Abstract
Context
Most initiatives for patient involvement in guideline development have been carried out for chronic diseases. The involvement of patients with incidental and non‐threatening diseases is more complicated. Little knowledge is available on how these patient groups can successfully be involved in guideline development.
Objective
To assess the effectiveness of the involvement of gynaecological patients in the guideline development for resumption of (work) activities after surgery.
Design
At three different stages patients were involved in the process: (i) three focus group discussions (FGDs) were organized, (ii) patients were involved for the instruction video, and (iii) patients tested the patient version of the clinical guideline. To assess the effectiveness, an evaluation framework was used. The guideline development process was divided into two parallel trajectories in which patients and professionals were consulted separately. Patients were primarily consulted for the development of the patient version, although their input also influenced the recommendations for resumption of (work) activities after surgery. Professionals were mainly involved in the development of the recommendations of the clinical guideline.
Discussion and conclusions
The involvement of gynaecological patients in the guideline development for resumption of (work) activities after surgery was successful in many respects. Consultation of individual patients by means of FGDs and with regular feedback moments has been rather effective for a guideline development process related to an incidental, non‐threatening disease for which there is no patient organization. Patients' input contributed to applicability of the clinical guideline in daily practice. Increased patient involvement could be achieved by integration of the two parallel trajectories with additional participatory activities, such as a dialogue meeting.
Keywords: clinical guideline, guideline development, gynaecological patients, patient involvement, patients with an incidental and non‐threatening disease
Introduction
Patients are increasingly involved in clinical guideline development. Their involvement is generally motivated by three arguments.1 Firstly, the experiential knowledge of patients – acquired by their daily personal experience with the disease – could complement scientific evidence and thereby increase the rationality of decisions and ultimately the quality of clinical guidelines.2, 3, 4, 5 Secondly, the involvement of patients could enhance the practical implementation of clinical guidelines.6 Thirdly, it can be argued that patients have the moral right to participate in decisions that could affect their lives.7
Most initiatives for patient involvement in clinical guideline development have been carried out for chronic diseases.8 These patient groups are often united in patient organizations, are usually motivated to participate and are therefore relatively easily accessible. In the Netherlands, the inclusion of one or two patient representatives in a guideline workgroup (recruited through the patient organization) is the most common approach to patient involvement.1, 2, 5, 9, 10, 11, 12 This approach is often complemented with participatory activities to explore patients' needs and preferences from a broader perspective, for example focus group discussions (FGDs), a literature search into patient preferences and sometimes dialogue meetings in which patients (representative) and professionals meet.5, 8, 11, 13
The involvement of patients in guideline development with incidental and non‐threatening diseases (e.g. hysterectomy, treatment of pneumonia or concussion) is more complicated.9 These patient groups are most often not united in patient organizations, and patients are only ‘patient’ for a limited period of time. As a consequence, the inclusion of patient representatives in a guideline workgroup cannot easily be realized and is less appropriate because patients lack the broader input from the collective knowledge of the patient organization and the experiences between individual patients differ greatly. Moreover, after recovery, patients most often want to forget their (negative) disease experiences and want to continue with their life. Little knowledge is available on how patients with incidental and non‐threatening diseases can most effectively be involved in clinical guideline development.
In this study, we address the above‐mentioned challenges by assessing the effectiveness of the involvement of patients with an incidental and non‐threatening disease in clinical guideline development by analysing a specific case concerning the involvement of gynaecological patients in an innovative guideline development process for resumption of (work) activities after surgery.
Case description
The development of the clinical guideline was initiated by the department of Obstetrics and Gynaecology and the EMGO Institute for Health and Care Research, both of the VU University Medical Center (Amsterdam, the Netherlands). The guideline development process was part of a larger project in which also a multidisciplinary peri‐operative care programme was developed and an extended pilot study among patients was executed.14 The results of the development of the care programme are published elsewhere.15
A researcher and two project leaders monitored the process and combined the data obtained by consultation of professionals and patients. For substantive and practical tasks, they were supported by an advisory committee. This clinical guideline for resumption of (work) activities after gynaecological surgery distinguishes itself from other clinical guidelines; it can be characterized as a transmural agreement among professionals with consensus‐based recommendations. As a consequence, the development process differs from more traditional clinical guidelines.
The guideline development process consisted of two parallel trajectories, which resulted in two products: (i) a clinical guideline with recommendations for resumption of (work) activities after gynaecological surgery 16 and (ii) a web‐based patient version of the clinical guideline.15 Both trajectories comprised several steps and were interconnected. In Fig. 1, the entire process is visualized. The web‐based patient version of the clinical guideline is an e‐health intervention. The aim of this intervention is to apply the recommendations for resumption of (work) activities into practice. As such, the two trajectories were interconnected. The recommendations of the clinical guideline are integrated into the e‐health intervention, and patients' needs and preferences influenced the topics of the recommendations (Fig. 1). Professionals were mainly involved in the development of the clinical guideline, while patients were primarily consulted for the development of the web‐based patient version of the guideline. The researcher and project leaders integrated the obtained data.
Figure 1.
