Abstract
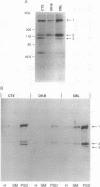
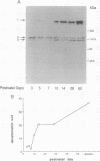
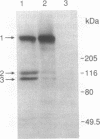
Duchenne muscular dystrophy (DMD) is a common, lethal, chromosome X-linked inherited disease. Moderate cognitive impairment is a feature of DMD, but the underlying mechanisms are unknown. DMD is characterized by a defect in a protein, dystrophin, that is located predominantly in muscle but has been detected in brain. We sought to directly localize dystrophin within the complex synaptic structure of the cerebral cortex by focusing on the postsynaptic density (PSD), which appears to be central to synaptic function. We report that a specific anti-dystrophin antibody (anti 6-10) recognizes three distinct proteins in the purified PSD: the 400-kDa dystrophin and two previously unidentified dystrophin-related proteins of 120 and 110 kDa. These proteins exhibited differential regional expression in PSDs from cerebral cortex, cerebellum, and olfactory bulb. In the cortical PSD, the 400-kDa dystrophin was predominant, whereas the 120-kDa protein was the major species in cerebellum and olfactory bulb PSDs. The three proteins were differentially expressed in the PSD during cortical development: the 400-kDa protein exhibited a selective 9-fold increase during postnatal days 7 to 10, suggesting a normal physiological role in synaptic maturation. The PSD from the mdx mouse, a model of human DMD, contained no detectable 400-kDa dystrophin but expressed the two dystrophin-related proteins. Our results indicate that brain dystrophins are localized to the PSD, potentially as three isoforms, and raise the possibility that cognitive abnormalities in DMD are attributable to synaptic dysfunction associated with deficits in brain dystrophin molecules.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonilla E., Samitt C. E., Miranda A. F., Hays A. P., Salviati G., DiMauro S., Kunkel L. M., Hoffman E. P., Rowland L. P. Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell. 1988 Aug 12;54(4):447–452. doi: 10.1016/0092-8674(88)90065-7. [DOI] [PubMed] [Google Scholar]
- Boyce F. M., Beggs A. H., Feener C., Kunkel L. M. Dystrophin is transcribed in brain from a distant upstream promoter. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1276–1280. doi: 10.1073/pnas.88.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulfield G., Siller W. G., Wight P. A., Moore K. J. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers T. J., Kunkel L. M., Watkins S. C. The subcellular distribution of dystrophin in mouse skeletal, cardiac, and smooth muscle. J Cell Biol. 1991 Oct;115(2):411–421. doi: 10.1083/jcb.115.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. P., Kahl S. D. Association of dystrophin and an integral membrane glycoprotein. Nature. 1989 Mar 16;338(6212):259–262. doi: 10.1038/338259a0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. S., Pearlman J. A., Muzny D. M., Gibbs R. A., Ranier J. E., Caskey C. T., Reeves A. A. Expression of the murine Duchenne muscular dystrophy gene in muscle and brain. Science. 1988 Mar 18;239(4846):1416–1418. doi: 10.1126/science.3347839. [DOI] [PubMed] [Google Scholar]
- Chelly J., Hamard G., Koulakoff A., Kaplan J. C., Kahn A., Berwald-Netter Y. Dystrophin gene transcribed from different promoters in neuronal and glial cells. Nature. 1990 Mar 1;344(6261):64–65. doi: 10.1038/344064a0. [DOI] [PubMed] [Google Scholar]
- Davison M. D., Critchley D. R. alpha-Actinins and the DMD protein contain spectrin-like repeats. Cell. 1988 Jan 29;52(2):159–160. doi: 10.1016/0092-8674(88)90503-x. [DOI] [PubMed] [Google Scholar]
- Feener C. A., Koenig M., Kunkel L. M. Alternative splicing of human dystrophin mRNA generates isoforms at the carboxy terminus. Nature. 1989 Apr 6;338(6215):509–511. doi: 10.1038/338509a0. [DOI] [PubMed] [Google Scholar]
