Abstract
Background
Clinicians, older adults and caregivers frequently meet to make decisions around treatment and lifestyle during an acute hospital admission. Patient age, psychological status and health locus of control (HLC) influence patient preference for consultation involvement and information but overall, a shared‐decision‐making (SDM) approach is favoured. However, it is not known whether these characteristics and the presence of cognitive impairment influence SDM competency during family meetings.
Objective
To describe meetings between older adults, caregivers and geriatricians in intermediate care and explore patient and meeting characteristics associated with a SDM communication style.
Methods
Fifty‐nine family meetings involving geriatricians, patients in an intermediate care setting following an acute hospital admission and their caregivers were rated using the OPTION system for measuring clinician SDM behaviour. The geriatric depression scale and multidimensional HLC scale were completed by patients. The mini‐mental state exam (MMSE) assessed patient's level of cognitive impairment.
Results
Meetings lasted 38 min (SD 13) and scored 41 (SD 17) of 100 on the OPTION scale. Nine (SD 2.2) topics were discussed during each meeting, and most were initiated by the geriatrician. Meeting length was an important determinant of OPTION score, with higher SDM competency displayed in longer meetings. Patient characteristics, including MMSE, HLC and depression did not explain SDM competency.
Conclusion
Whilst SDM can be achieved during consultations frail older patients and their caregivers, an increased consultation time is a consequence of this approach.
Keywords: decision making, frail elderly, health services for aged, physician‐patient relations
Introduction
Family meetings are a cornerstone of care of frail older adults. Combinations of medical, nursing and therapy staff frequently meet with older adults and caregivers to make important decisions about driving, moving into nursing homes and end‐of‐life care.1 These meetings can occur during intermediate care, which provides short‐term support to frail older adults not ready to return home following an acute hospital admission to allow further recovery and decision making. Family meetings differ from consultations with younger patients as older adults may have cognitive impairment and third party decision makers such as family members are often present. Whilst most clinicians regard these consultations as a forum for sharing information and decision making,2, 3 the impact of differing communication approaches is unclear.
Older adults undergoing frequent transition across institutions and systems of care are a population vulnerable to care fragmentation and subsequently poor quality of care.4 Associated risks include breakdown of care planning, discord between previous and successive medication regimens and deficiencies in communication of advanced care directives.4 Empowering families using a patient‐/caregiver‐centred approach represents an important potential strategy to facilitate transfer of information across sites and improve care quality. However, patient cognitive impairment can limit comprehension of discharge instructions and impact on the effective preparation for this new role.5 Use of a question prompt list (QPL) may encourage patient involvement during consultations and has previously assisted patients and caregivers asking questions around prognosis and end‐of‐life care in an oncology setting.6 A Cochrane systematic review across a range of clinical settings concluded that inclusion of a patient QPL or coaching before a consultation resulted in a small increase in frequency of questions asked by patients during consultations and patient satisfaction, but no statistically significant change in other outcome measures including patient anxiety, patient knowledge and consultation length.7
Patients from a wide range of clinical settings appear to favour a shared‐decision‐making (SDM) approach over a passive or autonomous role in their care, although there is some evidence to suggest that older and less educated patients may have an increased preference for passive roles.8 SDM involves both patient and clinician being explicit in their values and preferences and arriving on a mutually agreed decision.9 Tools have been developed to assess the extent clinician behaviours in consultations encourage SDM across settings, including general practice10 and oncology.11
The appropriateness of SDM in consultations is influenced by a variety of characteristics, including disease severity, disease nature (i.e. chronic or acute), medical urgency, number of treatment options and uncertainty about treatment efficacy.12 Furthermore, there is evidence that clinicians vary the level of SDM behaviour during consultations according to multiple factors, such as patient‐initiated medication requests and practice setting.13 Higher SDM competency is displayed in longer consultations in depression care13 and general practice.14 Whilst there are evaluations of decision making in other fields including general practice,14 oncology15 and psychiatry,16 investigation into SDM in geriatrics is scarce. No evaluations exist of the extent of SDM competency displayed by geriatricians in family meetings. There is increasing focus on effective communication between clinicians, older adults and caregivers during care transitions4 and acknowledgement of cognitive impairment as a possible barrier to achieving successful transitions across residential and health‐care sites.5 Evaluation of SDM behaviour in an intermediate care setting where care transfers between hospital and residential settings occur is therefore warranted.