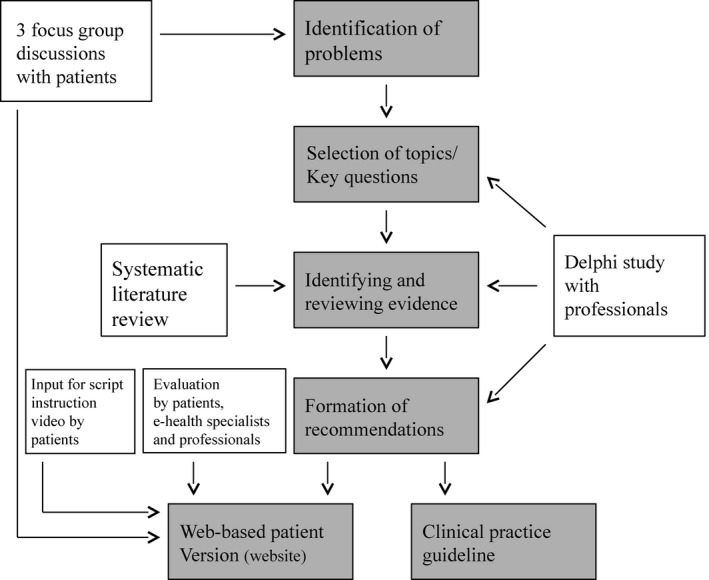
Visualization of the development process of the clinical guideline for resumption of (work) activities after gynaecological surgery.14
The project team acknowledged the asymmetry between the types of knowledge of patients and professionals; the experiential knowledge of patients vs. the expert knowledge of professionals. Therefore, the project team felt that consultation of patients and professionals in two separate trajectories would be most appropriate to thoroughly identify these different types of knowledge. They also felt that the establishment of a guideline workgroup would not be suitable. This is further stressed, because patients were not united in a patient organization and no patient representative could be appointed. The results of the consultation of patients and professionals were processed in parts of the guideline development for which the knowledge was considered most relevant. As a result, the perspective of patients had a more central role in the development of the patient version, although their input also influenced the recommendations for resumption of (work) activities after surgery. Professionals were mainly involved in the development of the recommendations of the clinical guideline.
Methodology
Patient involvement
At three different stages in the guideline development process, patients were involved: (i) three FGDs were organized to identify patients' perceived problems and needs concerning received peri‐operative care and counselling in resumption of (work) activities, (ii) patients were involved in the development of the script for an instruction video, which was part of the web‐based patient version of the clinical guideline, and (iii) patients tested the web‐based patient version of the clinical guideline.
Focus group discussions
Three FGDs with 21 participants (seven participants per FGD) were organized in the period May – June 2009. For the involvement of these patients, both the researcher and the project leaders of the guideline development process were aware of the large differences among patients. Purposeful sampling was used to capture the broadest set of information, and to aim at maximum variation. Participants were recruited from the patient files of the VU University Medical Center. Broad inclusion criteria were used as follows: (i) age between 18 and 65 years, (ii) a history of a gynaecological surgery (i.e. hysterectomy or laparoscopic adnexal surgery) and (iii) the presence of a paid or unpaid job of at least 8 h a week.15 Participants were sampled for delayed, intermediate and rapid resumption of (work) activities, to create homogeneity within the FGDs but heterogeneity between the groups.
To meet the purposes of FGDs and the specific aims of the consultation, a tailor‐made design of the meeting is required.17, 18 The aims of the FGDs were to identify (i) patients' problems, needs and preferences regarding peri‐operative care and counselling in resumption of (work) activities, and (ii) patients' ideas for the development of the web‐based patient version to empower patients during the peri‐operative period and resumption of (work) activities (the patient version of the clinical guideline). To achieve the aims of the FGDs, specific tools were used to steer the discussion. Participants were actively involved through a structured step‐by‐step process with several individual and joint assignments. The facilitator ensured all participants were included in the discussion using post‐its and go‐rounds. The issues discussed were visualized on flip charts. The focus group design comprised four different steps:
Step 1. Problems and needs in received peri‐operative care and counselling in resumption of (work) activities were identified. These problems were divided in two categories – ‘before surgery’ and ‘after surgery’ – and were subsequently prioritized by appointing a top five.
Step 2. Possible solutions and improvements, which could overcome the mentioned problems, were discussed.
Step 3. Patients brainstormed about favourable designs and content of the web‐based patient version for empowering patients during the peri‐operative period and resumption of (work) activities.
Step 4. At the end of each FGD, patients filled out a questionnaire containing factual questions regarding their gynaecological procedure, their personal recovery period and their (work) activities.
Focus group discussions were planned until no new perspectives emerged (data saturation), which was after three FGDs. All discussions were audio‐taped and verbatim transcribed by the note taker of the FGDs and checked by the researcher who observed the FGDs. A summary was sent to participants for member check (a process in which the participants are invited to react and reflect on the researcher's interpretations of the FGD). The verbatim transcripts were analysed using an inductive approach, by means of Atlas.ti. software,19 comprising three steps: (i) open coding (identifying, categorizing and describing of concepts), (ii) axial coding (creating subthemes by relating codes to each other) and (iii) selective coding (developing storyline by relating subthemes to main themes). The analysis of the FGDs formed input for the development of the e‐health intervention (content and design) and the Delphi Study among professionals (selection of topics for the recommendations of the clinical guideline).
Results of the FGDs
In Box 1, the results of the FGDs are presented. Furthermore, the FGD results showed that patients would appreciate the development of an instruction video for patients and employers to stimulate communication and to illustrate common pitfalls during reintegration. It was therefore decided to develop such an instruction video and integrate it into the web‐based patient version of the clinical guideline.
Box 1. Outcomes of the focus group discussions1 .
The main experienced problems and needs during the peri‐operative care and resumption of (work) activities were related to insufficient information supply. Participants considered realistic information supply tailored to individual characteristics and conditions most essential for good recovery and resumption of (work) activities. Additionally, they experienced problems regarding the communication between professionals of different disciplines. The identified problems and corresponding solutions were divided into three main categories: (i) information supply before surgery, (ii) information supply after surgery and (iii) communication between professionals of different disciplines. Regarding the website participants recommended functionalities which could provide detailed and personalized instructions for resumption of daily and work activities. Below, the results of the FGDs are described in more detail per category.
Before gynaecological surgery
A main topic of discussion was the information supply regarding surgical procedures (e.g. logistic procedures in the hospital from admission to discharge, anaesthesia, specific technical aspects of the surgery). Participants pointed out that they had received no or insufficient information about these procedures before surgery. As a consequence, some felt anxious during their time in hospital, while others had unrealistic expectations about the impact of the surgical intervention. Several participants specifically pointed out they received insufficient or even contradictory information concerning anaesthesia. For example, in some patients, the impact of the general anaesthesia was bigger than they expected, and they were not prepared for that.