- Hammonds R. G., Jr Protein sequence of DMD gene is related to actin-binding domain of alpha-actinin. Cell. 1987 Oct 9;51(1):1–1. doi: 10.1016/0092-8674(87)90002-x. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Kunkel L. M. Dystrophin abnormalities in Duchenne/Becker muscular dystrophy. Neuron. 1989 Jan;2(1):1019–1029. doi: 10.1016/0896-6273(89)90226-2. [DOI] [PubMed] [Google Scholar]
- Koenig M., Hoffman E. P., Bertelson C. J., Monaco A. P., Feener C., Kunkel L. M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987 Jul 31;50(3):509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lidov H. G., Byers T. J., Watkins S. C., Kunkel L. M. Localization of dystrophin to postsynaptic regions of central nervous system cortical neurons. Nature. 1990 Dec 20;348(6303):725–728. doi: 10.1038/348725a0. [DOI] [PubMed] [Google Scholar]
- Moser H. Duchenne muscular dystrophy: pathogenetic aspects and genetic prevention. Hum Genet. 1984;66(1):17–40. doi: 10.1007/BF00275183. [DOI] [PubMed] [Google Scholar]
- Nudel U., Robzyk K., Yaffe D. Expression of the putative Duchenne muscular dystrophy gene in differentiated myogenic cell cultures and in the brain. Nature. 1988 Feb 18;331(6157):635–638. doi: 10.1038/331635a0. [DOI] [PubMed] [Google Scholar]
- Nudel U., Zuk D., Einat P., Zeelon E., Levy Z., Neuman S., Yaffe D. Duchenne muscular dystrophy gene product is not identical in muscle and brain. Nature. 1989 Jan 5;337(6202):76–78. doi: 10.1038/337076a0. [DOI] [PubMed] [Google Scholar]
- Rosman N. P., Kakulas B. A. Mental deficiency associated with muscular dystrophy. A neuropathological study. Brain. 1966 Dec;89(4):769–788. doi: 10.1093/brain/89.4.769. [DOI] [PubMed] [Google Scholar]
- Sicinski P., Geng Y., Ryder-Cook A. S., Barnard E. A., Darlison M. G., Barnard P. J. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989 Jun 30;244(4912):1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- Siekevitz P. The postsynaptic density: a possible role in long-lasting effects in the central nervous system. Proc Natl Acad Sci U S A. 1985 May;82(10):3494–3498. doi: 10.1073/pnas.82.10.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K., Black I. B. Regulation of molecular components of the synapse in the developing and adult rat superior cervical ganglion. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8687–8691. doi: 10.1073/pnas.84.23.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K., Black I. B. Transsynaptic impulse activity regulates postsynaptic density molecules in developing and adult rat superior cervical ganglion. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6207–6210. doi: 10.1073/pnas.85.16.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K., Carlin R., Siekevitz P. Binding of L-[3H]glutamate to fresh or frozen synaptic membrane and postsynaptic density fractions isolated from cerebral cortex and cerebellum of fresh or frozen canine brain. J Neurochem. 1986 Mar;46(3):831–841. doi: 10.1111/j.1471-4159.1986.tb13047.x. [DOI] [PubMed] [Google Scholar]
- Wu K., Huang Y., Adler J., Black I. B. On the identity of the major postsynaptic density protein. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3015–3019. doi: 10.1073/pnas.89.7.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K., Sachs L., Carlin R. K., Siekevitz P. Characteristics of a Ca2+/calmodulin-dependent binding of the Ca2+ channel antagonist, nitrendipine, to a postsynaptic density fraction isolated from canine cerebral cortex. Brain Res. 1986 Nov;387(2):167–184. doi: 10.1016/0169-328x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Zubrzycka-Gaarn E. E., Bulman D. E., Karpati G., Burghes A. H., Belfall B., Klamut H. J., Talbot J., Hodges R. S., Ray P. N., Worton R. G. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature. 1988 Jun 2;333(6172):466–469. doi: 10.1038/333466a0. [DOI] [PubMed] [Google Scholar]