Evidence from general practice indicates patient preference for involvement decreases in severe and chronic conditions and in patients with a high external health locus of control (HLC), whilst patient preference for information during the consult decreases as depression scores and fatalistic external HLC scores rise.17 However, the relationship between decreased preference for involvement and patient characteristics was explained by increasing age overall in this setting. Interestingly, clinicians were able to successfully predict patient preference for involvement and information, presumably on the basis of characteristics of the patient including age.17 However, it is not known what consultation or patient characteristics (such as age, HLC and depression) influence actual SDM competency in practice in intermediate care settings. Furthermore, cognitive impairment is common in intermediate care settings, and the impact of cognitive impairment on SDM competency in consultations is yet to be investigated.
The aim of this study was to describe key aspects of a pre‐discharge family meeting in a (post‐acute) intermediate care setting following an inpatient hospital stay due to illness or injury. The impact of meeting and patient characteristics including cognitive impairment on SDM competency was also assessed. We hypothesized that higher‐shared‐decision‐making behaviour would be displayed by geriatricians during longer consultations with younger patients with a high internal HLC, less severe cognitive impairment and lower depression scores.
Methods
This paper presents analysis of consultations that occurred during a randomized controlled trial focused on improving the quality of transitions over a 12‐month period following a hospitalization. The primary aim of the main trial was to investigate the effectiveness of a patient and caregiver coaching intervention on quality of care transition at 3 months and health service utilization after 12 months. This study was registered with the Australian New Zealand Clinical Trial Registry (ACTRN12607000638437). The trial occurred in a post‐acute residential care facility which provided transition care (up to 12 weeks of low intensity, goal focused therapy and nursing care focused on returning home). This type of setting is similar in structure and purpose to intermediate care settings in the UK and skilled nursing facilities in the United States. Part of the intervention involved providing families and older adults with a meeting with a geriatrician prior to discharge to encourage them in their role as care coordinators.18 Audio recordings of baseline family meetings between patient, caregiver/s and a geriatrician, used primarily as an information aid for patients and their families, were coded for the current secondary analysis. This study informs on the typical family meeting which occurs in this population, including the common issues discussed, and explores the influence of meeting and patient characteristics on clinicians SDM behaviour in this setting. Ethics approval for the trial was obtained from the Repatriation General Hospital Human Research Ethics Committee (no. 90/07).
Participants
Clinicians
Two senior geriatricians conducted the family meetings according to their usual clinical practice. They were allocated to family meetings according to availability with the two geriatricians conducting 53% and 47%, respectively. Geriatricians had not received any formal training in SDM before conducting the family meetings, and although they agreed to the meetings being recorded and coded, they were unaware of the specific focus of this study on SDM at the time of the meeting. The geriatricians were subsequently informed of the full purpose of this secondary analysis and agreed to the use of audio recordings prior to this analysis.
Patients
Patients were eligible to be included in the trial if they had an informal caregiver or family member willing to participate and were admitted to a facility for residential intermediate care (Adelaide, Australia) between May 2008 and March 2010. The nature of the service has previously been described.19 Patients and caregivers unable to communicate in English were excluded due to project budget limitations; however, patients with cognitive impairment were included if proxy, and caregiver consent to participation was obtained.
Procedure and study design
All eligible patients and their caregivers were approached for consent. For patients unable to give informed consent due to significant cognitive impairment, proxy consent from the legal guardian or family caregiver was obtained. Of the 230 patient–caregiver dyads included in the trial, 116 were randomly allocated to the intervention group by permuted block randomisation by a statistician and pharmacist external to the study.20 The remaining 114 were allocated to a usual care group which did not include meetings with geriatricians and specialist nurses and were therefore not included in the current analysis. The two groups were similar in age, gender, place of residence, type of community care services received, reason for acute admission, length of hospital stay, cognition level [mini‐mental state exam (MMSE)], physical functional level and depression scores. The intervention group had lower ratings of quality of life on the EQ‐5D21 (0.42 vs. 0.51, P = 0.03) and also lower ratings of internal HLC (23.14 vs. 24.65, P = 0.03) than the control group.
Older adults and caregivers in the intervention arm of the trial were invited to take part in a family meeting with a senior geriatrician and a nurse specialist. Meetings occurred between 12 and 83 days after admission to intermediate care, with a mean of 30 days (SD 12). Meetings were originally offered in weeks 4 and 6 of the transition care programme; timing of individual meetings was affected by both planned and unplanned transfers such as early discharge or readmission to acute care following deterioration. The purpose of this meeting was to prepare for discharge and encourage older adults and caregivers to take an active role in future health care. Although meetings were structured loosely and were able to cover broad ranges of topics according to individual patient needs, medical conditions and medications were always discussed as key components of Coleman's Care Transition Intervention.18 Geriatricians were provided with a checklist of possible topics important to inform patients and caregivers about to prepare for their future role as care transition facilitators, including red flags indicative of a worsening condition and future care options. The week before the meeting patients and caregivers were provided with a QPL to encourage general involvement and discussion about sensitive issues such as diminished capacity, mental health and long‐term care options. The QPL and geriatrician checklist were developed following discussion with expert geriatricians and loosely adapted from Coleman's Care Transition Intervention.18 See Table 1, for a summary of items from the geriatrician checklist and patient–caregiver QPL.