Participants also indicated that transparency in planning could be improved. Furthermore, the results revealed difficulties in estimating realistic recovery periods. Different disciplines provided contradictory information or professionals disagreed about convalescence recommendations. Also, the lack of information about the psychological consequences of a gynaecological surgery was mentioned.
After gynaecological surgery
Central in the discussions were the experienced problems regarding the provided recommendations about resumption of (work) activities. On the one hand, participants emphasized the importance of uniform recommendations by different professionals because they experienced inconsistency in convalescence advice. On the other hand, they mentioned the significance of tailored instructions, because of different types of surgery and specific individual characteristics and (work) conditions. Furthermore, participants felt they were not well‐informed about the discharge policy. Participants emphasized that more information regarding the occurrence of possible complications was desired. Participants who suffered from complications could not remember receiving any information about potential risks. They stressed that in case of complications, medical doctors should clarify directly the situation, the potential risks and the further procedure.
Communication between professionals of different disciplines
Participants indicated they experienced several difficulties with the communication between professionals of different disciplines (e.g. the gynaecologist, the anaesthesiologist, the occupational physician, the general practitioner) and inadequate handover of patients to other professionals. Professionals were not fully informed about the entire procedure, and participants experienced inconsistency in convalescence recommendations.
Recommendations for web‐based patient version
Participants were enthusiastic about the development of a patient website to empower patients during the peri‐operative period and resumption of (work) activities. The web‐based patient version of the clinical guideline should provide in the need for more information about earlier mentioned problems and for written instructions. The FGD results revealed the following desired content and specific functionalities for a website:
‐ (technical) Information about gynaecological procedures and recovery (including admission, anaesthetics and complications).
‐ Reliable detailed and personalized recommendations for resumption of (work) activities. It was emphasized that these instructions should be practical and related to daily activities.
‐ Functionalities to develop a personalized reintegration plan.
‐ Tools to communicate with other patients, employers and medical doctors (e.g. a forum).
‐ Instruction video for patients and employers to stimulate communication and to illustrate common pitfalls during reintegration.
‐ Frequently Asked Questions (FAQ).
The FGD results are also published elsewhere
For the development of a script for the instruction video, the FGD results were converted into common pitfalls for patients and employers during the reintegration period. In addition, the scenario‐writer worked closely with three patients who participated in the FGDs to make the video more geared to the perception of patients. The main reason to involve patients in this part of the clinical guideline was to provide the scenario‐writer more background information about gynaecological surgery and resumption of (work) activities. These patients voluntarily enlisted to be involved (convenience sample). They had different experiences with a gynaecological procedure, but did not represent the entire patient group.
During the meeting, the three patients and the scenario‐writer talked about a most desirable script for the instruction video.
Testing of the web‐based patient version
The patient version of the clinical practice guideline was developed with the aim to apply the recommendations for resumption of (work) activities into practice. An e‐health intervention was chosen as an appropriate tool because it has the ability to provide tailor‐made information relatively easy in several forms to patients and to enhance interaction between patients and health‐care professionals.20, 21, 22, 23, 24 In this guideline development process, testing of the patient version was included to assess the usability of the e‐health intervention and the recommendations in practice. Twenty participants of the FGDs agreed to test the web‐based patient version. Of them, 15 participants completed a questionnaire regarding feasibility, content and design. Moreover, it was asked if the topics discussed in the FGDs are well reflected in the web‐based patient version. The results were used to optimize design and content of the e‐health intervention.
Monitoring and evaluation of patient involvement
To assess the effectiveness of the involvement of gynaecological patients in the clinical guideline for resumption of (work) activities, an evaluation framework was developed based on a literature review and comprising pre‐defined evaluation criteria detailing the participation process and generated outcomes.25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 These main criteria were divided in several subcriteria. Process criteria were subdivided in involvement of stakeholders, process structure and process management. For outcome criteria, direct and indirect outcomes were distinguished. The criteria are described in more detail in Box 2. The involvement of gynaecological patients is considered effective when it meets the evaluation criteria of the framework. Data concerning this evaluation were gathered and validated by means of a triangulated approach, involving (direct) observations, document analysis, semi‐structured interviews, informal conversations with patients and evaluation forms after the FGDs.
Box 2. Evaluation framework.
Process criteria
Involvement of stakeholders
To successfully involve patients in guideline development processes, attention should be paid to the balance between involved patients (or patients' representatives) and professionals. In addition, diversity among a patient population (e.g. demographics, ethnicity, and severity and duration of the disorder) should be acknowledged and effort should be made to take diversity into account. Also attention should go to representativeness.25, 35
Process structure
A guideline development process needs to be structured clearly and transparently.36 It is important that patients are informed about what is expected from them, what the aim of the overall project is, in which activities they participate, and what influence they have on the process and the clinical guideline.9, 28, 35, 36 Additionally, to ensure rationality of the guideline development process, involvement of patients from start to completion should be affirmed, as well as direct interaction between patients and professionals.9, 29, 37 Patients should be involved in significant aspects of decision making, so to ensure actual use of patients' input.9, 25, 38
Process management
Independent facilitation of patient involvement activities is crucial for equal treatment of patients (as compared to professionals) and should create an open and respectful atmosphere to enable patients to share their viewpoints.25, 27, 29, 36, 39 Good process management also includes the offer for support and the adjustment of the guideline development process to the abilities of patients. Moreover, there should be support among the involved professionals and the project team of the guideline development towards patient involvement.9
Outcome criteria
Direct outcomes
Consensus on the content of a clinical guideline is an important indicator for success. To reach consensus, the outcomes – in this case the web‐based patient version of the clinical guideline ‐ should reflect the input and perspectives of involved patients.29, 40 Also important is the degree to which guideline developers responded to patients' input. What aspects are incorporated in the guideline, and why? Moreover, patients must be satisfied with the end‐result and they have to recognize the clinical guideline as relevant.25, 27, 29 Additionally, dissemination of the clinical guideline is considered important.
