Google Scholar]
- 3. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross‐sectional study. Lancet, 2012; 380: 37–43. [DOI] [PubMed] [Google Scholar]
- 4. France EF, Wyke S, Gunn JM, Mair FS, McLean G, Mercer SW. Multimorbidity in primary care: a systematic review of prospective cohort studies. The British Journal of General Practice: the Journal of the Royal College of General Practitioners, 2012; 62: e297–e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caughey GE, Roughead EE, Vitry AI, McDermott RA, Shakib S, Gilbert AL. Comorbidity in the elderly with diabetes: Identification of areas of potential treatment conflicts. Diabetes Research and Clinical Practice, 2010; 87: 385–393. [DOI] [PubMed] [Google Scholar]
- 6. Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA: the Journal of the American Medical Association, 2005; 294: 716–724. [DOI] [PubMed] [Google Scholar]
- 7. Goldberg RW, Kreyenbuhl JA, Medoff DR, Dickerson FB, Wohlheiter K, Fang LJ, et al Quality of diabetes care among adults with serious mental illness. Psychiatric Services, 2007; 58: 536–543. [DOI] [PubMed] [Google Scholar]
- 8. Halanych JH, Safford MM, Keys WC, Person SD, Shikany JM, Kim Y‐I, et al Burden of Comorbid Medical Conditions and Quality of Diabetes Care. Diabetes Care, 2007; 30: 2999–3004. [DOI] [PubMed] [Google Scholar]
- 9. Bae S, Rosenthal M. Patients with Multiple Chronic Conditions Do Not Receive Lower Quality of Preventive Care. Journal of General Internal Medicine, 2008; 23: 1933–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Millett C, Bottle A, Ng A, Curcin V, Molokhia M, Saxena S, et al Pay for performance and the quality of diabetes management in individuals with and without co‐morbid medical conditions. Journal of the Royal Society of Medicine, 2009; 102: 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higashi T, Wenger N, Adams J, Fung C, Roland M, McGlynn E, et al Relationship between number of medical conditions and quality of care. The New England Journal of Medicine, 2007; 356: 2496–2504. [DOI] [PubMed] [Google Scholar]
- 12. Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care, 2006; 29: 725–731. [DOI] [PubMed] [Google Scholar]
- 13. Pentakota SR, Rajan M, Fincke BG, Tseng CL, Miller DR, Christiansen CL, et al Does diabetes care differ by type of chronic comorbidity? An evaluation of the Piette and Kerr framework. Diabete Care, 2012; 35: 1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Voorham J, Haaijer‐Ruskamp FM, Wolffenbuttel BH, de ZD, Stolk RP, Denig P. Differential effects of comorbidity on antihypertensive and glucose‐regulating treatment in diabetes mellitus–a cohort study. PLoS ONE, 2012; 7: e38707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Woodard LD, Urech T, Landrum CR, Wang D, Petersen LA. Impact of comorbidity type on measures of quality for diabetes care. Medical care, 2011; 49: 605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Glasgow RE, Wagner EH, Schaefer J, Mahoney LD, Reid RJ, Greene SM. Development and validation of the Patient Assessment of Chronic Illness Care (PACIC). Medical Care, 2005; 43: 436–444. [DOI] [PubMed] [Google Scholar]
- 17. Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. The Milbank Quarterly, 1996; 74: 511–544. [PubMed] [Google Scholar]
- 18. Coleman K, Austin BT, Brach C, Wagner EH. Evidence on the chronic care model in the new millennium. Health Affairs, 2009; 28: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. Journal of the American Medical Association, 2002; 288: 1909–1914. [DOI] [PubMed] [Google Scholar]
- 20. AIHW . Australia's health 2010. Australia's health. Canberra: AIHW; 2010. [Google Scholar]
- 21. Donald M, Dower J, Ware R, Mukandi B, Parekh S, Bain C. Living with diabetes: rationale, study design and baseline characteristics for an Australian prospective cohort study. BMC Public Health, 2012; 12: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. David M, Ware R, Donald M, Alati R. Assessing generalisability through the use of disease registers: findings from a diabetes cohort study. BMJ Open, 2011; 1: e000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diabetes Australia and the RACGP . Diabetes management in general practice: Guidelines for type 2 diabetes 2011/12 In: Harris P, Mann L, Phillips P, Bolger‐Harris H, Webster C. (eds), 17th ed Diabetes Australia; 2011. Available at: http://www.racgp.org.au, accessed October 2013 [Google Scholar]
- 24. Vrijhoef HJM, Berbee R, Wagner EH, Steuten LMG. Quality of integrated chronic care measured by patient survey: identification, selection and application of most appropriate instruments. Health Expectations, 2009; 12: 417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brechner RJ, Cowie CC, Howie LJ, Herman WH, Will JC, Harris MI. Ophthalmic examination among adults with diagnosed diabetes mellitus. JAMA: the Journal of the American Medical Association, 1993; 270: 1714–1718. [PubMed] [Google Scholar]
- 26. Tapp RJ, Zimmet PZ, Harper CA, de Courten MP, Balkau B, McCarty DJ, et al Diabetes care in an Australian population: frequency of screening examinations for eye and foot complications of diabetes. Diabetes Care, 2004; 27: 688–693. [DOI] [PubMed] [Google Scholar]
- 27. Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Narayan KM. A diabetes report card for the United States: quality of care in the 1990s. Annals of internal medicine, 2002; 136: 565–574. [DOI] [PubMed] [Google Scholar]
- 28. Wylie‐Rosett J, Walker EA, Shamoon H, Engel S, Basch C, Zybert P. Assessment of documented foot examinations for patients with diabetes in inner‐city primary care clinics. Archives of Family Medicine, 1995; 4: 46–50. [DOI] [PubMed] [Google Scholar]
- 29. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al Management of hyperglycaemia in type 2 diabetes: a patient‐centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia, 2012; 55: 1577–1596. [DOI] [PubMed] [Google Scholar]
- 30. Kerr EA, Heisler M, Krein SL, Kabeto M, Langa KM, Weir D, et al Beyond comorbidity counts: how do comorbidity type and severity influence diabetes patients' treatment priorities and self‐management? Journal of general internal medicine, 2007; 22: 1635–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Petersen LA, Woodard LD, Henderson LM, Urech TH, Pietz K. Will hypertension performance measures used for pay‐for‐performance programs penalize those who care for medically complex patients? Circulation, 2009; 119: 2978–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rick J, Rowe K, Hann M, Sibbald B, Reeves D, Roland M, et al Psychometric properties of the patient assessment of chronic illness care measure: acceptability, reliability and validity in United Kingdom patients with long‐term conditions. BMC Health Services Research, 2012; 12: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rhim B, Harkless L. Prevention: can we stop problems before they arise? Seminars in Vascular Surgery, 2012; 25: 122–128. [DOI] [PubMed] [Google Scholar]
- 34. Nohr EA, Frydenberg M, Henriksen TB, Olsen J. Does low participation in cohort studies induce bias? Epidemiology, 2006; 17: 413–418. [DOI] [PubMed] [Google Scholar]