Table 1.
Items on geriatrician checklist and patient–caregiver question prompt list
| Geriatrician checklist topics |
| Medical conditions (previous and current) |
| Medications (purpose, precautions) |
| Red flags (physical indicators the patient should seek clinical advice) |
| Depression |
| Falls |
| Continence |
| Dementia |
| Behaviour |
| Nutrition |
| Discharge destination (and risks) |
| Decision‐making capacity (guardianship and power of attorney) |
| Good palliative care plan (advanced care planning) |
| Patient–caregivers question prompt list |
| The following list provides a starting point for thinking about the questions that are important to you and that you may choose to discuss with the geriatrician or another staff member. |
| Here are some examples of the type of questions that you may wish to ask: |
| I'm not sure what the best decision is for the future, in terms of living arrangements… |
| Some days I'm okay, other times I'm unsteady on my feet. What can I do to help this problem? |
| I'm worried about Dad's memory and if it's safe for him to continue living alone… |
| I don't know if this is normal after a stroke, but I'm concerned that Mum seems to have given up on life… |
| Where do I go for advice about Power of Attorney and Guardianship? |
Audio from meetings was recorded with permission of the patient and family members present. Of the 116 patient–caregiver dyads allocated to the intervention arm, 11 did not attend the family meeting (deceased n = 3, returned to hospital n = 2 and declined n = 6). There were 74 family meetings available on audio files for analysis. Due to project budget limitations, only 60 audio files were randomly selected and transcribed verbatim. One file was removed due to incomplete recording of the meeting, leaving a total of 59 family meetings used in this study. The two geriatricians completed 28 and 31 meetings each.
Analysis
Topics and patient–caregiver initiator score
All family meetings were analysed by two research assistants who were blinded to the patient's other outcome measures. Raters read the hard copy of the transcript whilst listening to the audiotape to pick up para‐verbal cues.
Raters received a list of topics available for Geriatricians to discuss in the meeting and were asked to assess the appearance of these topics in the meeting and list any other topics discussed. Raters also noted if the topic of discussion was initiated by the patient or caregiver.
Shared decision making
The OPTION coding system10 was used to evaluate the practitioner's SDM behaviour directed at patient and caregiver/s in the meeting. The OPTION scale consists of 12 items, which assess key competencies displayed by clinicians. These items are as follows:
draws attention to an identified problem as one that requires a decision‐making process;
states that there is more than one way to deal with the identified problem (equipoise);
assesses the patient's preferred approach to receiving information to assist decision making (e.g. discussion in consultations, read printed material, assess graphical data, use videotapes or other media);
lists options, which can include the choice of ‘no action’;
explains the pros and cons of options to the patient (taking ‘no action’ is an option);
explores the patient's expectations (or ideas) about how the problem(s) are to be managed;
explores the patient's concerns (fears) about how problem(s) are to be managed;
checks that the patient has understood the information;
offers the patient explicit opportunities to ask questions during the decision‐making process;
elicits the patient's preferred level of involvement in decision making;
indicates the need for a decision‐making (or deferring) stage;
indicates the need to review the decision (or deferment).
A 5‐point scale is used to rate each individual item for a single index problem from the consultation, ranging from ‘the behaviour is not observed’ (0) to ‘the behaviour is exhibited to a very high standard’ (4). For each item, a score of 2 indicates the behaviour displayed by the clinician has reached baseline requirements for SDM competency. Scores from the 12 items are combined to give a total score ranging from 0 to 48 and converted into a scale from 0 to 100, with higher scores indicating greater SDM competency by the clinician. Where multiple problems were presented during the meeting, the problem with the highest degree of patient involvement in decision making was selected for analysis. The OPTION scale has good reliability and validity.10, 22 The two raters were provided with a published manual for the OPTION coding system and were trained prior to coding. Training included joint and independent practice coding sessions (using example audiotapes provided with the manual) which were reviewed until a good level of agreement was achieved. Raters then rated the family meetings independently. A high level of inter‐rater reliability was achieved, with an intraclass correlation coefficient of 0.824 (95% CI = 0.570–0.916) between the two raters. Both research assistants rerated 10% of the sample to assess intrarater stability, intraclass correlation coefficients were 0.912 (95% CI = 0.482–0.987) for rater 1 and 0.96 (95% CI = 0.725–0.994) for rater 2.