Indirect outcomes
Indirect outcomes are related to the stimulation of learning processes and the achievement of mutual learning, resulting in changes of thinking of both patients and professionals.27, 28 Mutual learning implies learning in a substantive way (concerning content‐related matters), in a procedural way (concerning participatory approaches) and in a reflexive way (concerning their own and each others' knowledge, perspectives or roles).31, 41
Observations were used to gain more insight in participatory aspects of the guideline development process. Direct observations were used for the three FGDs with gynaecological patients. The meetings with professionals as part of the Delphi study were indirectly observed by listening to the audio records (a detailed description of the Delphi study is published in a separate paper16). Research logs were kept to document the observations. Minutes of meetings (including the meetings for the Delphi study and of the advisory committee), focus group reports (including the results of the questionnaire), the results of the testing of the web‐based patient version (questionnaires), the clinical guideline and the web‐based patient version were analysed to examine the influence of patients' input on the products. Two in‐depth evaluative interviews were held: one interview with the researcher of the guideline development process concerning experiences with and expectations about the involvement of gynaecological patients in the process, and one interview with the scenario‐writer of the instruction video to gain insight in his experiences with the involvement of patients in the development of the script. Throughout the guideline development process, various informal conversations took place with the researcher, the project leaders and patients. The aim of these conversations was to discuss and reflect on the participatory activities and results. These conversations were documented in the research logs by two researchers who were responsible for the evaluation of patient involvement.1 At the end of each FGD, patients filled out an evaluation form. The evaluation form gave participants the opportunity to give feedback on how they experienced the FGD and to give possible recommendations for improvement of future FGDs.
Results
In this section, the findings of our study concerning patient involvement are presented. First, we describe the processes and the outcomes of the participatory activities in this guideline development process for both trajectories. Second, we reflect on the effectiveness of patient involvement along the lines of the evaluation framework.
Description of processes and outcomes
Processing of patients' input
In this section, we will describe how the FGD results were integrated in the clinical guideline and in the web‐based patient version.
Clinical guideline
The Delphi study among professionals was the main method to arrive at the recommendations for the clinical guideline.16 Although patient involvement was primarily appointed to the trajectory for the development of the web‐based patient version, the FGD results were processed into the clinical guideline to some extent. The researcher and the project leaders brought up some topics derived from the FGDs that in their opinion complemented the topics suggested by professionals that is, the improvement of information supply for recovery and resumption of (work) activities (Box 1). These topics were not directly of use as input for the Delphi study, which required advice for when work activities are thought to be medically safe to resume. Therefore, the topics brought in by the researcher and project leaders were extracted from the arguments patients gave to support their point of views. Ultimately, the topics selected by professionals included several issues brought up by patients. These topics concerned mostly advices regarding resumption of daily activities (e.g. taking a bath, jumping). The topics of professionals mainly concerned movements like lifting, walking and bowing.
Patient version of the clinical guideline
The ideas brought forward in the FGDs regarding design, topics and functionalities were leading in the development of the web‐based patient version.2 Box 3 shows which functionalities were included in the e‐health intervention and reveals that the input of patients in the FGDs (Box 1) substantially contributed to the development of the e‐health intervention. Extra attention was paid to providing detailed and tailored instructions for resumption of both work and daily activities. A tool was included to compose tailored instructions for resumption of normal and daily activities including a personalized work integration plan. Moreover, interactive tools (e.g. a forum, FAQ) were included to exchange experiences between patients and to provide information regarding (frequently asked) medical questions and common complications. The content of these tools was derived from the recommendations of the clinical guideline (the outcomes of the Delphi study), literature, patient leaflets and clinical experience.
Box 3. Design of the web‐based patient version.
The web‐based patient version aims at empowering patients during the peri‐operative period and resumption of (work) activities by increasing the applicability of the clinical guideline in daily practice. The website was designed with the following functionalities:
‐ Tool to compose a tailored work reintegration plan. Based on personalized characteristics and conditions, the tool provides the patient with a tailored advice for when work activities are thought to be medically safe to resume. Recommendations are based on the outcomes of the Delphi study.16
‐ Safe resumption of normal activities. This tool comprised a tailored plan for the gradual resumption of daily activities. Recommendations are derived from the Delphi study.42
‐ A tool to signal complications with advice about what to do and who to contact.
‐ (self) Monitoring of recovery and offering assistance when relevant
‐ Instruction video for patients and employers to stimulate communication and to illustrate common pitfalls during reintegration.
‐ Recommendations for communication between patients and employers
‐ Detailed instructions and illustrations on various gynaecological surgical procedures
‐ Frequently asked questions.
‐ Glossary. Explanation of frequently used medical terms.
‐ Links to websites regarding gynaecological surgery and recovery.
‐ A forum where patients can exchange experiences.
Instruction video
Part of the web‐based patient version is the instruction video for patients and employers to stimulate communication and to illustrate common pitfalls during reintegration. In the instruction, video potential reintegration problems are discussed by showing two cases of a good and bad interaction between patients, employers and occupational physicians. The scenario‐writer appreciated the ideas brought forward by the three involved patients. He asked them about their experiences with the reintegration period and emphasized the contact with the employer and occupational physicians during the peri‐operative period. Based on the experiences of the patients, it became clear to him that there is not one common ground; patients have different experiences and needs. He responded to this by showing two cases in the video. In this way, he tried to gear the instruction video towards patients' perception. The involved patients enjoyed working on the development of the instruction video and felt their input was taken serious. They were happy with the end‐result; their input was well integrated in the video.
Testing of the web‐based patient version
In total, 15 participants of the FGDs were involved in the evaluation of the web‐based patient version. The testing revealed how participants of the FGDs perceive feasibility, design and content of the website. The results also showed to what extent participants are satisfied with the processing of the FGD results. As the content of the website is also based on the recommendations of the clinical guideline, the testing results also gave insight in the applicability of the recommendations in practice as well.
Participants experienced a strong connection between the FGD results and the web‐based patient version. Except for one participant, participants indicated they would recommend the website to other patients. They indicated that almost all topics introduced in the FGDs were integrated. Especially in the FAQ and the recommendations for resumption of (work) activities, the FGD results have been picked up well. Also the forum was recognized. Moreover, they mentioned that the website could improve the communication between employers and patients. Participants indicated that the content of the instruction video was clearly related to the discussions in the FGDs.
Some recommendations for the improvement of the web‐based patient version were mentioned. Two participants emphasized that still more specific and tailored recommendations regarding resumption of daily activities would be desired to the currently provided information. Moreover, it was indicated that it would be preferred if more information about common complications would be offered. One participant suggested including positive experiences with resumption of work activities. In her opinion, the current patient version was mainly focused on pain. On the other hand, another participant considered the patient version to be too cheerful. She preferred more attention for problems regarding resumption of activities. Furthermore, a participant recommended providing more attention to possible causes for delayed recovery.
Process and outcome analysis
To assess the successfulness of the involvement of gynaecological patients in this guideline development process, patient involvement was evaluated along the lines of the evaluative framework (Box 1).
Process criteria
Involvement of stakeholders
Next to professionals (representatives of the medical boards of gynaecologists, general practitioners and occupational physicians), individual patients and a patient representative of an umbrella patient organization (The Federation of Patients and Consumer Organisations) were invited to participate in the guideline development process. The patient representative was not acquainted with the specific experiences of gynaecological patients. However, she was familiar with perspectives of surgical patients with resumption of (work) activities in general. The balance between patients and professionals was of less importance, because patients and professionals were consulted in parallel trajectories. In this way, they could not influence each other directly.
After each FGD participants filled out a questionnaire to identify the contextual situation of the participants regarding their gynaecological history and their resumption of work activities. The results of these questionnaires reveal diversity regarding differences in type of gynaecological surgery, course of resumption of work activities, load of work activities, age and educational level among participants. Six of 21 participants indicated a high load of activities on their work. The age of participants ranged from 20 to 40 years with an average of 29, and the educational level differed from low educated participants to high educated participants (equally divided). However, all participants were recruited through the VU University Medical Center, where most often patients with more complicated cases are treated. Nevertheless, there was variety in representation among involved participants observed; patients with minor and severe complications were involved.
Process structure
The focus group technique appeared to be successful for involving individual patients that are not united in a patient organization. The interactive character of FGDs stimulated co‐construction of meaning and understanding, and as a result provided broad and in‐depth information regarding the peri‐operative period and resumption of (work) activities. In addition, the FGD results provided recommendations for the development of the web‐based patient version and the instruction video. With the development of the instruction video (for which patients provided input), the researcher and the two project leaders specifically anticipated on the needs of patients. The testing of the web‐based patient version gave participants the opportunity to reflect on the processing of their input. Participants indicated that they very much appreciated this testing. They felt taken seriously and the reflection contributed to increased support for the intervention among patients. However, participants were only able to reflect by means of a questionnaire, which provides little room for own input or argumentation.
The researcher and the project leaders had a central position in the guideline development process. They were responsible for supervision and decision making. Patients were involved on the level of consultation and had no role in integrative activities and in translation of the FGD results. The researcher and the project leaders were responsible for integrating patients' input. There was no interaction between involved parties. However, patients had the opportunity to verify the processing of their input as well as the input of professionals by the evaluation of the patient version of the clinical guideline. As a result, not only knowledge transfer was realized, but also knowledge exchange.
During a participatory process, transparency towards all participants is important. By the involvement of FGD patients in the development of the instruction video and the testing of the web‐based patient version, transparency was created, because participants could verify the processing of their input. However, transparency regarding goals of the project was not for all participants clear. Although the goals of the FGDs and their contribution to the development of an e‐health intervention (the web‐based patient version of the guideline) were clear, participants were only partly aware of the overall process (including the clinical guideline). The overall process was addressed briefly, in contrast to their contribution to the web‐based patient version. As the evaluation forms participants filled out after the FGDs revealed, participants' expectations towards their participation in the FGDs were quite general, for example ‘sharing of experiences’ and ‘Contribute to improvement of care by the development of a website’. In addition, there was little attention for discussion of expectations of patients and professionals concerning their contribution to activities and end‐products.
Patients and professionals were not equally involved. Patients were involved on the level of consultation, while professionals were involved on a higher level. By their participation in the Delphi study, professionals had decision‐making power about the recommendations for resumption of (work) activities.
Process management
The researcher and the project leaders of the guideline development process were responsible for process management. As a consequence, the independent role of the managers was at stake. However, they took the input of patients seriously and adjusted the participatory activities to the needs of patients. Participation in the FGDs or the testing did not require specific skills of the patients. Patients could participate without preparation, their involvement required little efforts, and only their experiential knowledge was addressed.
During the FGD meetings, it was observed that the participants felt at ease, and the discussions were not affected by one or more dominant participants. Observations revealed that due to the FGD design, participants were able to formulate recommendations for resumptions of (work) activities by addressing their own experiences. The topic of discussion – information supply regarding peri‐operative care and recovery – was a topic participants could easily relate to and was important for them. They shared their experiences easily and reacted positively to each other's input, even if these did not correspond to their own experiences or visions.
The evaluation forms participants filled out after the FGDs revealed that participants were very positive about the design, focus and facilitation of the FGDs. They indicated: ‘It is a good thing that attention is given to this’, ‘I am convinced that a website with the possibility to contact fellow‐patients is very desirable’ and ‘I hope these meetings result in even better care’. Participants indicated they were pleased that they could share their experiences and that their input could be of use for improvement of information supply. Furthermore, they felt taken seriously. Also, participants indicated that all relevant topics were discussed.
The researcher and the project leaders were positive towards patient involvement in this guideline development process. They appreciated the experiential knowledge of patients and were confident about the added value of this knowledge to the development of the web‐based patient version. They believed specific medical and professional knowledge were required for the formulation and interpretation of the recommendations. As a consequence, the involvement of patients was mainly restricted to the web‐based patient version. The contribution of the representative of the umbrella organization was appreciated by the other members of the advisory committee. They considered her as equal and professional.