Patient measures
Information on the patient's age, gender and admission dates was collected at trial entry. Patient cognitive state was assessed using the MMSE,23 a validated and widely used measure comprising of 11 questions which assess orientation, registration, attention and calculation, recall, and language. The maximum score is 30, with scores of 24 or below indicative of cognitive impairment and decreasing scores associated with increasing severity of symptoms. The MMSE is commonly used as a measure of cognition in evaluation of medical decision‐making capacity.24
As presence of depression and HLC has previously been found to influence patient preference for SDM in consultations,17 these were included in this study. The geriatric depression scale (GDS)25 was completed at trial entry by patients to assess self‐reported depression symptoms. Scores on the 15‐item scale range from 0 to 15, where higher scores indicate more severe depressive symptoms. A score of 5 is suggestive of possible depression, whilst a score of 10 or more indicates a high likelihood of depression.
Patient perceived control over health at trial entry was assessed using the multidimensional HLC scale.26 The patient is asked to rate 18 belief statements about their medical condition (e.g. I am in control of my health) on a 6‐point scale from ‘strongly disagree’ to ‘strongly agree’ which inform three independent subscales: internal HLC, powerful others external HLC and chance external HLC. A higher score indicates a greater perceived influence on control over health outcomes (i.e. a high score on the internal HLC indicates greater perceived control of health outcomes by the patient). All assessments at trial entry were conducted by a member of research staff independent of the geriatrician who attended the family meeting and other members of the patient's clinical team.
Statistical analysis
Data analyses were conducted using the Statistical Packages for Social Sciences (SPSS) version 17.01 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were used to explore the data. The relationship between SDM and patient and meeting characteristics was assessed using hierarchical multiple linear regressions. All regression analysis entered the geriatrician participating in the meeting at Step 1. The change in variance in OPTION score explained after entering patient and/or meeting characteristics at Step 2 was then determined. Separate multiple regression analysis was conducted to examine the contribution of meeting characteristics and patient characteristics to OPTION score. To generate a final regression model, all selected factors (P ≤ 0.10) from the regression analysis were entered together into a final multiple regression. Selected factors were entered in order from lowest to highest P‐value. Significance was set at P < 0.05.
Results
Patient demographics
A summary of the demographic characteristics of patients is shown in Table 2. Presence and degree of cognitive impairment varied in the group, with MMSE scores ranging from 10 to 30.
Table 2.
Characteristics of patients included in analysis (n = 59a)
| Mean | SD | |
|---|---|---|
| Age, years | 85 | 7.4 |
| Mini‐mental state examination (MMSE) | 24 | 4.8 |
| Health locus of control (HLC) | ||
| Internal | 24 | 5.9 |
| External | 21 | 6.1 |
| Chance | 24 | 5.7 |
| Geriatric depression scale (GDS) | 5.0 | 3.1 |
| Admissions 12 monthsb | 2.3 | 1.6 |
| Admission length of stay, daysc | 27 | 17 |
| n | % | |
| Male | 24 | 40.6 |
| Admission type | ||
| Musculoskeletal | 33 | 55.9 |
| Neurological | 6 | 10.2 |
| Infection | 5 | 8.5 |
| Other | 15 | 25.4 |
| Lived alone, pre‐morbid | 34 | 57.6 |
| Caregiver, pre‐morbid | 47 | 79.7 |
| Community services, pre‐morbid | 34 | 57.6 |
n = 59 except for health locus of control where n = 58.
Number of hospital admissions 12 months prior to admission to intermediate care.
Length of stay of hospital admission directly prior to intermediate care admission.
Family meeting summary
The meeting lasted 38 min (SD 13, range from 17 to 89 min). An overall mean of 9.0 (SD 2.2) topics was discussed during each meeting, and the majority were initiated by the geriatrician; patients and caregivers initiated discussion on 0.6 (SD 0.7) and 1.6 (SD 1.6) topics per meeting, respectively.
The most commonly discussed topics were medical problems (n = 59), medications (n = 59), advanced care planning (n = 55) and discharge destination (n = 53) (Table 3). Although often discussed, advanced care planning was initiated by the geriatrician in the majority of meetings (n = 54). Discussion topics commonly initiated by patients and caregivers included discharge destination (n = 17), medications (n = 14), continence issues (n = 11) and descriptions of current medical problems (n = 10). Discharge destination was the only highly initiated topic which appeared on the question prompt list distributed to patients/caregivers before the meeting, although the other topics from the QPL (falls, dementia/memory, depression and decision making) were all initiated by patients and caregivers in some cases.
Table 3.
Frequency and ranking of meeting topics (n = 59)
| Issue | Discussed | Initiated by patient | Initiated by caregiver | |||
|---|---|---|---|---|---|---|
| n | Rank | n | Rank | n | Rank | |
| Medical conditions | 59 | 1 | 3 | 3 | 7 | 3 |
| Medications | 59 | 1 | 3 | 3 | 11 | 1 |
| Advanced care planning | 55 | 3 | 1 | 6 | 0 | 11 |
| Discharge destination | 53 | 4 | 5 | 1 | 11 | 1 |
| Decision making | 38 | 5 | 0 | 10 | 6 | 4 |
| Falls | 36 | 6 | 1 | 6 | 3 | 7 |
| Red flags | 29 | 7 | 0 | 10 | 0 | 11 |
| Dementia | 27 | 8 | 1 | 6 | 3 | 7 |
| Continence | 24 | 9 | 5 | 1 | 6 | 4 |
| Nutrition | 23 | 10 | 2 | 5 | 3 | 7 |
| Depression | 17 | 11 | 1 | 6 | 2 | 10 |
| Behaviour | 8 | 12 | 0 | 10 | 4 | 6 |
Shared decision making in family meetings
Index problems selected for rating on the OPTION scale included advanced care planning (n = 47), discharge destination and residential care entry (n = 8), diagnosis and treatment (n = 3) and power of attorney (n = 1). The mean OPTION score was 41 (SD 17) of 100. Figure 1 shows the mean score for each OPTION item across the 59 family meetings. Geriatricians performed above baseline skill level (a score of 2) on ‘drawing attention to an identified problem’ (Item 1), ‘listing options available’ (Item 4), ‘offering the patient opportunities to ask questions’ (Item 9) and ‘indicating the need for decision making’ (Item 11). Mean scores for ‘assessing the patient's preferred approach to receiving information’ (Item 3) and ‘exploring patient's fears or concerns’ (Item 7) fell below a score of 1 or ‘a minimal attempt is made to exhibit the behaviour’.
Figure 1.
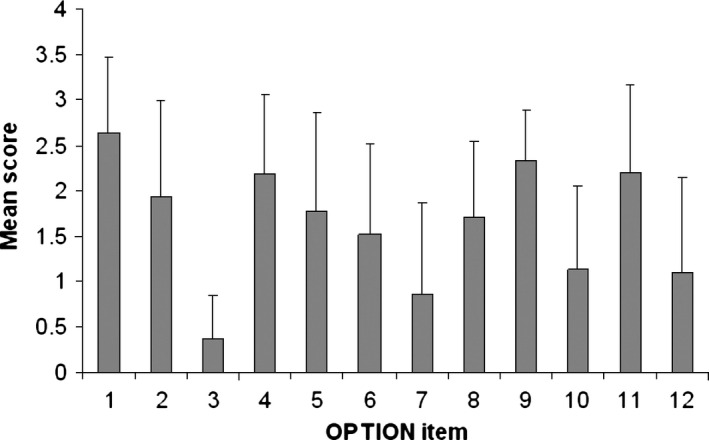
Mean score and SD for each OPTION item (n = 59).
The influence of meeting and patient characteristics on OPTION score was investigated using multiple hierarchical regression. Firstly, exploratory regression analysis was conducted to determine which patient and meeting factors should be entered in the final model. The patient characteristics age, gender, cognitive state (MMSE), HLC (internal, chance and powerful others subscales) and depression (GDS) were entered into the regression. Meeting length, number of topics discussed and frequency of patient and caregivers initiation of topics were entered into a separate regression of meeting characteristics which might influence SDM. As the main index problem assessed for the OPTION scale was a categorical variable, it was entered as dummy variables in a separate regression. Meeting length, frequency of topics initiated by caregivers, patient age and patient depression scores (GDS) were the only variables with P ≤ 0.10 from the exploratory regressions and were entered into the final model (Table 4). Other patient and meeting characteristics, including patient MMSE, did not significantly influence OPTION scores in this sample. After inclusion of significant characteristics in the final model, the total variance explained was 48% F(5, 54) = 12.026, P < 0.001. These characteristics explained an additional 12% of the variance (R 2 change = 0.119, F change (4, 54) = 3.407, P = 0.015) after controlling for the geriatrician involved at Step 1. In the final model, meeting length was the only statistically significant patient or meeting characteristic, with higher‐shared‐decision‐making competency displayed in longer meetings (β= 0.413, P = 0.017).
Table 4.