Outcome criteria
Direct outcomes
For the web‐based patient version, the contribution of patients to the intervention was substantial. Almost all topics introduced in the FGDs were integrated. The central position of the web‐based patient version in this guideline development process contributed to the applicability of the clinical guideline in daily practice. Patients' input for the clinical guideline contributed to the formulation of some additional topics for the recommendations, mainly being reflected in the tailoring of the topics for recommendations to more complex daily activities. This was considered a valuable input by all parties.
Indirect outcomes
The absence of direct interaction between patients and professionals prevented optimal mutual learning, because the two groups did not meet at any point in time during the process. As a consequence, mutual learning occurred only indirectly. The guideline development process resulted to some extent in changes in thinking of involved parties (i.e. patients, professionals, the researcher and the two project leaders). Mutual learning occurred regarding medical content – professionals, the researcher and the two project leaders got acquainted with problems and needs of patients, and patients learned about medical‐related procedures by evaluating the web‐based patient version – and in a procedural way – learning occurred concerning patient involvement procedures and methods.
It was also observed that the diversity among participants stimulated co‐creation of solutions. At several moments, it was observed that interaction between participants in the FGDs resulted in a broad variety of topics and in‐depth reflection. Moreover, during the FGDs, there was specific attention for interaction by the use of statements. These statements stimulated participants to react and to start a discussion. Furthermore, the statements appeared useful to stimulate the articulation of solutions.
Discussion and conclusions
Our findings reveal that consultation of individual patients by means of FGDs and with regular feedback moments has been quite successful for a guideline development process related to an incidental, non‐threatening disease for which there is no patient organization. There was diversity among participants of the FGDs, and there was saturation of the results. In addition, the involvement of FGD patients in the development of the instruction video and the testing of the web‐based patient version afterwards was valuable, because it gave patients the opportunity to verify the processing of their input and assured continuity of patient involvement. As a result, not only knowledge transfer, but also knowledge exchange was realized between professionals and patients. Moreover, the web‐based patient version was considered very valuable by patients, and as consequence, the external validity can be regarded as high.
Patients were well able to participate in this clinical guideline development process, because their involvement did not require specific skills; only their experiential knowledge was addressed. This is in favour of the discussion where scholars argue whether training and support of patients in order to be able to participate on an equal level is desirable.8 Furthermore, literature on implementation of clinical guidelines reports poor implementation with almost non‐existing implementation among patients.1, 43, 44, 45 The development of a web‐based patient version may contribute to enhanced applicability of the clinical guideline in daily practice and to dissemination among patients. In a follow‐up study, a randomized controlled trial (RCT) started in the autumn of 2011 to further evaluate the effects and effectiveness of the recommendations of the clinical guideline and the web‐based patient version.14 Part of the RCT will be a process evaluation to assess (i) the extent to which the web‐based patient version and convalescence recommendations are used and followed up (compliance) and (ii) the appreciation of the different tools of the e‐health intervention. The results of the process evaluation will be used to optimize the web‐based patient version and will contribute to enhanced implementation of the clinical guideline.
The guideline development process was divided into two parallel trajectories in which patients and professionals were consulted separately. Patients were primarily consulted for the development of the web‐based patient version of the clinical guideline, while professionals were mainly involved for the development of the recommendations of the clinical guideline. This division was an explicit choice of the project team, who appreciated the experiential knowledge of patients and valued their input for the web‐based patient version. However, they believed expert knowledge was required for the formulation and interpretation of the recommendations. With this division, the project team followed a more Governance Discourse, as described by Boivin et al.42, which has an emphasis on the synthesis of scientific evidence to clinical decision making, predominantly informed by evidence‐based medicine. Although the followed approach turned out to be quite successful, one could question to what extent a more interactive process would have had an added value on the quality of the recommendations. When another discourse was followed in which shared decision‐making and patient‐centred care have a more central place,42, 46 patients would have been more involved in the development of the recommendations. To ensure the motivated involvement of an unorganised patient population, like gynaecological patients, the involvement of a skilled facilitator is required. The developed web‐based version is able to monitor the actual achieved resumption of normal and work‐related activities, enabling future adjustments of these recommendations. In addition, the experiences of patients and care‐providers will be registered in a randomised study evaluating the effect of this web‐based version on return to work (RTW), quality of life (QOL) and pain. Patients reported experiences will be part of the final clinical guideline.
The FGD results were taken along in the formulation (and selection) of the topics of the recommendations of the professionals. The influence the FGD results had on these topics reveal that patients' input complemented the input of professionals and increased the applicability of the recommendations in daily practice. One might argue that a higher degree of involvement in this trajectory could have resulted in recommendations even more aligned to the daily practice. In participatory approaches for agenda setting in chronic disease domains together with active patient organizations, interaction between patients and professionals proved to stimulate mutual learning. Consultations complemented by collaboration breed partnerships and could, as a result, contribute to increased quality and relevance of health research.47 Applied for clinical guideline development processes, these insights could contribute to better tuning of clinical guidelines to daily practice and a higher appreciation of each others' input. Particularly because this clinical guideline concerns a transmural agreement with consensus‐based recommendations, patient involvement might more easily be achieved.1 The development of such a clinical guideline is less dependent on scientific evidence, and therefore, there tends to be more room (and need) for experience‐based knowledge from both professionals and patients. Increased patient involvement could be achieved by integration of the two parallel trajectories with additional dialogue meetings with patients and professionals. However, follow‐up research is required to assess the added value of these additional dialogue meetings, in which aspects like (practical and financial) feasibility and diversity among individual patient should be taken into account.