Hierarchical regression analysis of OPTION score based on meeting and patient characteristics: final model (n = 59)
| Beta | SE | Stand. Beta | P | |
|---|---|---|---|---|
|
Step 1: R
2 = 0.397 F(1, 58) = 39.9 |
0.000 | |||
| Geriatrician | −20.3 | 3.21 | −0.638 | 0.000 |
|
Step 2: R
2 change = 0.119 F change (4, 54) = 3.407 |
0.015 | |||
| Geriatrician | −16.7 | 3.38 | −0.526 | 0.000 |
| Length (min) | 0.413 | 0.169 | 0.321 | 0.017 |
| Carer topic initiation | −1.307 | 0.656 | −0.151 | 0.177 |
| Age (years) | −0.274 | 2.42 | −0.118 | 0.263 |
| GDS | 0.370 | 0.576 | 0.067 | 0.523 |
SE, standard error; stand, standardized; GDS, geriatric depression scale.
Discussion and conclusion
Discussion
This is the first study to explore clinician–patient communication and SDM behaviours in family meetings with older adults. A key finding from this study is that although clinician SDM competency increased with increasing meeting length, it did not differ across cognitive level of the patients. It is likely that as patients become more cognitively impaired and the caregiver plays a more active role in the decision‐making process, clinician SDM behaviours are directed away from the patient in favour of the caregiver.
We found that higher clinician SDM competency was displayed in meetings of longer duration. This appears to be a key finding across a range of settings, including depression care,13 general practice,14 oncology16 and family medicine27 but not in cardiology.28 Effective communication between clinicians, patients and caregivers on the complex and multiple problems that occur in older people following an acute hospital admission takes time. Previously, increased proportion of family speaking time during family meetings was associated with increased satisfaction with clinician communication and less family–physician conflict in end‐of‐life care,29 a commonly discussed topic during our family meetings. When taking a SDM approach to problems in the current study, geriatricians may have spent more time listening to patients and their families and supporting expression of their views, increasing patient–family speaking time and therefore the length of the meeting. Alternatively, time pressures and fluctuations in daily workload may have influenced the amount of time which geriatricians, patients and carers had available to complete the meeting, and SDM behaviour was limited as a result.
Communication style strengths of geriatricians included informing the patient and caregivers about the current problem (Item 1), listing options available (Item 4) and indicating the consult had reached a decision‐making stage (Item 11). Lower scores were reported for eliciting the patient and caregiver's preferred approach to receiving information (Item 3) and exploring concerns and fears (Item 7) and have also been previously reported in psychiatry,16 cardiology28 and family medicine.27 As part of the intervention, patients and caregivers were provided with information face to face, via audio recordings and written summaries which included images, so it could be argued that Item 3 was less relevant in the context of this study. Overall, it is possible that clinicians feel that asking specific questions in relation to patient involvement in decision‐making and consultation structure is unnecessary. Clinicians have previously indicated they prefer to intuitively ‘feel’ when the patient would like to participate in the decision‐making process and then respond accordingly.16 Evidence from the general practice setting that clinicians are successfully able to predict patient preferences for level of involvement in a consultation supports this,17 and these intuitive behaviours in clinician–patient communication could be explored further.
The family meetings conducted in this group aimed to encourage patient and caregivers involvement in future health care partly by use of a patient–caregiver QPL. Although family meetings included discussion of multiple topics, overall patient and caregiver initiation of discussion topics was low. Previously, cancer patients had indicated that whilst they often prefer a paternalistic decision‐making style, they do value inclusion of a QPL to aid in decision making.30 Inclusion of a QPL may result in increased satisfaction of patients during the consultation despite little detectable change in overall patient ownership of discussion in consultations.
Although, patients and caregivers did initiate some discussion of topics listed on the QPL in this study, discussions around topics not included in the QPL such as medical problems, medications and continence were frequently initiated by patients and caregivers. These may reflect topics which patients and caregivers perceive as personally important and related to their health‐care needs, or perhaps topics that patients and caregivers feel most familiar with and comfortable initiating discussion on. In contrast, whilst advanced care planning was a common topic of discussion, this was rarely initiated by patients and caregivers. Family caregivers of residential care recipients have expressed positive opinions towards advanced care planning previously; however, the recipient's reluctance to discuss the topic and presence of dementia are perceived as barriers to discussion.31 These barriers may have played a role in the limited patient–caregiver initiation of advanced care planning seen in this study.
The results of this study should be interpreted in light of some limitations. The influence of caregiver characteristics on SDM competency was not investigated. The small number of geriatricians involved in the family meetings may have limited the consultation style observed in analysis. Previous evidence points towards clinician characteristics influencing SDM competency, such as younger clinicians displaying more SDM behaviours.13 Although our analysis focussed on patient and meeting characteristics and we accounted for the geriatrician involved in our analysis, it is possible that the relationships observed between patient–meeting characteristics and OPTION score could vary depending on clinician characteristics such as level of experience.
The OPTION scale used for evaluation of SDM assesses behaviours displayed by the clinician for one index problem by an outside observer. Multiple problems were often presented during these complex family meetings. In this case, the problem with greatest patient involvement in decision making was assessed; however, SDM competency may have varied across problems within a single consultation. Furthermore, it is plausible that clinician behaviour earlier in the consultation may influence patient behaviour later on during a decision‐making process, and this was not captured in the current analysis. Furthermore, there is some evidence of discord between observations by outside coders and perceptions by patients of decision‐making involvement.32
The patients involved in this study are older than those in previous studies of SDM in psychiatry,16 oncology33 and cardiology28 but similar to previous studies in an intermediate care setting.18, 19 The inclusion of participants with cognitive impairment is also unique in investigation into SDM competency to date. The patients included in analysis were from a heterogeneous population with a wide range of conditions, which is reflective of previous samples in an intermediate care setting.18, 19 However, it is possible that SDM characteristics may differ between patient subgroups. It should also be noted that only English‐speaking patients and caregivers were included which may have limited the patient sample, but is reflective of investigations into SDM in other populations.14, 33 Overall, through inclusion of older adults across residential settings, clinical conditions and cognitive levels, results from this study are generalizable to the population of older adults who have not fully recovered from an acute hospital admission on discharge and are admitted to an intermediate care setting.
Conclusion
Family meetings in a residential intermediate care setting involve detailed discussions on prognosis including medical condition, function and overall. Whilst SDM can be achieved during meetings with frail older patients and their caregivers, an increased consultation time is a consequence of this approach.
Sources of funding
This study was funded by a National Health and Medical Research Council Health Services Research Grant entitled Transition care: innovation and evidence (402791). SM is the recipient of an Australian Postgraduate Award through Flinders University, Adelaide, Australia.
Conflicts of interest
We declare no conflicts of interest.
Acknowledgements
The authors would like to thank members of the COACH trial team including Helen White, Maryann Duffield, Prue Smith, Leah Couzner, Kelly Bond and Cara McGrain. Thank you to Jacqueline Peters for assistance with analysis.
References
- 1. Griffith JC, Brosnan M, Lacey K, Keeling S, Wilkinson TJ. Family meetings – a qualitative exploration of improving care planning with older people and their families. Age and Ageing, 2004; 33: 577–581. [DOI] [PubMed] [Google Scholar]
- 2. Yaffe MJ, Klvana J. Physician perspectives on the elderly patient‐family caregiver‐physician encounter. Israel Medical Association Journal, 2002; 4: 785–789. [PubMed] [Google Scholar]
- 3. Hudson P, Thomas T, Quinn K, Aranda S. Family meetings in palliative care: are they effective? Palliative Medicine, 2009; 23: 150–157. [DOI] [PubMed] [Google Scholar]
- 4. Coleman EA. Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. Journal of the American Geriatrics Society, 2003; 51: 549–555. [DOI] [PubMed] [Google Scholar]
- 5. Chugh A, Williams MV, Grigsby J, Coleman EA. Better transitions: improving comprehension of discharge instructions. Frontiers of Health Services Management, 2009; 25: 11–32. [PubMed] [Google Scholar]
- 6. Clayton JM, Butow PN, Tattersall MH et al Randomized controlled trial of a prompt list to help advanced cancer patients and their caregivers to ask questions about prognosis and end‐of‐life care. Journal of Clinical Oncology, 2007; 25: 715–723. [DOI] [PubMed] [Google Scholar]
- 7. Kinnersley P, Edwards AGK, Hood K et al Interventions before consultations for helping patients address their information needs. Cochrane Database of Systematic Reviews, 2007; Issue 3, Art. No.: CD004565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deber RB, Kraetschmer N, Urowitz S, Sharpe N. Do people want to be autonomous patients? Preferred roles in treatment decision‐making in several patient populations. Health Expectations, 2007; 10: 248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elwyn G, Edwards A, Mowle S et al Measuring the involvement of patients in shared decision‐making: a systematic review of instruments. Patient Education and Counseling, 2001; 43: 5–22. [DOI] [PubMed] [Google Scholar]
- 10. Elwyn G, Edwards A, Wensing M, Hood K, Atwell C, Grol R. Shared decision making: developing the OPTION scale for measuring patient involvement. Quality and Safety in Health Care, 2003; 12: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown RF, Butow PN, Ellis P, Boyle F, Tattersall MH. Seeking informed consent to cancer clinical trials: describing current practice. Social Science & Medicine, 2004; 58: 2445–2457. [DOI] [PubMed] [Google Scholar]