Limitations of the study
Patient involvement in guideline development processes is highly contextualized, and our results are therefore difficult to generalize. Characteristics of this specific guideline development process and of the Dutch context might have shaped our findings. First, this guideline development process concerns an innovative process, in which individual patients and professionals were consulted separately in two different trajectories. Secondly, there was a specific emphasis on the development of a web‐based patient version. As a consequence, this clinical guideline development process, including the patient involvement, differs from the more traditional development processes in the Netherlands and abroad, in which the inclusion of one or two patient representatives in a guideline workgroup is the most common approach.1, 2, 5, 8, 9, 10, 11, 12 Although these factors might influence the dissemination of our findings to a broader context, we believe our findings may contribute to valuable directions for future guideline development processes in which patient groups are not united, for example for incidental and non‐threatening diseases.
Conclusion
In conclusion, the involvement of gynaecological patient in an innovative guideline development for resumption of (work) activities after surgery can be regarded as quite successful; the consultation of individual patients by FGDs, as well as the testing of the web‐based patient version resulted in meaningful input. Furthermore, patients' input contributed to applicability of the clinical guideline in daily practice and to implementation among patients. Although the choices for two parallel trajectories are legitimate and resulted in end‐products aligned to patients' daily practice, we suggest that more patient involvement in the development of the recommendations of the clinical guideline may result in increased relevance and quality of the recommendations.
Conflicts of interest
The authors declare that they have no competing interests.
Funding
The conducted research is funded by the Netherlands Organisation for Health research and Development (ZonMw).
Acknowledgements
We would especially like to thank prof. Maurits van Tulder and Lia van der Ham for their involvement in the project and critical reflection on our work, and Monique Brood for the facilitation of the FGDs (voluntary as an independent doctor). Also, we would like to thank the Netherlands Organisation for Health research and Development (ZonMw) for providing funding. Most of all we would like to thank all patients, who participated in this guideline development process and shared their experiences with us.
Notes
The project team comprised two groups. One group was involved with the execution of the guideline development process and the development of the web‐based patient version. The other group was responsible for the evaluation of patient involvement.
The Internet address of the web‐based patient version is www.ikherstel.nl, which means ‘I am recovering’.
References
- 1. Boivin A, Currie K, Fervers B et al Patient and public involvement in clinical guidelines: international experiences and future perspectives. Quality & Safety in Health Care, 2010; 19: e22. [DOI] [PubMed] [Google Scholar]
- 2. Kelson M. Patient involvement in clinical guideline development – where are we now? The Journal of Clinical Governance, 2001; 9: 169–174. [Google Scholar]
- 3. Owens DK. Patient preferences and the development of practice guidelines. Spine, 1998; 23: 1073–1079. [DOI] [PubMed] [Google Scholar]
- 4. Rankin N, Newell S, Sanson‐Fisher R, Girgis A. Consumer participation in the development of psychosocial clinical practice guidelines: opinions of women with breast cancer. European Journal of Cancer Care, 2000; 9: 97–104. [DOI] [PubMed] [Google Scholar]
- 5. van Wersch A, Eccles M. Involvement of consumers in the development of evidence based clinical guidelines: practical experiences from the North of England evidence based guideline development programme. Quality in Health Care, 2001; 10: 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boivin A, Legare F. Public involvement in guideline development. Canadian Medical Association Journal, 2007; 176: 1308–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rogers WA. Are guidelines ethical? Some considerations for general practice. British Journal of General Practice, 2002; 52: 663–668. [PMC free article] [PubMed] [Google Scholar]
- 8. Bovenkamp H, Trappenburg M. Reconsidering patient participation in guideline development. Health Care Analysis, 2009; 17: 198–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Broerse JEW, van der Ham L, van Veen S, Pittens C, van Tulder M. Inventarisatie van patientenparticipatie bij richtlijnontwikkeling. Amsterdam: VU University Amsterdam, 2010. [Google Scholar]
- 10. Kwaliteitsinstituut voor de Gezondheidszorg CBO en NPCF. Richtlijnontwikkeling ‐ Praktische handleiding voor patientvertegenwoordigers. Utrecht: Kwaliteitsinstituut voor de Gezondheidszorg CBO en NPCF, 2008. [Google Scholar]
- 11. National Institute for Health and Clinical Excellence . The Guidelines Manual, 2009. London: National Institute for Health and Clinical Excellence; Available at: http://www.nice.org.uk/media/5f2/44/the_guidelines_manual_2009_-_all_chapters.pdf, accessed 14 August 2013. [Google Scholar]
- 12. Vereniging Integrale Kankercentra. Draaiboek richtlijnen. Ontwikkelen, implementeren en evalueren van richtlijnen. Utrecht: Vereniging Integrale Kankercentra, 2010. [Google Scholar]
- 13. Krahn M, Naglie G. The next step in guideline development – incorporating patient preferences. Jama‐Journal of the American Medical Association, 2008; 300: 436–438. [DOI] [PubMed] [Google Scholar]
- 14. Vonk Noordegraaf A, Huirne JAF, Brolmann HAM et al Effectiveness of a multidisciplinary care program on recovery and return to work of patients after gynaecological surgery; design of a randomized controlled trial. BMC Health Services Research, 2012; 12: 29–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vonk Noordegraaf AV, Huirne JAF, Pittens CACM, et al eHealth program to empower patients in returning to normal activities work after gynecological surgery: intervention mapping as a useful method for development. Journal of Medical Internet Research, 2012; 14: e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vonk Noordegraaf A, Huirne JAF, Brolmann HAM, van Mechelen W, Anema JR. Multidisciplinary convalescence recommendations after gynaecological surgery: a modified Delphi method among experts. BJOG: An International Journal of Obstetrics and Gynaecology, 2011; 118: 1557–1567. [DOI] [PubMed] [Google Scholar]