- 12. Muller‐Engelmann M, Keller H, Donner‐Banzhoff N, Krones T. Shared decision making in medicine: the influence of situational treatment factors. Patient Education and Counseling, 2011; 82: 240–246. [DOI] [PubMed] [Google Scholar]
- 13. Young HN, Bell RA, Epstein RM, Feldman MD, Kravitz RL. Physicians' shared decision‐making behaviors in depression care. Archives of Internal Medicine, 2008; 168: 1404–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weiss MC, Peters TJ. Measuring shared decision making in the consultation: a comparison of the OPTION and informed decision making instruments. Patient Education and Counseling, 2008; 70: 79–86. [DOI] [PubMed] [Google Scholar]
- 15. Butow P, Juraskova I, Chang S, Lopez AL, Brown R, Bernhard J. Shared decision making coding systems: how do they compare in the oncology context? Patient Education and Counseling, 2010; 78: 261–268. [DOI] [PubMed] [Google Scholar]
- 16. Goossensen A, Zijlstra P, Koopmanschap M. Measuring shared decision making processes in psychiatry: skills versus patient satisfaction. Patient Education and Counseling, 2007; 67: 50–56. [DOI] [PubMed] [Google Scholar]
- 17. Schneider A, Korner T, Mehring M, Wensing M, Elwyn G, Szecsenyi J. Impact of age, health locus of control and psychological co‐morbidity on patients' preferences for shared decision making in general practice. Patient Education and Counseling, 2006; 61: 292–298. [DOI] [PubMed] [Google Scholar]
- 18. Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Archives of Internal Medicine, 2006; 166: 1822–1828. [DOI] [PubMed] [Google Scholar]
- 19. Crotty M, Whitehead CH, Wundke R, Giles LC, Ben‐Tovim D, Phillips PA. Transitional care facility for elderly people in hospital awaiting a long term care bed: randomised controlled trial. BMJ, 2005; 331: 1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beller EM, Gebski V, Keech AC. Randomisation in clinical trials. Medical Journal of Australia, 2002; 177: 565–567. [DOI] [PubMed] [Google Scholar]
- 21. Rabin R, de Charro F. EQ‐5D: a measure of health status from the EuroQol Group. Annals of Medicine, 2001; 33: 337–343. [DOI] [PubMed] [Google Scholar]
- 22. Elwyn G, Hutchings H, Edwards A et al The OPTION scale: measuring the extent that clinicians involve patients in decision‐making tasks. Health Expectations, 2005; 8: 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Molloy DW, Alemayehu E, Roberts R. Reliability of a Standardized Mini‐Mental State Examination compared with the traditional Mini‐Mental State Examination. American Journal of Psychiatry, 1991; 148: 102–105. [DOI] [PubMed] [Google Scholar]
- 24. Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision‐making capacity? JAMA, 2011; 306: 420–427. [DOI] [PubMed] [Google Scholar]
- 25. Yesavage JA, Brink TL, Rose TL et al Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research, 1982; 17: 37–49. [DOI] [PubMed] [Google Scholar]
- 26. Wallston KA, Wallston BS, DeVellis R. Development of the Multidimensional Health Locus of Control (MHLC) Scales. Health Education Monographs, 1978; 6: 160–170. [DOI] [PubMed] [Google Scholar]
- 27. Pellerin MA, Elwyn G, Rousseau M, Stacey D, Robitaille H, Legare F. Toward shared decision making: using the OPTION scale to analyze resident‐patient consultations in family medicine. Academic Medicine, 2011; 86: 1010–1018. [DOI] [PubMed] [Google Scholar]
- 28. Langseth MS, Shepherd E, Thomson R, Lord S. Quality of decision making is related to decision outcome for patients with cardiac arrhythmia. Patient Education and Counseling, 2012; 87: 49–53. [DOI] [PubMed] [Google Scholar]
- 29. McDonagh JR, Elliott TB, Engelberg RA et al Family satisfaction with family conferences about end‐of‐life care in the intensive care unit: increased proportion of family speech is associated with increased satisfaction. Critical Care Medicine, 2004; 32: 1484–1488. [DOI] [PubMed] [Google Scholar]
- 30. Brown RF, Shuk E, Leighl N et al Enhancing decision making about participation in cancer clinical trials: development of a question prompt list. Supportive Care in Cancer, 2011; 19: 1227–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stewart F, Goddard C, Schiff R, Hall S. Advanced care planning in care homes for older people: a qualitative study of the views of care staff and families. Age and Ageing, 2011; 40: 330–335. [DOI] [PubMed] [Google Scholar]
- 32. Saba GW, Wong ST, Schillinger D et al Shared decision making and the experience of partnership in primary care. Annals of Family Medicine, 2006; 4: 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith A, Juraskova I, Butow P et al Sharing vs. caring–the relative impact of sharing decisions versus managing emotions on patient outcomes. Patient Education and Counseling, 2011; 82: 233–239. [DOI] [PubMed] [Google Scholar]