- 17. Kupper F, Krijgsman L, Bout H, de Cock Buning T. The value lab: exploring moral frameworks in the deliberation of values in the animal biotechnology debate. Science and Public Policy, 2007; 34: 657–670. [Google Scholar]
- 18. Roelofsen A, Broerse J, Buning TdC, Bunders J. Engaging with future technologies: how potential future users frame ecogenomics. Science and Public Policy, 2010; 37: 167–179. [Google Scholar]
- 19. Scientific Software Development ATLAS.ti Version 6.2, Berlin: Scientific Software Development, 2010. [Google Scholar]
- 20. Powell JA, Darvell M, Gray JAM. The doctor, the patient and the world‐wide web: how the internet is changing healthcare. Journal of the Royal Society of Medicine, 2003; 96: 74–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cross M. How the internet is changing health care. British Medical Journal, 2008; 337: a883. [DOI] [PubMed] [Google Scholar]
- 22. Randeree E. Exploring technology impacts of Healthcare 2.0 initiatives. Telemedicine Journal and E‐Health, 2009; 15: 255–260. [DOI] [PubMed] [Google Scholar]
- 23. Griffiths F, Lindenmeyer A, Powell J, Lowe P, Thorogood M. Why are health care interventions delivered over the internet? A systematic review of the published literature. Journal of Medical Internet Research, 2006; 8: e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Forkner‐Dunn J. Internet‐based patient self‐care: the next generation of health care delivery. Journal of Medical Internet Research, 2003; 5: e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abelson J, Forest PG, Eyles J, Smith P, Martin E, Gauvin FP. Deliberations about deliberative methods: issues in the design and evaluation of public participation processes. Social Science & Medicine, 2003; 57: 239–251. [DOI] [PubMed] [Google Scholar]
- 26. Abelson J, Gauvin FP, Assessing the Impacts of Public Participation: Concepts, Evidence and Policy Implications. Ottawa: Canadian Policy Research Networks Inc, 2006. [Google Scholar]
- 27. Broerse JEW, de Cock Buning T, Roelofsen A, Bunders JFG. Evaluating interactive policy making on biotechnology: the case of the Dutch Ministry of Health, Welfare and Sport. Bulletin of Science, Technology & Society, 2009; 29: 447–463. [Google Scholar]
- 28. Caron‐Flinterman JF, Broerse JEW, Teerling J et al Stakeholder participation in health research agenda setting: the case of asthma and COPD research in the Netherlands. Science and Public Policy, 2006; 33: 291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Driessen PPJ, Glasbergen P, Verdaas C. Interactive policy‐making – a model of management for public works. European Journal of Operational Research, 2001; 128: 322–337. [Google Scholar]
- 30. Guston DH. Critical appraisal in science and technology policy analysis: the example of Science, the endless frontier. Policy Sciences, 1997; 30: 233–255. [Google Scholar]
- 31. Guston DH. Evaluating the first US consensus conference: the impact of the citizens' panel on telecommunications and the future of democracy. Science Technology & Human Values, 1999; 24: 451–482. [Google Scholar]
- 32. Laird FN. Participatory analysis, democracy, and technological decision‐making. Science Technology & Human Values, 1993; 18: 341–361. [Google Scholar]
- 33. Rowe G, Frewer LJ. Evaluating public‐participation exercises: a research agenda. Science Technology & Human Values, 2004; 29: 512–557. [Google Scholar]
- 34. Webler T. ‘Right’ discourse in citizen participation: an evaluation yardstick In: Renn O, Webler T, Wiedemann P. (eds) Fairness and Competence in Citizen Participation: Evaluating Models for Environmental Discourse. Dordrecht: Kluwer Academic Publishers, 1995: 35–86. [Google Scholar]
- 35. Webler T, Tuler S. Fairness and competence in citizen participation – theoretical reflections from a case study. Administration & Society, 2000; 32: 566–595. [Google Scholar]
- 36. Rowe G, Frewer LJ. Public participation methods: a framework for evaluation. Science, Technology & Human Values, 2000; 25: 3–29. [Google Scholar]
- 37. Weldon S. Public Engagement in Genetics: A Review of Current Practice in the UK. Lancaster: Lancaster University, 2004. [Google Scholar]
- 38. Arnstein SR. Ladder of citizen participation. Journal of the American Institute of Planners, 1969; 35: 216–224. [Google Scholar]
- 39. Hagendijk R, Irwin A. Public deliberation and governance: engaging with science and technology in contemporary Europe. Minerva, 2006; 44: 167–184. [Google Scholar]
- 40. Hagendijk R, Healey P, Horst M, Irwin A. Science, technology and governance in Europe: challenges of public engagement STAGE (HPSE‐CT2001‐50003) final report, 2005. Available at: http://www.stage-research.net/STAGE/index.html, accessed 14 August 2013. [Google Scholar]
- 41. Irvin RA, Stansbury J. Citizen participation in decision making: is it worth the effort? Public Administration Review, 2004; 64: 55–65. [Google Scholar]
- 42. Boivin A, Green J, van der Meulen J, Legare F, Nolte E. Why consider patients' preferences? A discourse analysis of clinical practice guideline developers. Medical Care, 2009; 47: 908–915. [DOI] [PubMed] [Google Scholar]
- 43. Beaulieu MD, Hudon E, Roberge D, Pineault R, Forte D, Legare J. Practice guidelines for clinical prevention: do patients, physicians and experts share common ground? Canadian Medical Association Journal, 1999; 161: 519–523. [PMC free article] [PubMed] [Google Scholar]
- 44. Rashidian A, Eccles MP, Russell I. Falling on stony ground? A qualitative study of implementation of clinical guidelines' prescribing recommendations in primary care. Health Policy, 2008; 85: 148–161. [DOI] [PubMed] [Google Scholar]
- 45. Oxman AD, Lavis JN, Fretheim A. Use of evidence in WHO recommendations – reply. Lancet, 2007; 370: 826–827. [DOI] [PubMed] [Google Scholar]
- 46. van der Weijden T, Legare F, Boivin A et al How to integrate individual patient values and preferences in clinical practice guidelines? A research protocol. Implementation Science, 2010; 5: doi:10.1186/1748‐5908‐5‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Abma TA, Broerse JEW. Patient participation as dialogue: setting research agendas. Health Expectations, 2010; 13: 160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]


